Introduction
Melanoma cells are transformed melanocytes of neural
crest origin. The aggressiveness and metastatic potential of
melanoma cells have been intensively studied, and yet the molecular
and cellular mechanisms through which melanoma cells behave
aberrantly remain to be elucidated. Existing data have shown that
human melanocytes (HMC) and melanoma cells have Ca2+
channels, neural Na+ channels, Cl− channels
and K+ channel (1–3). The activities of those channels have
been suggested to be associated with tumorigenesis and
metastasis.
Studies have shown that K+ channels
blockers induce a dose-dependent membrane depolarization, which
leads to the reduction of the driving force for the influx of
Ca2+ (4,5). The intracellular Ca2+
concentration [(Ca2+)i] is related to cell
proliferation because transition from G1 to S during mitosis
depends on an increase of [Ca2+]i in
mammalian cells (6). Thus, blocking
K+ channels may reduce [Ca2+]i and
attenuate tumorigenesis and metastasis.
Both HMCs and melanoma cells express Cav1 and Cav2
channels. Cav3 and T-type Ca2+ channels are only
expressed in melanoma cells and mediate constitutively
Ca2+ influx (7).
Ca2+ channels directly affect Ca2+ handling.
Previous reports have also demonstrated that another
Ca2+ channel named as the calcium release-activated
channel (CRAC) is present in 4 types of melanoma (1), although the function of those channels
is unknown.
The cardiac Na+ channel,
Nav1.5, has been found in human breast cancer cells and
human colon cancer cells. This channel possibly provides favorable
conditions for proteolytic activity of extracellular matrix
proteins in human breast cancer cells and drives colon cancer
invasion (8,9). While there is no report to show that
this channel is expressed in HMCs, neural Na+ channel,
Nav1.6, was found in melanoma cells. Nav1.6
participates in the control of podosome and invadopodia formation
and regulates cellular invasion in these cells (10). It is still unclear whether
Na+ channels affect cellular behavior by altering
Ca2+ homeostasis.
As Ca2+ uptake and release are
significantly increased during the action potentials (APs) in
cardiac myocytes, we assume that Na+ channels together
with other channels participate in the formation of APs
depolarization phase and increase Ca2+ influx and efflux
in HMC and melanoma cells. Studies have indicated that ultraviolet
(UV) light induces APs in both HMC and melanoma cells. UV light
depolarizes cell membrane through transient receptor potential A1
(TRPA1) channels, which can be blocked by a specific antagonist
HC-030031. The increase of [Ca2+]i occurs
during depolarization phase of APs, and intracellular
Ca2+ decays when the membrane is hyperpolarized
(11–13). UV light activates a
Gαq/11-coupled phototransduction pathway in HMCs
(12). However, the function of
Na+ channels in AP needs to be studied.
Here, we present a novel discovery that functional
cardiac Na+ channels, expressed in human melanoma cells
(WM 266-4) but not in HMCs, depolarize the resting membrane
potential (RMP) and decrease [Ca2+]i. Our
research provides insights into the understanding of the molecular
mechanism of the aggressiveness of melanoma cells which is related
with Ca2+ homeostasis and may lead to novel clinical
management of human melanoma like WM 266-4.
Materials and methods
Cell culture
Human skin melanocytes (ATCC®
PCS-200-012™, Manassas, VA) were cultured in 254 medium (Life
Technologies, Grand Island, NY, USA) with growth factor
supplements. Human melanoma WM 266-4 cells were maintained in DMEM
supplemented with 10% fetal bovine serum (FBS),
penicillin/streptomycin (1:100; all Sigma, St. Louis, MO, USA) and
100 mM sodium pyruvate, in a CO2 incubator at 37°C.
Immunofluorescence staining and
confocal microscopy
Cells were plated in 8-well chamber slides (Lab-Tek,
Nalge Nunc International, Naperville, IL, USA) and washed in
phosphate balanced saline (PBS) to remove traces of medium. The
cells were then fixed for 20 min in fresh 4% paraformaldehyde-PBS,
permeabilized and blocked with normal goat serum (diluted 1:10) for
2 h in PBS. Cells were then washed three times in PBS and incubated
with primary antibodies, anti-Nav1.1 (ASC-001),
-Nav1.2 (ASC-002), -Nav1.3 (ASC-004),
-Nav1.5 (ASC-005) (14),
and anti-Nav1.6 (ASC-009) (15) from Alomone Labs (Jerusalem, Israel)
1:100, 1 h at room temperature. Those antibodies were recommended
to be used in WB for human tissue, and also widely in
immunofluorescence studies (14,15). The
primary antibody incubation was followed by another three times PBS
wash and incubation in secondary antibodies, Alexa
Fluor® 594 goat anti-rabbit IgG (dilution in PBS 1:500)
and Alexa Fluor® 488 goat anti-mouse IgG (dilution in
PBS 1:500, green; Life Technologies) at room temperature for 1 h.
The cell nuclei were also stained with Hoechst 33342 (1 µg/ml in
PBS) for 10 min. The slides were mounted with anti-fade (Life
Technologies) and kept in the dark until viewing. The samples were
observed under a confocal microscope (Carl Zeiss AG, Oberkocken,
Germany) and images were captured by Zen 2009 Light Edition.
Western blot analysis
Cells were cultured in 6-well plates, treated under
respective treatments and then lysed using RIPA buffer. The lysates
were then run through 10% SDS-PAGE. The gels were run through
semi-dry transfer (Trans-blot SD Semi-dry Transfer Cell; Bio-Rad,
Hercules, CA, USA) onto PVDF membranes. Membranes were blotted with
primary antibodies 4°C over night. Anti-Nav1.1, -Nav1.2,
-Nav1.3, -Nav1.5, and anti-Nav1.6
are from Alomone Labs (1:1,000). Secondary antibodies,
IRDye® 680LT goat anti-mouse (dilution in PBS 1:10,000,
red) and IRDye® 800CW goat anti-rabbit (1:10,000; LI-COR
Biosciences, Lincoln, NE, USA). The secondary antibodies were
incubated for 1 h at room temperature. Beta actin was used as a
housekeeping control gene in western blotting. The proteins were
then detected with Li-COR Odyssey imaging system.
Electrophysiological recordings
Perforated whole-cell current-clamp by an
Axopatch-200B amplifier (Molecular Devices, Foster City, USA) was
used to record APs at room temperature. The glass pipettes were
filled with 120 mmol/l potassium gluconate, 20 mmol/l KCl, 5 mmol/l
NaCl, 5 mmol/l HEPES, and 5 mmol/l MgATP (pH 7.2). The
extracellular bathing solution (Tyrode solution) contained (in
mmol/l) 140 mmol/l NaCl, 5.4 mmol/l KCl, 1 mmol/l MgCl2,
10 mmol/l HEPES, 1.8 mmol/l CaCl2, and 5.5 mmol/l
glucose (pH 7.4). β-escin (50 µmol/l) was added to pipette
solution. Pipette resistances were ~5 MΩ. Records were low-pass
filtered at 10 kHz and digitized at 20 kHz. The exposure to 15
mJ/cm2 UV light (280–320 nm) with duration of 12s was
applied to induce depolarization of cells (11–13).
Na+ channel currents were measured by
using the whole-cell patch-clamp technique in the voltage-clamp
configuration at room temperature. Healthy and tightly attached
cells, with excellent morphological appearance, were used in this
experiment. To measure Na+ channel currents, pipettes
(1–2 MΩ) were filled with a pipette solution containing: 80 mmol/l
CsCl, 80 mmol/l cesium aspartate, 11 mmol/l EGTA, 1 mmol/l
MgCl2, 1 mmol/l CaCl2, 10 mmol/l HEPES, and 5
mmol/l Na2ATP (adjusted to pH 7.4 with CsOH). The bath
solution is Tyrode solution. The holding potential was −100 mV. A
voltage step of 200 ms ranging from −80 to +60 mV with steps of 10
mV was applied to establish the presence of Na+ channel
currents. The peak current density was used to plot I–V curves. The
holding potential was −120 mV for inactivation measurement. A
voltage step of 400 ms ranging from −120 to +30 mV with steps of 10
mV was applied before the final voltage −20 mV with duration of 40
ms to elicit the inactivation of Na+ currents. Low pass
filter was set as 10 kHz and currents were sampled at a frequency
of 20 kHz. Cell capacitance and series resistance (>80%) were
compensated (16).
Intracellular
(Ca2+)i measurement
Fluo-4 AM (2 µM; Thermo Fisher Scientific, Inc.,
Franklin, MA, USA) was load to cells for 20 min in Tyrode solution
at room temperature. Then cells were washed out three times by
Tyrode solution and followed by a 20 min de-esterification
(17). Cells were transferred onto
the stage of a real-time florescence microscope (Olympus IX81;
Olympus Corp., Tokyo, Japan). The images (2,048 × 2,048 pixels)
were acquired at a room temperature. Analysis of the signals was
performed with the software MetaMorph (version 7.8.11.0, Nashville,
TN, USA). Ca2+ transients are presented as
background-subtracted normalized fluorescence (F/Fo). Basal
cytosolic (Ca2+)i in those cells was
measured.
Statistics
Data are shown as the mean ± standard error. The
t-test was employed for two groups statistical analysis. Bonferroni
correction and analysis of variance were performed for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference. SigmaPlot (version 11.0; Systat Software,
Inc., San Jose, CA, USA) was used for statistical analysis.
Results
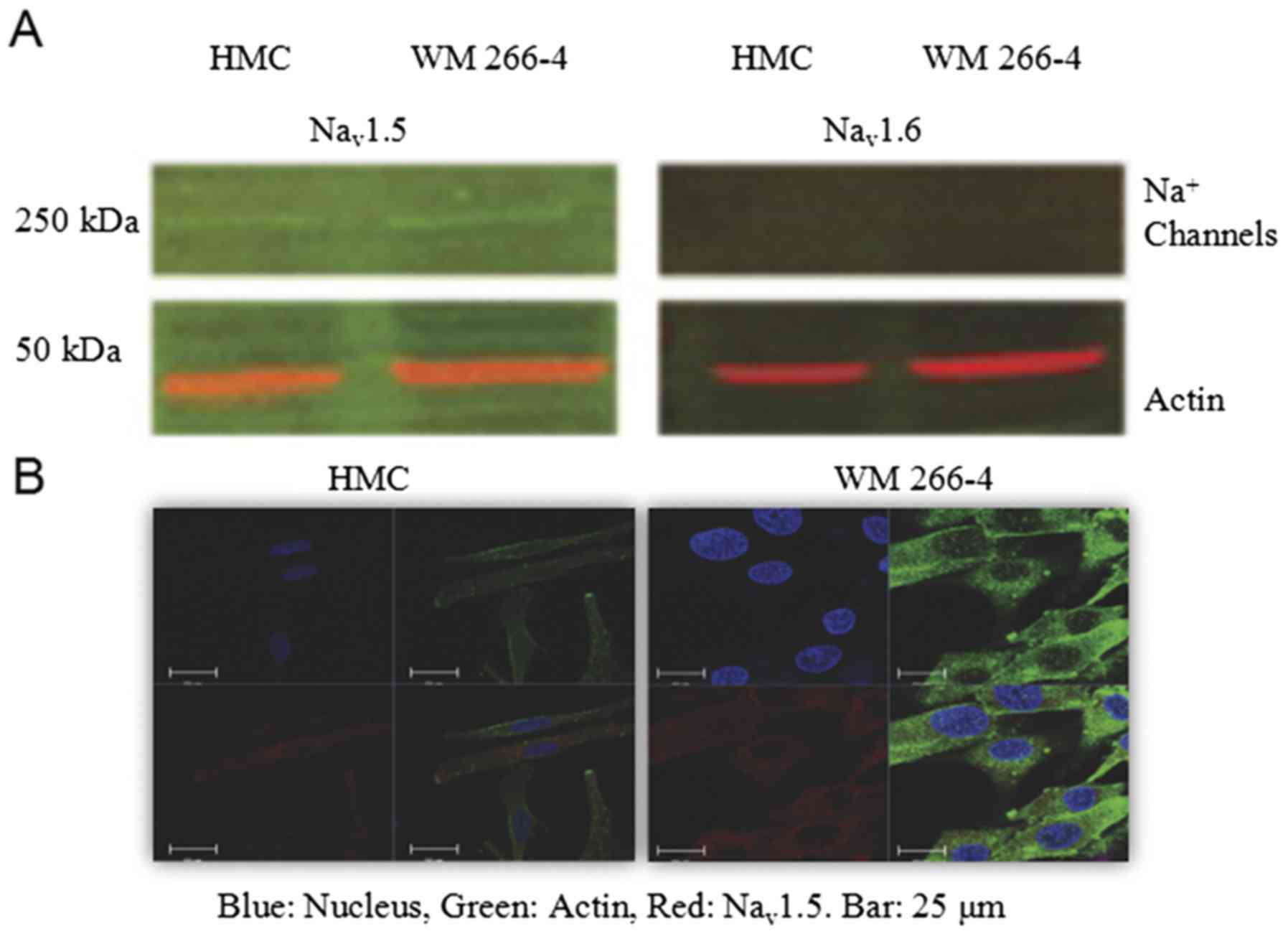
Expression of Na+ channels
in HMC and WM 266-4 cells
If Na+ channel expression changes are to
contribute to malignancy, their expression should differ between
benign and malignant cells. Therefore, we compared the expression
of various Na+ channels between HMCs and melanoma cells
(WM 266-4). Nav1.5 is cardiac Na+ channel.
Nav1.4 is expressed in skeletal muscle while
Nav1.1, Nav1.2, Nav1.3, and
Nav1.6 subtypes are found in central nerve system.
Nav1.7, Nav1.8 and Nav1.9 subtypes
are peripheral neural Na+ channels observed in dorsal
root ganglion (18). We first
investigated the expression of central neural Na+
channels and cardiac Na+ channels in both HMC and WM
266-4. Western blot analysis indicated that Nav 1.5 was
expressed in both HMC and WM 266-4, but much less pronouncedly in
HMCs. Nav1.6 subtype was not observed in either HMC or
WM 266-4 (Fig. 1A). Neither was
Nav1.1, Nav1.2, Nav1.3 (data not
shown). Confocal microscopy results confirmed that only
Nav1.5 was expressed in both HMC and WM 266-4. The
fluorescence intensity of Nav1.5 was almost evenly
distributed in the whole cell except the area of the nucleus
(Fig. 1B). Two different secondary
antibodies for actin in Western and Immunofluorescence from two
different sources were used. In western blot, actin is red [Goat
anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody,
Alexa Fluor Plus 488; cat. no. A32723], and in Immunofluorescence,
actin is green (Alexa Fluor® 488 goat anti-mouse IgG).
In Immunofluorescence or Confocal, those cells are derived from
pigment cells which have numerous vesicles. Therefore, those
actin-coated granules are likely melanosomes.
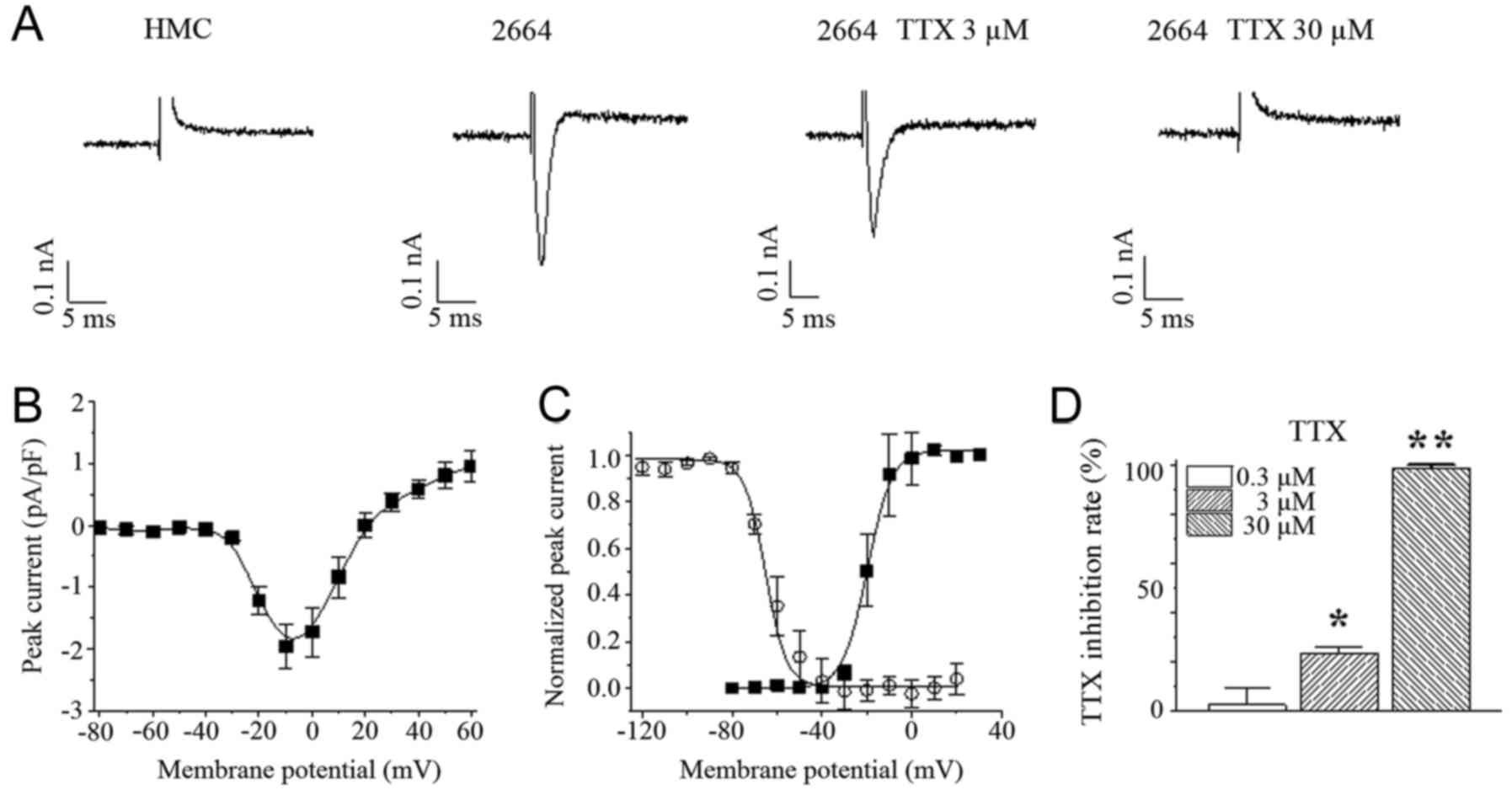
Cardiac Na+ currents
recorded in WM 266-4 cells
Then, we performed patch-clamp technique to measure
Na+ currents. The results showed that there was no
Na+ current recorded in HMCs. The maximum current
density of Na+ channels obtained in WM 266-4 was 1.7±0.3
pA/pF at −10 mV (Fig. 2A and B).
There was a tiny window of Na+ current from −60 to −20
mV (Fig. 2C). Na+ currents
recorded could be partially blocked (by 23.1±2.8%) by 3 µM of
tetrodotoxin (TTX) and almost totally blocked by 30 µM TTX in WM
266-4, while 300 nM TTX almost had no effect on Na+
currents in WM 266-4 (Fig. 2A and D).
This suggests that Nav1.5 play a predominant role in
Na+ currents in WM 266-4.
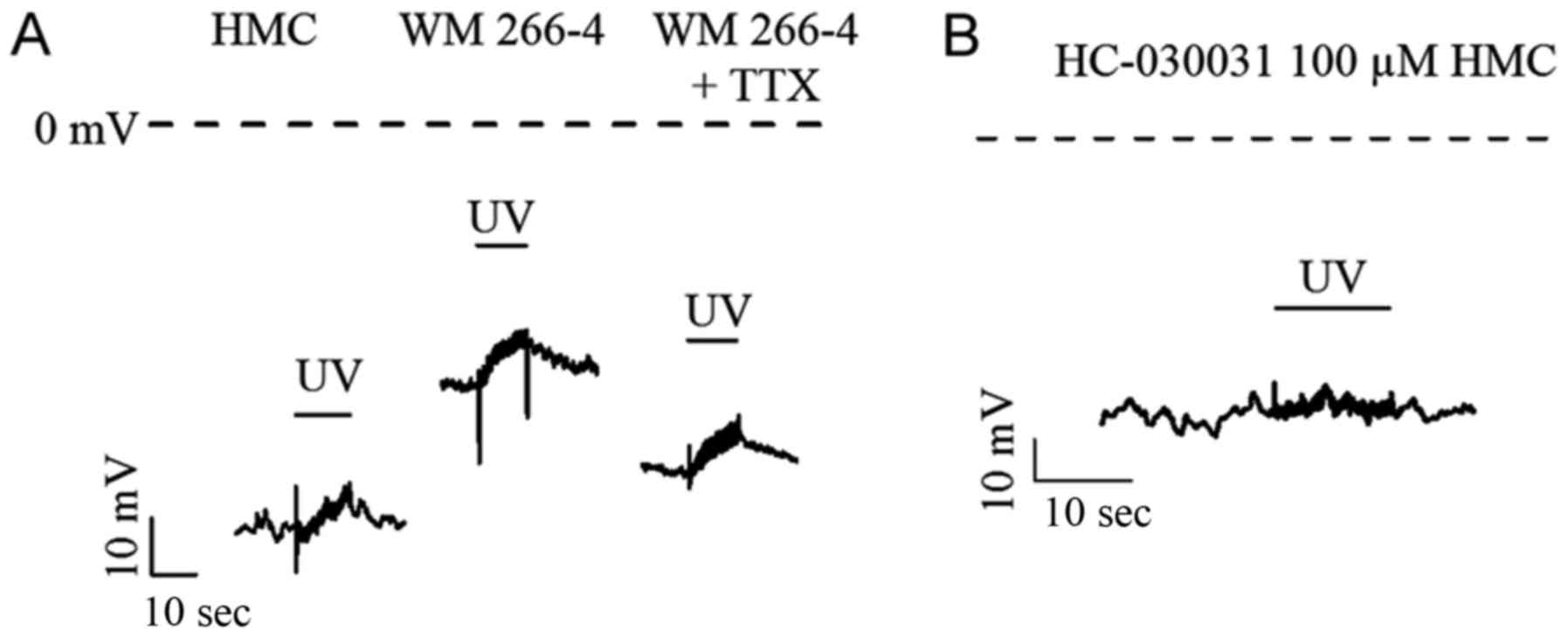
APs recorded in HMC and WM 266-4
Interestingly, melanoma cells had depolarized RMPs
of −50.5±2.7 mV, which would be expected to generate a cardiac
Na+ channel window current (Fig. 2C). The RMP could be significantly
hyperpolarized by 30 µM TTX. UV light, at 15 mJ/cm2
(280–320 nm) with duration of 12s, could induce similar APs in both
HMC and WM 266-4 (Fig. 3A and
Table I). There was no significant
difference in kinetic parameters (i.e., activation time constant
and inactivation time constant of APs). The amplitudes of APs in WM
266-4 were slightly smaller than those in HMC. TTX, 30 µM, could
partially increase the AP amplitude, time constants and prominently
hyperpolarize RMP in WM 266-4 (Table
I). AP recorded from HMCs was totally abolished by TRPA1
specific blocker, 100 µM HC-030031 (Fig.
3B). This suggests that the contribution of Na+
channels to APs is very limited.
 | Table I.APs evoked by UV light. |
Table I.
APs evoked by UV light.
| Group | RMP (mV) | APA (mV) | τrise
(sec) | τdecay
(sec) | n |
|---|
| HMC | −70.3±4.1 | 9.7±1.1 | 8.8±1.7 | 28.2±12.5 | 9 |
| WM 266-4 |
−50.5±2.7a | 8.0±0.5 | 6.6±0.9 | 15.3±5.8 | 6 |
| WM 266-4 + TTX |
−61.0±2.9b | 8.7±1.0 | 7.4±0.8 | 23.4±5.1 | 6 |
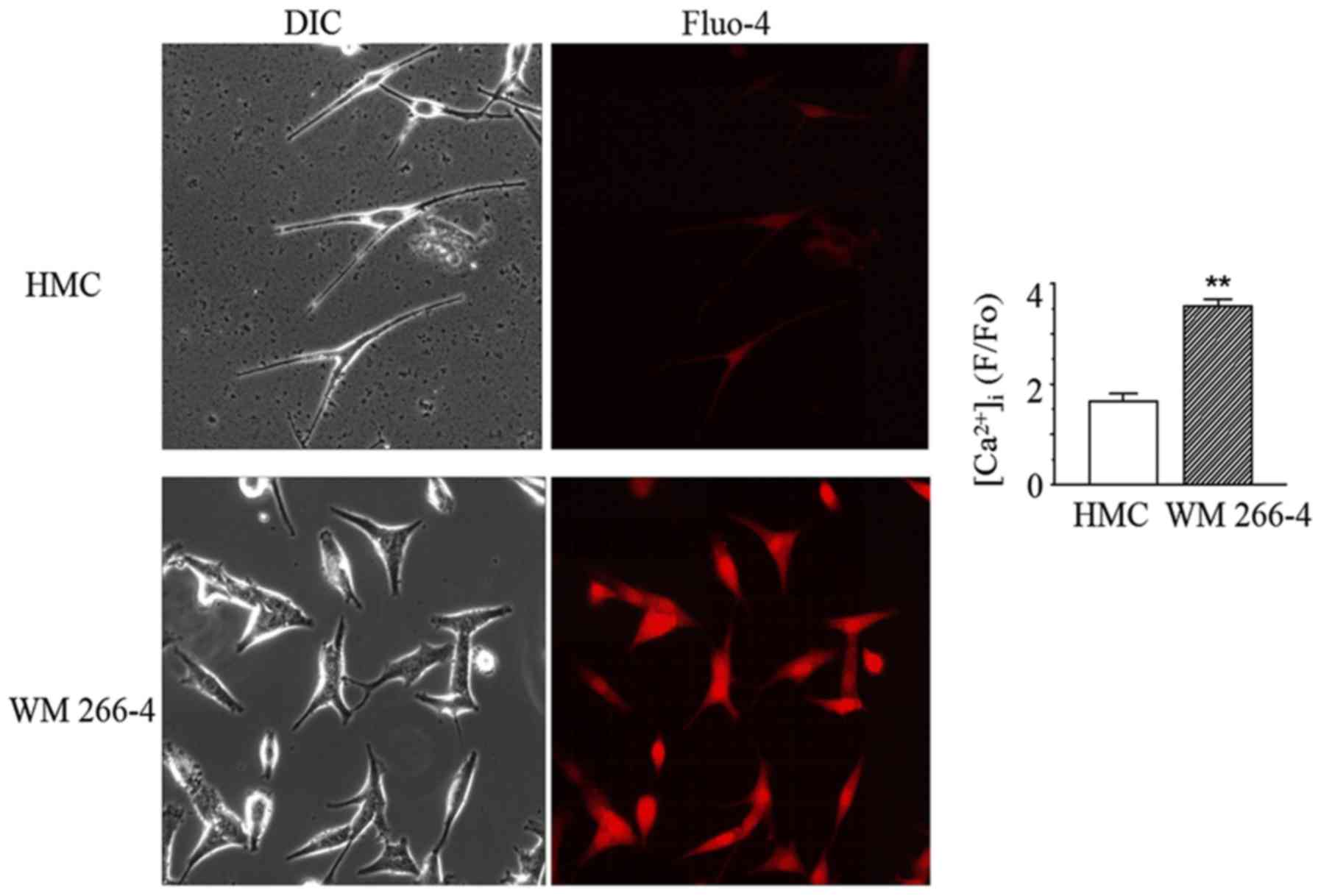
The cytoplasmic Ca2+ images
in both HMC and WM 266-4
One possibility for how the presence of Na channels
can affect malignancy is that they alter Ca2+ handling.
Therefore, we measured cytoplasmic Ca2+ in both HMC and
WM 266-4 cells. The results showed that the normalized fluorescence
intensity (F/Fo) of HMC was significantly less than that in WM
266-4 (Fig. 4). Compared to WM 266-4
cells, HMCs had only 47% of the basal Ca2+. In WM 266-4
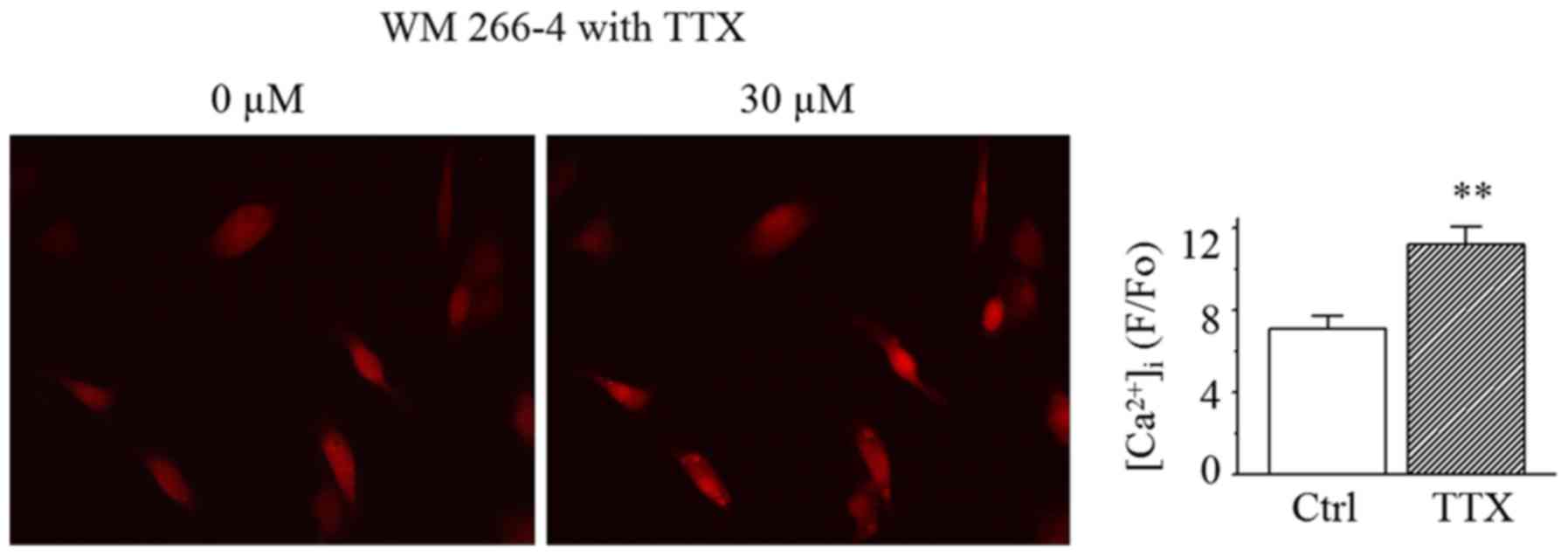
cells but not HMCs, the normalized fluorescence intensity increased
robustly from 7.2±0.6 to 11.2±0.9 after 30 µM TTX was applied into
bath solution for 15 min (Fig. 5).
This suggests that Na+ channels are contributing to
reduce Ca2+ entry in melanoma cells.
Discussion
It had been reported consistently that increase of
[Ca2+]i can enhance oncogene-induced
tumorigenesis and metastasis. Intracellular
[Ca2+]i is related to cell proliferation
because transition from G1 to S during mitosis depends on an
increase of [Ca2+]i in mammalian cells
(6). Our data showed that WM 266-4
melanoma cells have a higher intracellular calcium concentration
than that in normal human skin melanocytes, thus supporting the
above notion.
However, existing publications about the
relationship between RMP and oncogene-induced tumorigenesis and
metastasis are inconsistent. It has been argued that enhanced
expression of Ca2+-activated K+ channels
during cell proliferation provides a positive-feedback mechanism
with a result of long-term changes in [Ca2+]i
that are required for the G1-S transition in the cell cycle
(5,6).
There is a reverse linear correlation between
[Ca2+]i and membrane potential in the range
from −100 to +50 mV. Thus, overexpression of K+ channels
could hyperpolarize membrane and increase Ca2+ influx
driving force and lead to enhancement of
[Ca2+]i (5). On
the contrary, if Kir4.1 is overexpressed, the hyperpolarized
membrane potential could reduce tumor induction by two canonical
oncogenes (19). Therefore, forced
hyperpolarization is suggested to reduce tumor incidence.
In the present study, we found that the current
density of cardiac Na+ channels is small but sufficient
to depolarize the cell membrane to voltages within the
Na+ channel window current. In WM 266-4 melanoma cells,
RMP was −50±2.7 mV, which is in the voltage range of Na+
channel window current, from −60 to −20 mV. The TTX experiments
demonstrate that the Na+ channel current contributes to
the depolarization of WM 266-4 cells' RMP. When Na+
channels were blocked, RMP is hyperpolarized.
Our experimental results then suggest that RMP
determines the driving force for basal Ca2+ uptake.
Ca2+ uptake through the membrane is voltage dependent by
affecting the Ca2+ uptake driving force. In our case, it
seems that cardiac Na+ channel opening depolarizes the
cell membrane and reduces Ca2+ uptake driving force.
This would protect cells from Ca2+ overload. If
Na+ channels are blocked, RMP becomes more
hyperpolarized, and Ca2+ is increased. This may result
in the induction of tumorigenesis and metastasis.
The APs induced by UV light are assumed by inward
Na+ currents. But our experiment results prove that the
induced APs are caused by TRPA1 channels. The APs induced by UV
light in HMC and WM 266-4 cells are similar. These results suggest
that the difference of [Ca2+]i in HMC and WM
266-4 is determined by RMP, not the APs evoked by UV light.
Na+ channel-specific antagonist, TTX, is
used as a therapeutic agent for cancer-related pain (20). The administration of TTX at doses
below those that interfere with the generation and conduction of
APs in normal (non-injured) nerves has been used in humans and
experimental animals under different pain conditions. Nanomolar
concentrations of TTX block Nav1.1, Nav1.2,
Nav1.3, Nav1.4, Nav1.6, and
Nav1.7 subtypes (TTX-sensitive Na+ channel),
whereas significantly higher (micromolar) concentrations are needed
to block Nav1.5, Nav1.8 and Nav1.9
subtypes (TTX-resistant Na+ channel) (21). Therefore, there is no deleterious side
effect on melanoma when nanomolar TTX is applied as a pain-killer.
On the other hand, TTX is used to identify Na+ channel
subtypes (22,23). While Nav1.5 had a median
effective concentration of TTX at 5.7 µM, the median effective
concentrations of TTX to Nav1.8 and Nav1.9
are 60 and 40 µM respectively (18).
In our experiments, Na+ currents recorded in WM 266-4
cells were almost completely blocked by 30 µM TTX and 300 nM TTX
almost had no effect on Na+ currents. This result
suggests that almost all Na+ currents recorded in WM
266-4 cells are from Nav1.5.
We also performed test on another cell line, A375,
using same culture conditions was also performed and results showed
no Na+ current. Melanoma cell lines are extremely
heterogeneous, Studies showed that 40% of C8161 and C8146 cells
have a voltage-activated Na+ channel, and SK28 and C832C do not
have Na+ channel as A375 cells we used (1). 100% of WM 266-4 cells have
voltage-activated Na+ channels and we had identified
them as a Nav1.5 subtype. During patch clumping procedure, the test
solution for both melanocytes and melanoma cells was the same. The
recordings were similar. Thus we assume that culture medium we used
is not a factor for channel expression, and tests on more cell
lines should be performed in the future.
In conclusion, functional cardiac Na+
channels are only expressed in WM 266-4 melanoma cells. Cardiac
Na+ currents depolarize cell membrane, reduce
Ca2+ uptake driving force and have no relationship with
UV light elicited APs that work through TRPA1 channels. WM 266-4
cells have an increased [Ca2+]i which is
suggested to facilitate tumorigenesis and metastasis. The blocking
of cardiac Na+ channels in WM 266-4 cells hyperpolarizes
cell membrane and increases Ca2+ influx driving force
and enhanced [Ca2+]i. Thus, cardiac
Na+ channels work to reduce melanoma cell proliferation
and act as a protective function. In the meantime, cardiac
Na+ channel is a biomarker of carcinoma such as WM 266-4
melanoma as it can depolarize cell membrane. While further studies
are still ongoing, this novel discovery provides insights into the
understanding of the complex cellular and molecular mechanisms of
the aggressive behavior of various forms of melanoma.
Acknowledgements
Not applicable.
Funding
This research project was supported by a grant from
the National Institute of Health (grant no. P20RR016457; from the
INBRE Program of the National Center for Research).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
AX, HDC, YC and YW conceived and designed the
study. AX, BG, HG, SCD, AG, MC, AM and FF performed research. AX,
SCD and YW analyzed the data. AX and HG wrote the manuscript. AX,
HG, YW, HDC, YC and SCD revised the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Allen DH, Lepple-Wienhues A and Cahalan
MD: Ion channel phenotype of melanoma cell lines. J Membr Biol.
155:27–34. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ekmehag B, Persson B, Rorsman P and
Rorsman H: Demonstration of voltage-dependent and TTX-sensitive
Na(+)-channels in human melanocytes. Pigment Cell Res. 7:333–338.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zahradníková A, Zahradník I and Rýdlová K:
Single-channel potassium currents in human melanoma cells. Gen
Physiol Biophys. 7:109–112. 1988.PubMed/NCBI
|
|
4
|
Lepple-Wienhues A, Berweck S, Böhmig M,
Leo CP, Meyling B, Garbe C and Wiederholt M: K+ channels and the
intracellular calcium signal in human melanoma cell proliferation.
J Membr Biol. 151:149–157. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nilius B and Wohlrab W: Potassium channels
and regulation of proliferation of human melanoma cells. J Physiol.
445:537–548. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nilius B, Schwarz G and Droogmans G:
Control of intracellular calcium by membrane potential in human
melanoma cells. Am J Physiol. 265:C1501–C1510. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Das A, Pushparaj C, Bahí N, Sorolla A,
Herreros J, Pamplona R, Vilella R, Matias-Guiu X, Martí RM and
Cantí C: Functional expression of voltage-gated calcium channels in
human melanoma. Pigment Cell Melanoma Res. 25:200–212. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gillet L, Roger S, Besson P, Lecaille F,
Gore J, Bougnoux P, Lalmanach G and Le Guennec JY: Voltage-gated
sodium channel activity promotes cysteine cathepsin-dependent
invasiveness and colony growth of human cancer cells. J Biol Chem.
284:8680–8691. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
House CD, Vaske CJ, Schwartz AM, Obias V,
Frank B, Luu T, Sarvazyan N, Irby R, Strausberg RL, Hales TG, et
al: Voltage-gated Na+ channel SCN5A is a key regulator of a gene
transcriptional network that controls colon cancer invasion. Cancer
Res. 70:6957–6967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carrithers MD, Chatterjee G, Carrithers
LM, Offoha R, Iheagwara U, Rahner C, Graham M and Waxman SG:
Regulation of podosome formation in macrophages by a splice variant
of the sodium channel SCN8A. J Biol Chem. 284:8114–8126. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bellono NW, Kammel LG, Zimmerman AL and
Oancea E: UV light phototransduction activates transient receptor
potential A1 ion channels in human melanocytes. Proc Natl Acad Sci
USA. 110:2383–2388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bellono NW, Najera JA and Oancea E: UV
light activates a Gαq/11-coupled phototransduction pathway in human
melanocytes. J Gen Physiol. 143:203–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bellono NW and Oancea E: UV light
phototransduction depolarizes human melanocytes. Channels (Austin).
7:243–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu Q, Patel D, Zhang X and Veenstra RD:
Changes in cardiac Nav1.5 expression, function, and acetylation by
pan-histone deacetylase inhibitors. Am J Physiol Heart Circ
Physiol. 311:H1139–H1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anderson LL, Hawkins NA, Thompson CH,
Kearney JA and George AL Jr: Unexpected efficacy of a novel sodium
channel modulator in dravet syndrome. Sci Rep. 7:16822017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu M, Sanyal S, Gao G, Gurung IS, Zhu X,
Gaconnet G, Kerchner LJ, Shang LL, Huang CL, Grace A, et al:
Cardiac Na+ current regulation by pyridine nucleotides. Circ Res.
105:737–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mackenzie L, Bootman MD, Laine M, Berridge
MJ, Thuring J, Holmes A, Li WH and Lipp P: The role of inositol
1,4,5-trisphosphate receptors in Ca(2+) signalling and the
generation of arrhythmias in rat atrial myocytes. J Physiol.
541:395–409. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee CH and Ruben PC: Interaction between
voltage-gated sodium channels and the neurotoxin, tetrodotoxin.
Channels (Austin). 2:407–412. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lobikin M, Chernet B, Lobo D and Levin M:
Resting potential, oncogene-induced tumorigenesis, and metastasis:
The bioelectric basis of cancer in vivo. Phys Biol. 9:0650022012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hagen NA, Lapointe B, Ong-Lam M, Dubuc B,
Walde D, Gagnon B, Love R, Goel R, Hawley P, Ngoc AH and du Souich
P: A multicentre open-label safety and efficacy study of
tetrodotoxin for cancer pain. Curr Oncol. 18:e109–e116. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nieto FR, Cobos EJ, Tejada MÁ,
Sánchez-Fernández C, González-Cano R and Cendán CM: Tetrodotoxin
(TTX) as a therapeutic agent for pain. Mar Drugs. 10:281–305. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang YQ, Yang H, Sun WD, Wang J, Zhang
BY, Shen YJ, Yin MQ, Liu YX, Liu C and Yun S: Ethanol extract of
Ilex hainanensis Merr. exhibits anti-melanoma activity by induction
of G1/S cell-cycle arrest and apoptosis. Chin J Integr Med.
24:47–55. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang H, Liu C, Zhang YQ, Ge LT, Chen J,
Jia XQ, Gu RX, Sun Y and Sun WD: Ilexgenin A induces B16-F10
melanoma cell G1/S arrest in vitro and reduces tumor growth in
vivo. Int Immunopharmacol. 24:423–431. 2015. View Article : Google Scholar : PubMed/NCBI
|