Introduction
Pancreatic ductal carcinoma is the fourth most
common cause of cancer-associated mortality in the United States of
America (1). Despite recent advances
in treatment modalities, the 5-year overall survival (OS) rate of
pancreatic cancer patients is <5% (2), reflecting the aggressive invasion and
early metastasis of the disease; the presence of extra-pancreatic
dissemination at diagnosis is typical for patients with pancreatic
cancer (3–5). Although a number of molecules have been
identified to serve roles in the progression and metastasis of
pancreatic cancer, the underlying molecular mechanisms remain
unclear.
One-carbon metabolism is a network of biological
reactions that serve critical roles in DNA methylation and DNA
synthesis, and facilitate cross talk between genetic and epigenetic
processes (6) (Fig. 1). One-carbon metabolism is also
referred to as folate-mediated one-carbon metabolism and can impact
genetic and epigenetic pro-carcinogenic processes, reflecting
critical roles in DNA methylation and DNA synthesis.
Methylenetetrahydrofolate dehydrogenase 2 (MTHFD2) is an enzyme of
the mitochondrial folate metabolic pathway that functions as a
methylenetetrahydrofolate dehydrogenase and a cyclohydrolase
(6). Previous studies demonstrated
that MTHFD2 was specifically upregulated in various types of
cancer, compared with in normal tissues, and MTHFD2 has been
identified as a novel drug target with the potential to block
cancer cell migration and invasion in breast cancer and melanoma
(7,8).
In the present study, the expression of MTHFD2 and the coupling
enzymes aldehyde dehydrogenase 1 family member L2 (ALDH1L2) and
serine hydroxymethyltransferase (SHMT2), and the
clinicopathological significance of these mitochondrial folate
metabolic pathway enzymes in pancreatic cancer were
investigated.
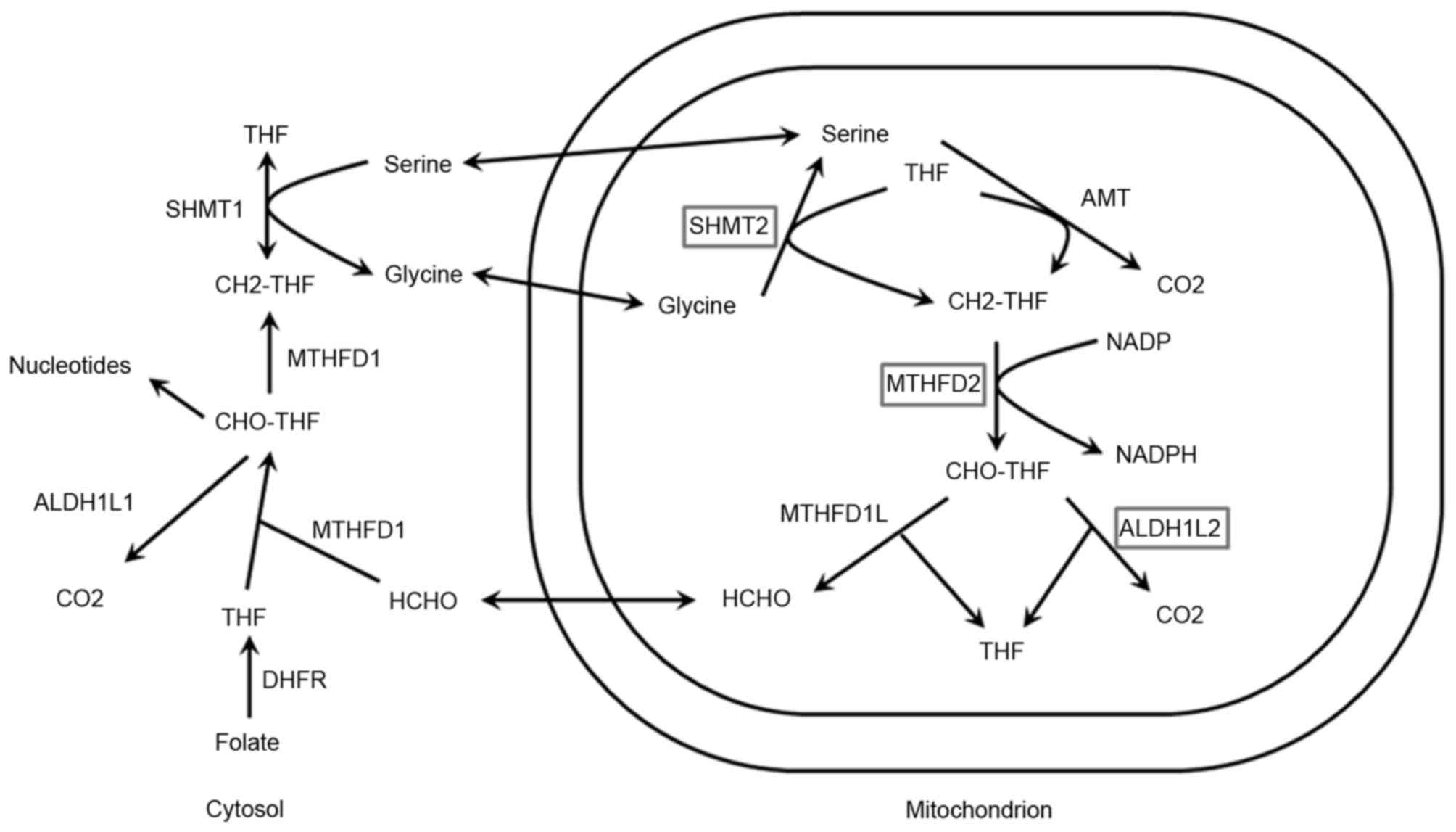 | Figure 1.Schematic of one-carbon metabolism.
Enzymes with polymorphisms investigated in the present study are
illustrated in the boxes. ALDH1L1, aldehyde dehydrogenase 1 family
member L1; ALDH1L2, aldehyde dehydrogenase 1 family member L2; AMT,
aminomethyltransferase; CH2-THF, Methylenetetrahydrofolate;
CHO-THF, formyl-tetrahydrofolate; DHFR, dehydrofolate reductase;
MTHFD1, methylenetetrahydrofolate dehydrogenase D1; MTHFD2,
methylenetetrahydrofolate dehydrogenase D2; HCHO, formaldehyde;
NADPH, nicotinamide adenine dinucleotide phosphate; SHMT1, serine
hydroxymethyltransferase 1; SHMT2, serine hydroxymethyltransferase
2; THF, tetrahydrofolate. |
Materials and methods
Patients and specimens
Between April 2007 and December 2013, a total of 103
patients underwent surgical resection for pancreatic ductal
adenocarcinoma (PDAC) at the Department of Surgery at Osaka
University Hospital (Osaka, Japan) and all patients were enrolled
in the present study (mean age, 67.2±9.6, range 38 to 84 years; 64
males and 39 females; Table I). The
clinicopathological characteristics of the enrolled patients are
presented in Table I. Patients who
underwent preoperative chemotherapy were included in this study.
The tumor stages were determined according to the 7th edition of
the American Joint Committee on Cancer/Unio Internationalis Contra
Cancrum (UICC) tumor-node-metastasis classification (9). Two pathologists examined all
histological slides, and the immunohistochemical (IHC) diagnoses of
tumors and degrees of differentiation were determined. The median
follow-up time was 60.9 months (range, 2 to 72 months), and the
regular follow-up included measurements of carcinoembryonic antigen
(CEA) and carbohydrate 19-9 (CA19-9). The present study was
approved by an institutional review board of Osaka University
School of Medicine (approved by Professor Y. Kaneda) and written
informed consent was provided by all patients.
 | Table I.Clinicopathological characteristics of
103 patients with pancreatic ductal adenocarcinoma. |
Table I.
Clinicopathological characteristics of
103 patients with pancreatic ductal adenocarcinoma.
| Variables | n (%) |
|---|
| Age (years) | 67.2±9.6 |
| Gender
(male:female) | 64 (62.1):39
(37.9) |
| Carcinoembryonic
antigen (ng/ml) | 6.68±32.9 |
| Cancer antigen 19-9
(U/ml) | 267.7±952.8 |
| Duke pancreatic
monoclonal antigen type 2 (U/ml) | 643.6±2014.5 |
| Operative procedure
(PD:DP:others) | 64 (62.1):37 (35.9):2
(2.0) |
| Preoperative
chemoradiotherapy (yes vs. no) | 63 (61.2):40
(38.8) |
| Adjuvant therapy (yes
vs. no) | 84 (81.6):19
(18.4) |
| T stage
(1:2:3:4) | 21 (20.2):9 (8.7):73
(71.2):0 (0) |
| N stage (0 vs.
1) | 68 (66.0): 35
(34.0) |
| UICC stage
(IA:IB:IIA:IIB:III:IV) | 17 (16.5):7 (6.8):44
(42.7):35 (34.0):0 (0):0 (0) |
| Histological type
(well:moderately:poor:other) | 7 (6.8):90 (87.4):5
(4.9):1 (1.0) |
| Median follow-up time
(months) | 60.9 |
Immunohistochemistry
IHC analyses were performed as described previously
(10). Briefly, surgical tissue
specimens were fixed in 10% formaldehyde, embedded in paraffin and
cut into 3.5-µm sections. The sections were then deparaffinized in
xylene, incubated at 95°C for 10 min with antigen retrieval buffer
(cat no. CTS014; Funakoshi Co., Ltd., Tokyo, Japan) and then
incubated with the following specific antibodies overnight at 4°C:
Anti-MTHFD2 (cat no. ab176016; rabbit polyclonal; dilution, 1:100),
anti-ALDH1L2 (cat no. ab170176; rabbit polyclonal; dilution, 1:200)
or anti-SHMT2 (cat no. ab64417; rabbit polyclonal; dilution, 1:300;
all Abcam, Cambridge, MA, USA). The sections were subsequently
visualized using avidin-biotin complex reagents by direct addition
(no dilution; ABC-HRP kit; Vector Laboratories, Inc., Burlingame,
CA, USA) and diaminobenzidine. The reaction was performed for 30
min at 23°C. Two reviewers performed IHC analyses independently in
a blinded manner. All IHC analyses were performed using a series of
tissue sections for each antibody.
Quantification of immunostaining
parameters
IHC analyses of MTHFD2, ALDH1L2 and SHMT2 were
performed at ×200 magnification using a light microscope. The
samples were scored according to the intensity of cytoplasmic
staining for MTHFD2 and were categorized as follows: 0, no
staining; 1, weak (weaker staining than the positive control); 2,
strong (equal to or stronger than the positive control, which
included advanced colorectal cancer tissues or normal pancreatic
acini tissues). IHC analyses of ALDH1L2 and MTHFD2 were scored in
the same manner. The positively stained cells were counted in four
representative fields of tumor regions at ×100 magnification.
Patients with scores of 1 or 0 were included in the low expression
group and those with scores of 2 were included in the high
expression group.
Statistical analysis
The associations between MTHFD2, ALDH1L2 and SHMT2
expression levels and other parameters were identified using
chi-squared tests, Fisher's exact tests or independent t-tests as
appropriate. The OS and disease-free survival (DFS) rates were
estimated using the Kaplan-Meier estimator method and were compared
using the log-rank test. Variables that were identified as
significant in univariate analyses were included in subsequent Cox
proportional hazards regression models. All statistical analyses
were performed using JMP Statistical Software (version 11; SAS
Institute Inc., Cary, NC, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
IHC analyses of one-carbon metabolism
enzymes in PDAC
The clinicopathological characteristics of the
patients included in the current study are presented in Table I. IHC analysis demonstrated that
MTHFD2, ALDH1L2 and SHMT2 were localized in the cytoplasm of
pancreatic cancer cells (Fig. 2). In
addition, MTHFD2 expression was greater in pancreatic epithelial
cancer tissue specimens compared with in surrounding non-epithelial
tissues (Fig. 2). Representative
images of ALDH1L2 scoring are demonstrated as: Score 0 in Fig. 2A, score 0 in Fig. 2B, score 2 in Fig. 2C, and score 2 in Fig. 2D. Examples of MTHFD2 scoring are
presented as: Score 0 in Fig. 2E,
score 1 in Fig. 2F, score 2 in
Fig. 2G, and score 2 in Fig. 2H. Representative SHMT2 scoring is
demonstrated as: Score 0 in Fig. 2I,
score 1 in Fig. 2J, score 2 in
Fig. 2K, and score 2 in Fig. 2L. With the exception of pathological N
stage, no significant differences were observed in the
clinicopathological parameters between the high and low expression
groups in all three enzymes (data not presented). High MTHFD2
expression was positively correlated with the incidence of lymph
node metastases (P=0.033; data not presented). IHC analyses using
advanced colorectal cancer tissues or normal pancreatic acini
tissues were used as positive controls as observed by two
pathologists. Following IHC analyses of MTHFD2, ALDH1L2 and SHMT2,
patients with scores of 1 or 0 were included in the low expression
group and those with scores of 2 were included in the high
expression group (Fig. 2).
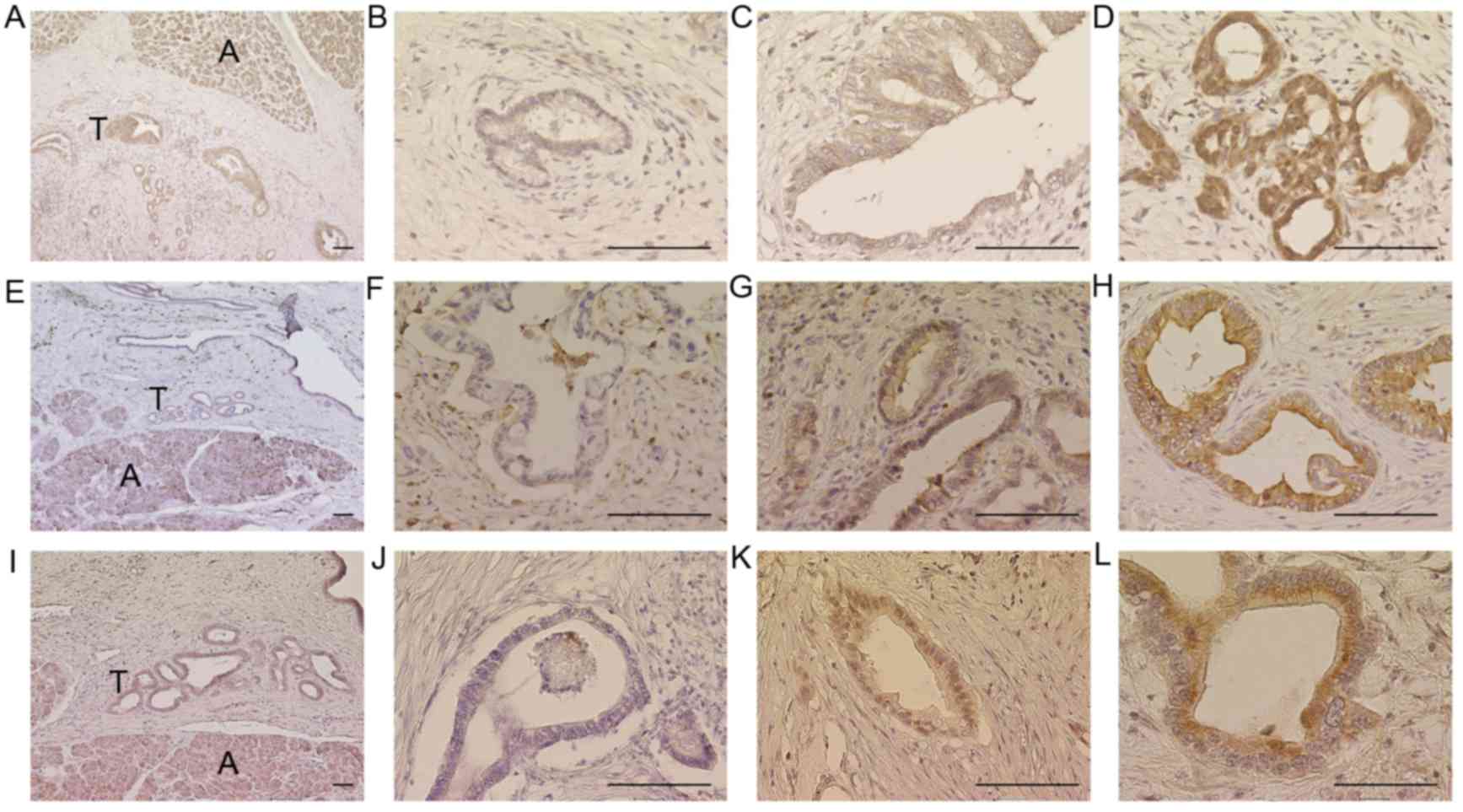 | Figure 2.Immunohistochemical analyses of
MTHFD2, ALDH1L2 and SHMT2. Representative images of ALDH1L2, (A)
score 0; (B) score 0; (C) score 2; (D) score 2. Representative
images of MTHFD2, (E) score 0; (F) score 1; (G) score 2; (H) score
2. Representative images of SHMT2, (I), score 0; (J) score 1; (K),
score 2; (L) score 2. Acini were used as positive controls. Scale
bar, 100 µm. A, Acini; T, Tumors; ALDH1L2, aldehyde dehydrogenase 1
family member L2; MTHFD2, methylenetetrahydrofolate dehydrogenase
D2; SHMT2, serine hydroxymethyltransferase 2. |
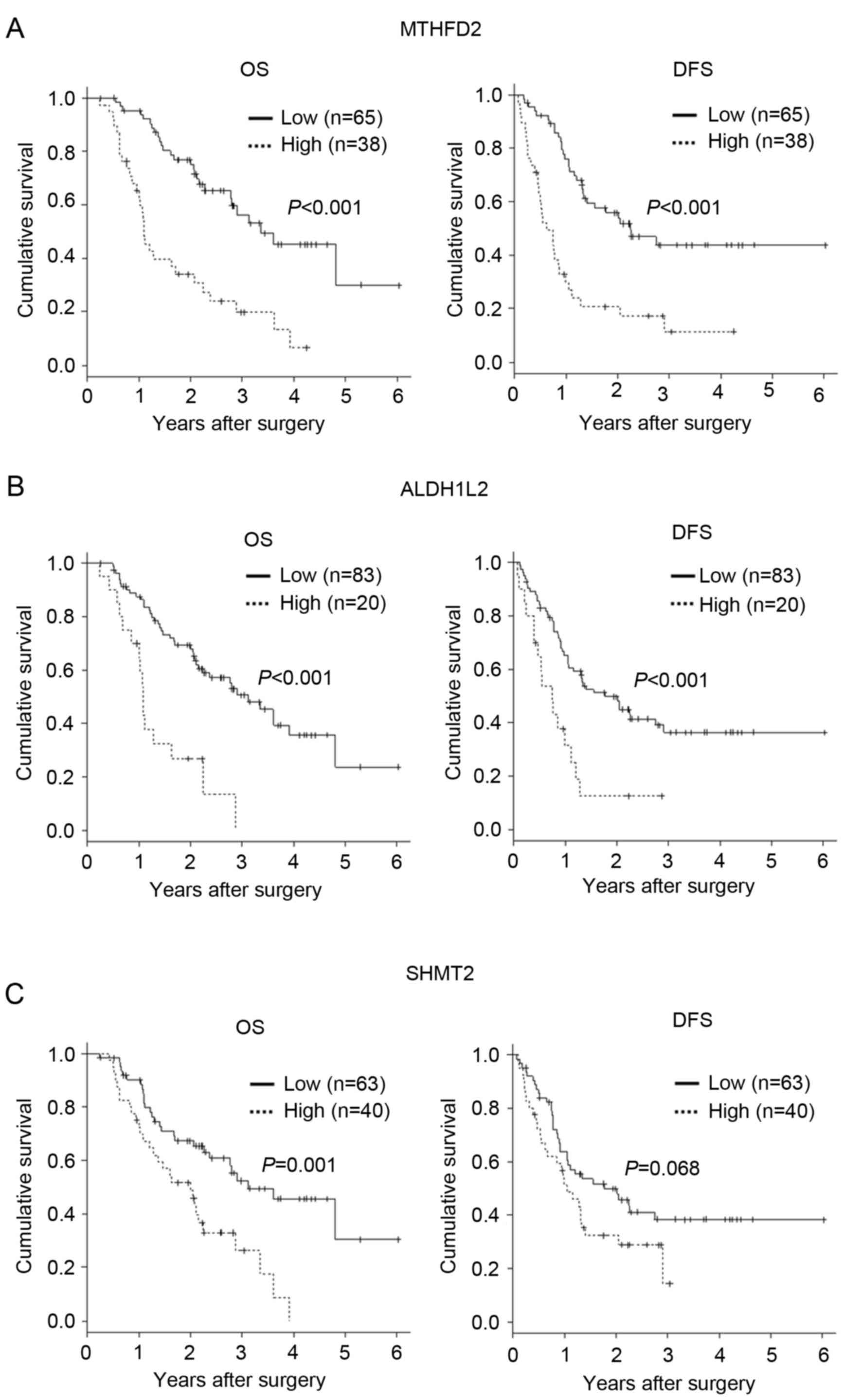
Expression of MTHFD2, SHMT2 and
ALDH1L2 in patients with PDAC correlates with poor outcomes
A previous report correlated the expression of the
mitochondrial folate metabolic pathway enzyme MTHFD2 with poor
clinical survival in breast cancer (7). Thus, we determined the association of
mitochondrial folate metabolic pathway enzymes, including MTHFD2,
ALDH1L2 and SHMT2, with clinical survival in patients with
pancreatic cancer using Kaplan-Meier estimator analyses.
Kaplan-Meier estimator curves demonstrated significantly lower DFS
among patients with high tumor MTHFD2 expression (P<0.001;
Fig. 3A). In addition, high ALDH1L2
expression was significantly associated with poor OS and DFS
(P<0.001; Fig. 3B). SHMT2
expression was significantly associated with poor OS (P=0.001), but
was not associated with DFS (P=0.068; Fig. 3C). These data suggest that one-carbon
metabolism serves an important role in the biologically malignant
features such as invasion and metastasis of pancreatic cancer. In
addition, based on the univariate analysis of OS (Table II), clinicopathological features that
correlated with poor patient survival included pathological N stage
(P<0.001), pathological UICC stage (P=0.007) and IHC scores for
MTHFD2 (P<0.001), ALDH1L2 (P<0.001) and SHMT2 (P=0.002).
Subsequent multivariate Cox proportional hazards analyses revealed
that pathological N stage and IHC scores for MTHFD2, ALDH1L2 and
SHMT2 were independent prognostic factors for OS (P=0.001, P=0.015
and P=0.017, respectively). Similarly, based on the univariate
analyses of DFS (Table III),
clinicopathological features that correlated with poor patient
survival included pathological N stage (P<0.001), pathological
UICC stage (P=0.034) and IHC scores for MTHFD2 (P<0.001) and
ALDH1L2 (P<0.001); however, SHMT2 expression was not associated
with patient survival (P=0.070). Finally, multivariate Cox
proportional hazards analyses demonstrated that pathological N
stage and IHC scores for MTHFD2 are independent prognostic factors
for OS (P=0.025 and P<0.001, respectively).
 | Table II.Multivariate analysis of overall
survival. |
Table II.
Multivariate analysis of overall
survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Clinicopathological
characteristic | HR | 95% CI |
P-valuea | HR | 95% CI | P-value |
|---|
| Age (years) | 1.05 | 0.98–1.05 | 0.262 |
|
|
|
| Gender (female vs.
male) | 0.84 | 0.50–1.42 | 0.523 |
|
|
|
| Carcinoembryonic
antigen (ng/ml) | 0.99 | 0.98–1.01 | 0.412 |
|
|
|
| Cancer antigen 19-9
(U/ml) | 0.99 | 0.99–1.00 | 0.443 |
|
|
|
| Duke pancreatic
monoclonal antigen type 2 (U/ml) | 1.00 | 0.99–1.00 | 0.241 |
|
|
| Preoperative
chemoradiotherapy (yes vs. no) | 0.97 | 0.58–1.66 | 0.939 |
|
|
|
| Adjuvant therapy
(yes vs. no) | 0.56 | 0.29–1.05 | 0.072 |
|
|
|
| T stage (3+4 vs.
1+2) | 1.76 | 0.94–3.28 | 0.077 |
|
|
|
| N stage (1 vs.
0) | 2.43 | 1.42–4.14 | 0.001 | 2.49 | 1.43–4.32 | 0.001 |
| UICC Stage (IA+IIA
vs. IB+IIB) | 2.07 | 1.22–3.51 | 0.007 |
|
|
|
| Histological type
(moderate vs. well+poor+muc) | 0.77 | 0.24–2.48 | 0.661 |
|
|
|
| MTHFD2 (high vs.
low) | 3.48 | 2.04–5.91 |
<0.001b | 2.22 | 1.17–4.20 | 0.015b |
| ALDH1L2 (high vs.
low) | 3.64 | 1.98–6.70 |
<0.001b | 2.35 | 1.16–4.74 | 0.017b |
| SHMTL2 (high vs.
low) | 2.35 | 1.38–4.02 | 0.002b | 1.39 | 0.75–2.57 | 0.299b |
 | Table III.Multivariate analysis of disease-free
survival. |
Table III.
Multivariate analysis of disease-free
survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Categories | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (years) | 1.02 | 0.99–1.05 | 0.230 |
|
|
|
| Gender (female vs.
male) | 0.78 | 0.48–1.29 | 0.336 |
|
|
|
| Carcinoembryonic
antigen (ng/ml) | 1.00 | 0.99–1.00 | 0.370 |
|
|
|
| Cancer antigen 19-9
(U/ml) | 1.00 | 0.99–1.00 | 0.242 |
|
|
|
| Duke pancreatic
monoclonal antigen type 2 (U/ml) | 1.00 | 0.99–1.00 | 0.297 |
|
|
|
| Preoperative
chemoradiotherapy (yes vs. no) | 1.28 | 0.76–2.13 | 0.353 |
|
|
|
| Adjuvant therapy (yes
vs. no) | 1.02 | 0.52–2.02 | 0.935 |
|
|
|
| T stage (3+4 vs.
1+2) | 1.48 | 0.84–2.62 | 0.175 |
|
|
|
| N stage (1 vs.
0) | 1.95 | 1.17–3.24 | 0.010 | 1.81 | 1.08–3.04 | 0.025 |
| UICC Stage (IA+IIA
vs. IB+IIB) | 1.71 | 1.04–2.80 | 0.034 |
|
|
|
| Histological type
(mod vs. well+poor+muc) | 1.13 | 0.41–3.11 | 0.820 |
|
|
|
| MTHFD2 (high vs.
low) | 3.28 | 1.99–5.41 | <0.001 | 2.59 | 1.47–4.55 | <0.001 |
| ALDH1L2 (high vs.
low) | 2.68 | 1.50–4.80 | <0.001 | 1.70 | 0.88–3.24 | 0.111 |
| SHMT2 (high vs.
low) | 1.57 | 0.96–2.61 | 0.070 |
|
|
|
Low expression of MTHFD2, SHMT2 and
ALDH1L2 correlates with improved survival among patients with
PDAC
Overexpression of MTHFD2, SHMT2 or ALDH1L2 was
associated with poor survival (Fig.
3); however, it remains unclear whether the upregulation of one
folate metabolic pathway enzyme directly enhances another folate
pathway. As tumors that depend on the folate metabolic pathway may
upregulate multiple folate metabolic pathway enzymes for
progression and survival, the clinical outcomes of patients with
PDAC with high expression of all three enzymes were compared with
those of patients with low expression of all three enzymes, or with
high expression of one or two of the enzymes. Kaplan-Meier
estimator analyses revealed that low expression levels of MTHFD2,
ALDH1L2 and SHMT2 correlated with improved OS and DFS in patients
with PDAC, as compared with in patients with high expression of one
or two of the enzymes (P<0.001; Fig.
4).
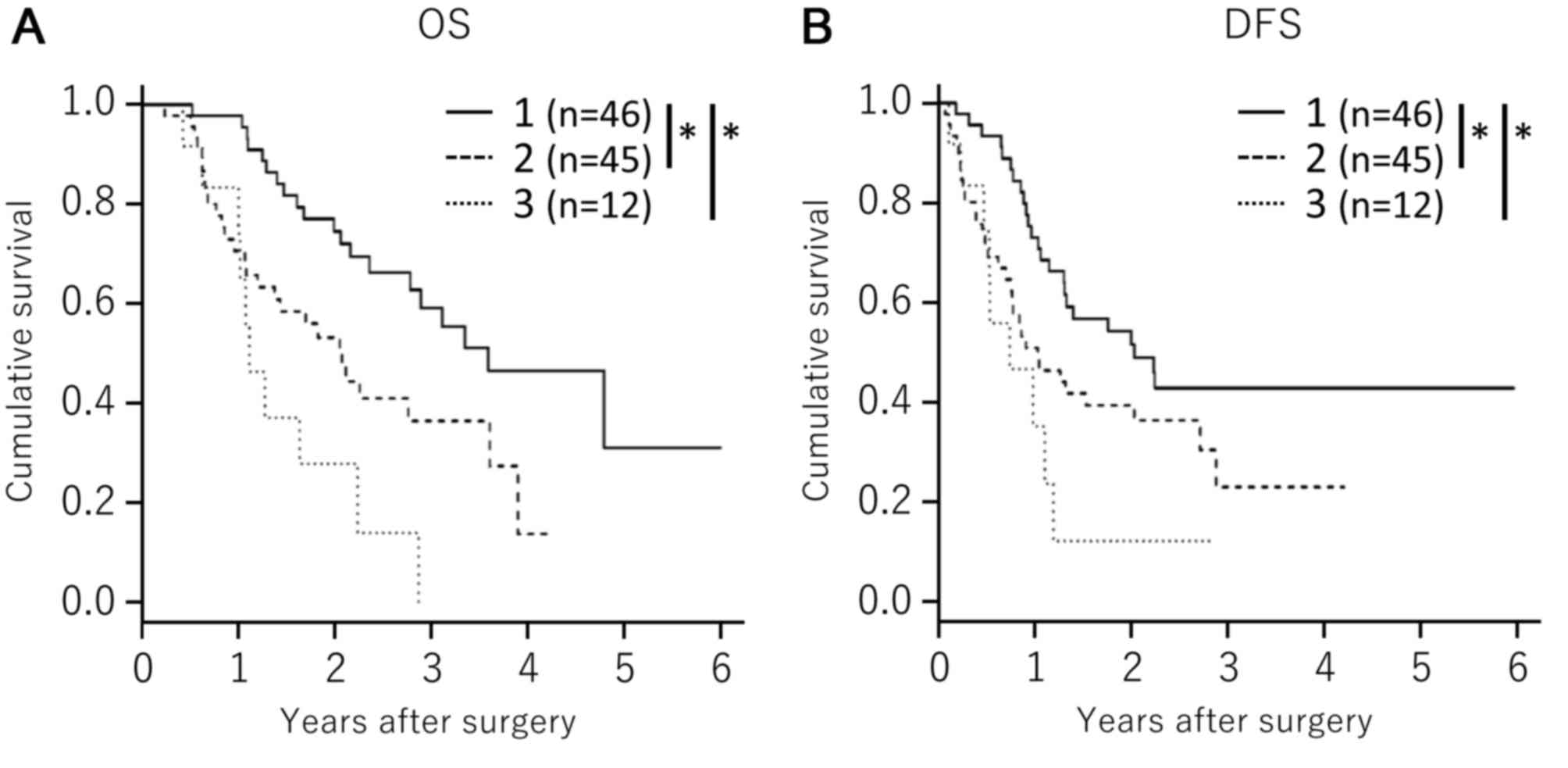 | Figure 4.Associations between patient survival
and the combined expression patterns of MTHFD2, ALDH1L2 and SHMT2.
Associations between (A) OS or (B) DFS and triple low expression of
MTHFD2, ALDH1L2 and SHMT2 (solid line; line 1); triple high
expression of MTHFD2, ALDH1L2 and SHMT2 (dotted line, line 3); and
other groups (broken line, line 2). Survival was estimated using
Kaplan-Meier estimator analyses. P-values were calculated using the
log-rank test. *P<0.001. MTHFD2, methylenetetrahydrofolate
dehydrogenase D2; SHMT2, serine hydroxymethyltransferase 2;
ALDH1L2, aldehyde dehydrogenase 1 family member L2; OS, overall
survival; DFS, disease-free survival. |
Discussion
In the present study, overexpression of each of the
one-carbon metabolic enzymes, MTHFD2, ALDH1L2 and SHMT2, which are
specifically located in the mitochondria, was associated with poor
clinical outcomes among patients with PDAC. However, high and low
expression levels of all three folate pathway enzymes were
associated with improved survival, compared with the high
expression of one or two of the three enzymes studied. Previously,
a high intake of folate and the upregulation of the folate pathway
were considered to reduce the risks of cancer due to the associated
antioxidant activities of the folate metabolic pathway (6). However, recent epidemiologic data
regarding the association between folate intake and pancreatic
cancer are inconsistent (11). In
addition, the expression of folate metabolic pathway enzymes,
including MTHFD2, was identified to be markedly elevated in
numerous types of cancer (12) and
MTHFD2 expression has previously been associated with poor clinical
outcomes (7). Therefore, one-carbon
metabolism is currently considered one of the most important
metabolic pathways for cancer progression (6).
Previous studies have revealed a number of molecular
mechanisms underlying the effects of the folate metabolic pathway
on tumorigenesis and metastasis (6).
As a source of one-carbon units that are necessary for DNA
replication and repair, folate protects normal tissues from
mutations and chromosomal damage. However, folate may enhance the
growth of pre-existing neoplastic lesions, as rapidly proliferating
tissues such as tumors have increased nucleotide demands.
Additionally, antioxidants have been demonstrated to promote
distant metastasis in immunodeficient mice, and the inhibition of
the folate pathway using low-dose methotrexate, ALDH1L2 knockdown
or MTHFD1 knockdown limited distant metastasis without
significantly affecting the growth of subcutaneous tumors in mice.
Therefore, oxidative stress inhibits the distant metastasis of
melanoma cells in vivo (8).
In the present study, the systematic downregulation
of individual folate metabolic pathway enzymes was demonstrated to
significantly correlate with improved survival. Recently,
innovative drugs have been developed that target one-carbon
metabolism. However, the majority of drugs targeted dihydrofolate
reductase (DHFR). When the DHFR enzyme was inhibited, there was a
metabolic flow of one-carbon metabolism. Therefore, the effect of
DHFR targeting drugs may be insufficient. Cytoplasmic one-carbon
metabolic enzymes, including MTHFD1, ALDH1L1 and SHMT1, and
mitochondrial enzymes, including MTHFD2, ALDH1L2 and SHMT2,
constitute the circinate metabolic flow, and this metabolic flow
does not have an alternative loophole pathway. Therefore, the
effect of targeting these enzymes may be sufficient. However,
normal pancreatic tissues have high expression levels of these
one-carbon metabolic enzymes. Thus, the drug targeting these
enzymes must be delivered using cancer-specific drug delivery
systems. Specifically, data from the present study demonstrated
that high expression levels of one or two of the enzymes MTHFD2,
ALDH1L2 and SHMT2 are associated with poor prognosis (Figs. 3 and 4),
suggesting that the activity of one carbon metabolism plays a role
in invasion and metastasis of pancreatic cancer cells and
associates with clinical survival of patients with pancreatic
cancer. These proteins may be independent prognostic factors and
potential therapeutic targets for pancreatic cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a
Grant-in-Aid for Scientific Research from the Ministry of
Education, Culture, Sports, Science and Technology (Tokyo, Japan;
grant nos. 23390199, 25112708, 25134711, 30253420 and 26670604), a
Grant-in-Aid from the Ministry of Health, Labour and Welfare
(Tokyo, Japan; grant no. H23-003), a grant from the National
Institute of Biomedical Innovation (Osaka, Japan; grant no. 12-4)
and a grant from the Osaka University Drug Discovery Fund (Osaka,
Japan). Partial support was also received from the Takeda Science
and Medical Research Foundation (Osaka, Japan; to Dr H Ishii), the
Princess Takamatsu Cancer Research Fund (Tokyo, Japan; to Dr H
Ishii), the Suzuken Memorial Foundation (to Dr M Konno), the Yasuda
Medical Foundation (to Dr M Konno), the Pancreas Research
Foundation (to Dr K Kawamoto), the Nakatani Foundation (to Dr H
Ishii) and the Nakatomi Foundation, Japan (to Dr M Konno).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KN, MK, HE, YD, MM, and HI conceived and designed
the study. KN, MK and HI developed the methodology. KN, MK, JK, NN,
KK, DY, TA, TN, HW, KG, DS, TK, TS, HE and HI acquired the data.
KN, MK, JK, NN and KK analyzed and interpreted the data. KN, MK,
HE, YD, MM and HI wrote, reviewed and revised the manuscript. MM
and HI supervised the study.
Ethics approval and consent to
participate
This study has been approved by the research ethics
committee of Osaka University (ID of the approval: 15149-2, by Dr.
Y. Kaneda).
Consent for publication
All patients provided written consent for the
publication of results.
Competing interests
Institutional endowments were received partially
from Taiho Pharmaceutical Co., Ltd. (Tokyo, Japan), Evidence-Based
Medical Research Center (Osaka, Japan), Chugai Co., Ltd. (London,
UK), Yakult Honsha Co., Ltd. (Tokyo, Japan) and Merck & Co.,
Ltd., Whitehouse Station, NJ, USA). These funding bodies had no
role in the main experimental equipment, the study design, data
collection and analysis, the decision to publish or the preparation
of this manuscript.
References
|
1
|
Howe HL, Wu X, Ries LA, Cokkinides V,
Ahmed F, Jemal A, Miller B, Williams M, Ward E, Wingo PA, et al:
Annual report to the nation on the status of cancer, 1975–2003,
featuring cancer among U.S. Hispanic/Latino populations. Cancer.
107:1711–1742. 2006.
|
|
2
|
Majumder K, Gupta A, Arora N, Singh PP and
Singh S: Premorbid obesity and mortality in patients with
pancreatic cancer: A systematic review and meta-analysis. Clin
Gastroenterol Hepatol. 14:355–368.e. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hashimoto D, Chikamoto A, Ohmuraya M,
Sakata K, Miyake K, Kuroki H, Watanabe M, Beppu T, Hirota M and
Baba H: Pancreatic cancer in the remnant pancreas following primary
pancreatic resection. Surg Today. 44:1313–1320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanda M, Fujii T, Takami H, Suenaga M,
Inokawa Y, Yamada S, Nakayama G, Sugimoto H, Koike M, Nomoto S and
Kodera Y: Combination of the serum carbohydrate antigen 19-9 and
carcinoembryonic antigen is a simple and accurate predictor of
mortality in pancreatic cancer patients. Surg Today. 44:1692–1701.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pandol S, Gukovskaya A, Edderkaoui M,
Dawson D, Eibl G and Lugea A: Epidemiology, risk factors, and the
promotion of pancreatic cancer: Role of the stellate cell. J
Gastroenterol Hepatol. 27(Suppl 2): S127–S134. 2012. View Article : Google Scholar
|
|
6
|
Konno M, Asai A, Kawamoto K, Nishida N,
Satoh T, Doki Y, Mori M and Ishii H: The one-carbon metabolism
pathway highlights therapeutic targets for gastrointestinal cancer
(Review). Int J Oncol. 50:1057–1063. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu F, Liu Y, He C, Tao L, He X, Song H
and Zhang G: Increased MTHFD2 expression is associated with poor
prognosis in breast cancer. Tumour Biol. 35:8685–8690. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piskounova E, Agathocleous M, Murphy MM,
Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ
and Morrison SJ: Oxidative stress inhibits distant metastasis by
human melanoma cells. Nature. 527:186–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC cancer staging manual 7. Springer; New
York, NY; 2010
|
|
10
|
Hasegawa S, Eguchi H, Nagano H, Konno M,
Tomimaru Y, Wada H, Hama N, Kawamoto K, Kobayashi S, Nishida N, et
al: MicroRNA-1246 expression associated with CCNG2-mediated
chemoresistance and stemness in pancreatic cancer. Br J Cancer.
111:1572–1580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin HL, An QZ, Wang QZ and Liu CX: Folate
intake and pancreatic cancer risk: An overall and dose-response
meta-analysis. Public Health. 127:607–613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nilsson R, Jain M, Madhusudhan N, Sheppard
NG, Strittmatter L, Kampf C, Huang J, Asplund A and Mootha VK:
Metabolic enzyme expression highlights a key role for MTHFD2 and
the mitochondrial folate pathway in cancer. Nat Commun. 5:31282014.
View Article : Google Scholar : PubMed/NCBI
|