Introduction
Liver cancer is a highly prevalent malignancy
worldwide, and is associated with high mortality. According to
Global Cancer Statistics (2012), liver cancer accounts for 521,000
and 224,500 mortalities among men and women, respectively (1). Hepatocellular carcinoma (HCC) accounts
for >70% of all primary liver cancer (2). Due to its asymptomatic nature, patients
with HCC are typically diagnosed at an advanced stage (3). Hence, there is an urgent requirement to
identify biomarkers for early detection of HCC to improve patient
prognosis (3).
Bone morphogenetic proteins (BMPs), which are
homodimeric members of the transforming growth factor-β (TGF-β)
superfamily, have been implicated in the pathogenesis of human
malignancies (4). To date, >20
human BMPs have been identified, among which BMP-7 levels have been
demonstrated to be closely associated with HCC (1). The anti-tumorigenic potential of BMP-7
has been detected in several human malignancies, including breast
cancer and glioblastoma (5–7). Hou et al (8) indicated that BMP-7 attenuates liver
fibrosis, which may develop into HCC. Furthermore, inhibition of
BMP-7 signaling was revealed to facilitate invasive growth of HCC
cells (9). The BMP family members
mediate signal transduction by binding to their receptors, which
triggers the phosphorylation of Smad proteins 1, 5, and 8
(Smad1/5/8) (10,11). However, the potential association
between BMP-7 and Smad signaling pathway in HCC has not yet been
fully elucidated.
Gremlin, an endogenous antagonist of BMP-7, inhibits
the binding of BMP-7 to its receptor, thereby, inhibiting Smad
phosphorylation and the downstream signaling cascade (12). Gremlin protein, secreted by
fibroblasts has been revealed to be a biomarker of liver fibrosis
(13). Gremlin promotes stem cell
maturation and proliferation via inhibition of the BMP signaling
pathway, which in turn accelerates hepatic fibrosis and
carcinogenesis (14). These findings
indicate that the BMP signaling pathway may serve as an endogenous
self-defense mechanism against carcinogenesis and progression of
HCC.
In the present study, it was hypothesized that the
BMP-7 and Smad signaling pathway may serve a pivotal role in the
prevention of HCC. Therefore, HCC samples with varied tumor
differentiation status and at different clinical stages were
included. Expression levels of BMP-7, gremlin, and Smad1/5/8 in
carcinomatous and their adjacent non-carcinomatous (normal) tissues
were assessed. The findings from the present study indicate the
clinical significance of these indices in predicting the disease
status of HCC.
Materials and methods
Reagents
Rabbit anti-human anti-BMP-7 antibody was obtained
from Abcam, Cambridge, UK (cat. no. ab56023). Rabbit anti-human
anti-gremlin antibody was purchased from Wuhan Boster Biological
Technology, Ltd., Wuhan, China (cat. no. BA2287). Rabbit anti-human
anti-p-Smad1/5/8 antibody was purchased from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA (cat. no. sc-12353-R). The
3,3′-diaminobenzidine tetrahydrochloride (DAB) kit was obtained
from ZSGB-Bio, Beijing, China. RNAstore solution, TIANScript
first-strand cDNA synthesis kit, and SuperReal PreMix Plus SYBR
Green were obtained from Tiangen Biotech Co., Ltd., Beijing, China.
RIPA lysis buffer and phenylmethylsulfonyl fluoride (PMSF) were
obtained from Beyotime Institute of Biotechnology, (Haimen,
China).
Patients
A total of 27 patients with HCC who underwent
curative surgical resection at the Department of General Surgery,
The Affiliated Hospital of Xuzhou Medical University, (Xuzhou,
China), between November 2014 and August 2015, were included in the
present study. Inclusion criteria are as follows: i) Diagnosis of
HCC according to the diagnostic criteria recommended by the
Professional Committee of Chinese Anti-Cancer Association of Liver
Cancer in 2001 (15); ii) not
received surgical, interventional or radiation therapy prior to
sample collection. Tumor-node metastasis (TNM) staging was
performed based on the criteria of the American Joint Committee on
Cancer (AJCC) (16). Pathological
examination was conducted and tumor differentiation was recorded.
Baseline demographic and clinical characteristics of patients are
listed in Table I. In addition, a
total of 7 healthy subjects were enrolled in the present study.
Written informed consent was obtained from all subjects prior to
their enrollment in the study. The study was approved by the Ethics
Committee at the Affiliated Hospital of Xuzhou Medical University
(Xuzhou, China).
 | Table I.Demographic and clinical
characteristics of subjects. |
Table I.
Demographic and clinical
characteristics of subjects.
| Parameters | Patients with HCC
(n=27) | Healthy subjects
(n=7) |
|---|
| Sex (%) |
|
|
| Male | 21 (78) | 5 (71) |
|
Female | 6 (22) | 2 (29) |
| Age, years | 57±11 | 54±14 |
| TNM staging (%) |
|
|
| I | 3 (11) | – |
| II | 12 (45) | – |
| III
A | 9 (33) | – |
| III
B | 3 (11) | – |
| Differentiation
(%) |
|
|
| Poor | 4 (15) | – |
|
Moderate | 15 (56) | – |
| High | 8 (29) | – |
Sample collection
A total of ~100 mg tumor tissues or para-carcinoma
tissues (3 cm away from the tumor margin) were collected following
curative surgical resection for polymerase chain reaction (PCR)
analysis. The tissues were washed in phosphate buffer solution
(PBS). The samples to be used for RNA extraction were incubated in
1 ml RNAstore solution at 4°C overnight and stored at −80°C until
further use. For western blot analysis, samples were grinded into
pieces, immediately immersed in liquid nitrogen (−196°C), followed
by long-term storage at −80°C. For histological examination, tissue
samples were fixed in 4% paraformaldehyde solution for 24 h at room
temperature. In addition, fasting venous blood samples were
collected from patients with HCC prior to operation and from
healthy subjects. The samples were incubated at room temperature
for 1–2 h, followed by centrifugation at 2,200 × g for 5 min at
room temperature. The serum was separated and stored at −80°C until
use.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
A total of ~100 mg of the tissue samples were
grinded, and the total RNA was extracted using TRIzol reagent
according to the manufacturer's protocol (Ambion; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Total RNA (1 µg) was reverse
transcribed using TIANScript first-strand cDNA synthesis kit,
according to the manufacturer's protocol. Synthesized cDNA was used
for RT-qPCR amplification using SuperReal PreMix Plus SYBRGreen,
and a sequence detection system (Roche Diagnostics, Basel,
Switzerland). The PCR primers were synthesized by Takara
Biotechnology Co., Ltd., (Dalian, China) and Sangon Biotech Co.,
Ltd., (Shanghai, China). The primer sequences for target genes were
as follows: BMP-7, forward, 5′-ACCAGAGGCAGGCCTGTAAGA-3′; and
reverse, 5′-CTCACAGTTAGTAGGCGGCGTAG-3′; gremlin, forward,
5′-GCCCTCGGGAGCCACAAACC-3′; and reverse,
5′-GCAGCAGGAGTCGCGGTGAG-3′; β-actin, forward,
5′-ACTAACCGCTTCTGTTACGG-3′; and reverse,
5′-ATGCAACGACACTGCTTCAC-3′. The PCR reaction system included 10 µl
2X SuperReal PreMix Plus, 0.6 µl forward primer, 0.6 µl reverse
primer, 1 µl template cDNA and 7.8 µl ddH2O to a total
volume of 20 µl. PCR reactions were performed by pre-denaturation
at 95°C for 15 min, followed by 40 cycles of 95°C for 10 sec, 60°C
for 20 sec and 72°C for 20 sec. The predicted product length was
107 bp for BMP-7, 197 bp for gremlin and 151 bp for β-actin. Each
experiment was conducted in triplicate. The relative mRNA
expression was calculated using the 2−ΔΔCq method
(17).
Immunohistochemistry
Tissue samples collected after curative surgical
resection were embedded in paraffin, and the sections (thickness, 4
µm) were prepared using an automatic tissue processor (RM2235;
Leica, Germany). Subsequently, the sections were deparaffinized in
xylene (twice, each for 10 min), and rehydrated in an ethanol
series (100% for 5 min, 90% for 2 min, 70% for 2 min and distilled
water for 2 min). The sections were heated in 0.01 M citrate buffer
(pH 6.0) in a microwave. After three washes in PBS, the samples
were incubated with 3% hydrogen peroxide at room temperature, to
block the endogenous peroxidase activity. After three washes with
PBS (5 for each time) at room temperature, the sections were
incubated with 100 µl primary antibodies, including rabbit
asti-BMP-7 (1:100) and rabbit anti-gremlin (1:200) at 4°C overnight
as described previously. Immunostaining was performed using rabbit
ultra-sensitive two-step kit according to manufacturer's protocol
(ZSGB-Bio, Beijing, China). The following day, the samples were
washed three times in PBS and probed with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG secondary antibody,
pre-diluted from the supplier, for 20 min at 37°C. After PBS
washes, the immunoreactivity was assessed by DAB staining for 3 min
at room temperature. Nuclei were counterstained with hematoxylin.
Subsequently, the sections were washed with running tap water for
10 min, followed by dehydration through 95% ethanol for 5 min and
100% ethanol for 5 min. After two washes of xylene (each for 5
min), the samples were mounted with neutral balsam.
Imaging
Immunoreactivity was analyzed under a phase contrast
microscope (BX51; Olympus Corporation, Tokyo, Japan) at −400
magnification. BMP-7 positive cells were identified by a
brown-yellow stained cytosol or nuclei. Gremlin-positive cells were
defined as those with brown-yellow-stained cell membrane, cytosol
or nuclei. A total of five fields of interest were randomly
selected from each sample for imaging in order to minimize bias as
described previously (18), and the
average integral optical density (OD) per area was calculated by
Image-Pro Plus software (version 6.0, Media Cybernetics, Inc.,
Rockville, MD, USA).
Western blotting
A total of ~100 mg of tissue samples were lysed in 1
ml RIPA lysis buffer, which contained 10 µl PMSF and 7 µl protease
inhibitor. Following centrifugation at 1,452 × g for 10 min at 4°C,
the supernatant was collected. For the determination of protein
concentration, bicinchoninic acid (BCA) protein assay kit (Beyotime
Institute of Biotechnology) was used. Equal amounts of total
protein (80 µg) per well were separated by 10% SDS-PAGE
electrophoresis. After electrophoresis, the proteins were
transferred on to PVDF membranes, blocked with 5% non-fat dry milk
dissolved in PBS for 3 h at room temperature (RT) and probed with
primary antibodies, including anti-BMP-7 (1:500 dilution) and
anti-p-Smad1/5/8 (1:500) antibodies, at 4°C overnight. Following
four washes with TBST (each for 5–10 min), the membranes were
incubated with goat anti-rabbit HRP-conjugated secondary antibodies
(1:4,000) for 2 h at room temperature. After washes with TBST,
immunoreactivity was assessed using an enhanced chemiluminescence
assay. The immunobands were exposed to X-ray films and scanned. The
density of bands was analyzed by Quantity One software 4.62 patch
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
ELISA for cytokine analysis
The serum BMP-7 level was examined by ELISA using a
human BMP-7 ELISA kit according to manufacturer's protocol
(RayBiotech Inc., GA, USA). The absorbance was measured at 450 nm
using a ClinBio128 microplate reader (Asys Hitech GmbH, Eugendorf,
Austria).
Statistical analysis
All data are expressed as the mean ± standard
deviation. The differences between the groups were assessed with
unpaired Student's t-test, and multiple group comparisons were
performed using one-way analysis of variance following
Student-Newman-Keuls post-hoc test. All statistical tests were
two-tailed with α=0.05. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression BMP-7 and gremlin in
carcinoma and non-carcinoma tissues of HCC
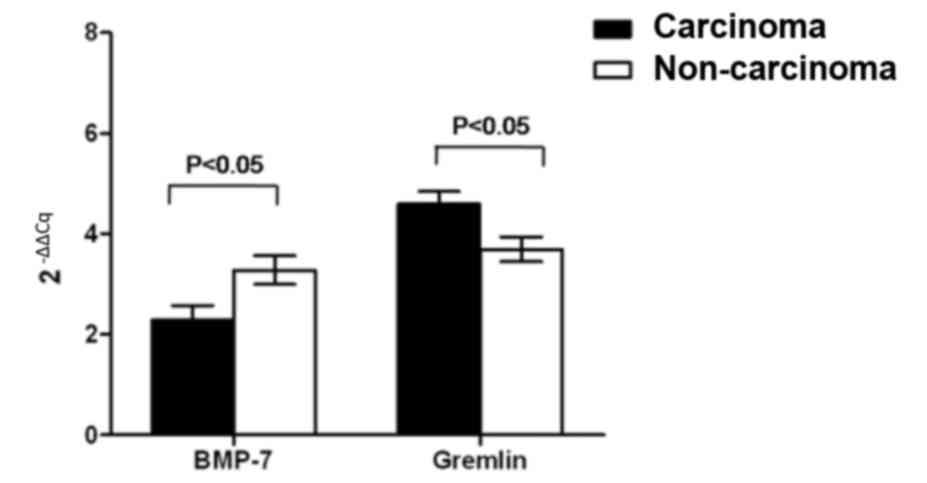
To investigate the involvement of BMP-7 during
carcinogenesis and progression of HCC, the mRNA levels of BMP-7 and
its endogenous antagonist gremlin were determined in carcinoma and
non-carcinoma tissues of HCC by RT-qPCR. BMP-7 mRNA expression was
significantly downregulated in tumor tissues, compared with
para-carcinoma tissues (P<0.05; Fig.
1). By contrast, the mRNA expression of gremlin in carcinoma
tissues was significantly increased compared with non-carcinoma
tissues (P<0.05; Fig. 1).
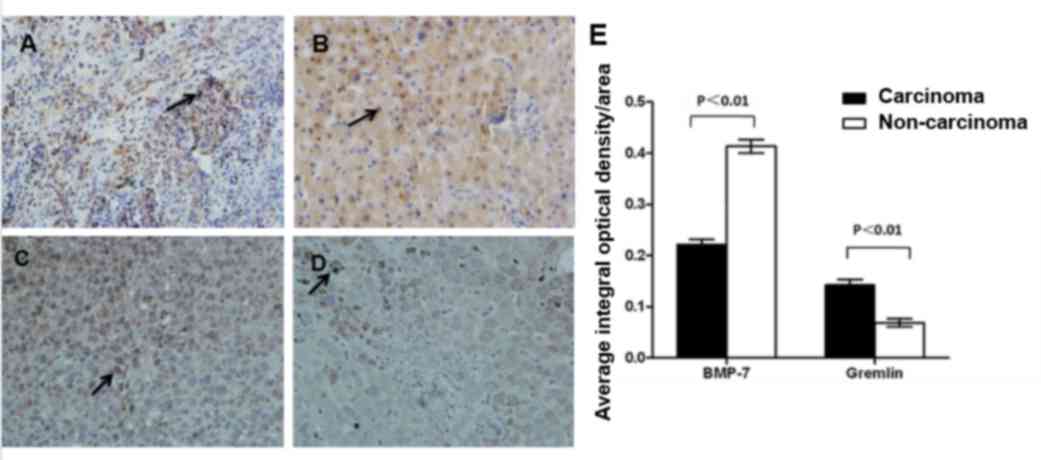
Immunohistochemical analysis revealed that BMP-7 was predominantly
expressed in the nucleus of tumor cells (Fig. 2A). The protein expression of BMP-7 was
greatly reduced in carcinoma tissues compared with the
non-carcinoma tissues (t=11.808, P<0.01; Fig. 2A and B). Gremlin exhibited a different
intracellular distribution pattern compared with BMP-7, as gremlin
expression was predominantly detected in the cell membrane and
cytosol (Fig. 2). Furthermore, by
contrast to BMP-7, gremlin protein was significantly increased in
carcinoma tissues compared with non-carcinoma tissues (t=−6.182;
P<0.01; Fig. 2C-E). These results
indicate that BMP-7 mRNA and protein expression levels were reduced
in HCC tissues, and that the level of BMP-7 was negatively
associated with that of gremlin.
Expression of BMP-7 and gremlin in HCC
tissues at various stages of differentiation
Expression levels of BMP-7 and gremlin in HCC
tissues with poor, moderate, and high differentiation status were
compared. It was observed that patients with highly differentiated
HCC had a relatively higher BMP-7 level but a lower level of
gremlin expression (BMP-7, F=42.29, P<0.01; gremlin, F=37.93,
P<0.01; Fig. 3). The data of BMP-7
and gremlin in para-carcinoma tissues are presented in Fig. 2. The results from the present study
indicate that BMP-7 may be used to predict the tumor
differentiation status.
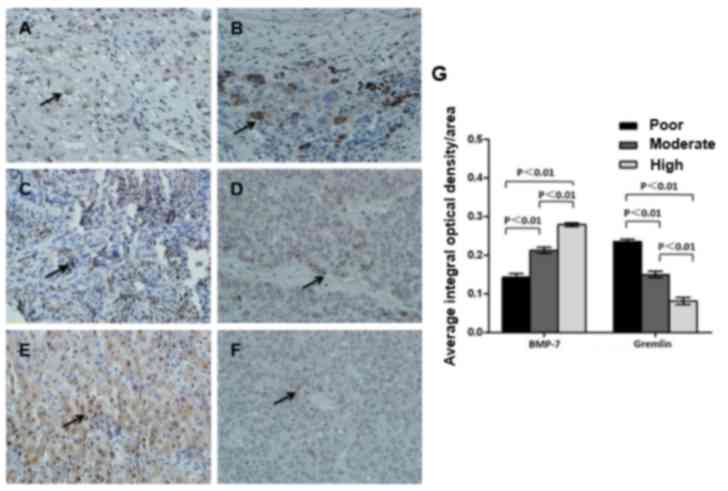 | Figure 3.Protein expression of BMP-7 and
gremlin in HCC tissue specimens with varied differentiation status.
Carcinoma tissue samples were obtained from patients with (A,B)
poor, (C,D) moderate or (E,F) high differentiation. Protein
expression of (A,C,E) BMP-7 and (B,D,F) gremlin were analyzed by
immunohistochemistry. Magnification, −400. (G) Mean integral
optical density per area was calculated. Protein expression of
BMP-7 and gremlin in carcinoma tissue samples derived from patients
at varied differentiation were compared. P<0.01. BMP-7, bone
morphogenetic protein-7; HCC, hepatocellular carcinoma. |
Expression of BMP-7, gremlin and
p-Smad1/5/8 in carcinoma tissues of HCC at different clinical
stages
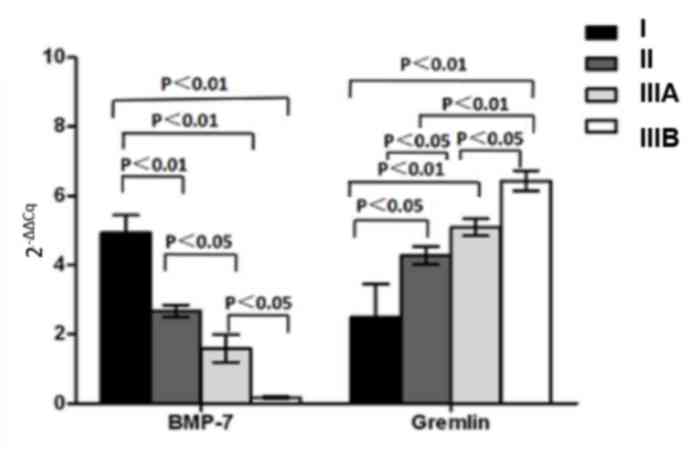
To elucidate the role of BMP-7 and gremlin in the
regulation of HCC development, gene expression levels in carcinoma
tissues at various clinical stages (I, II, IIIA and IIIB) were
examined. BMP-7 mRNA expression was gradually reduced with
increasing clinical stage (F=18.45, P<0.01), while the level of
gremlin was elevated in advanced staged HCC tissue samples
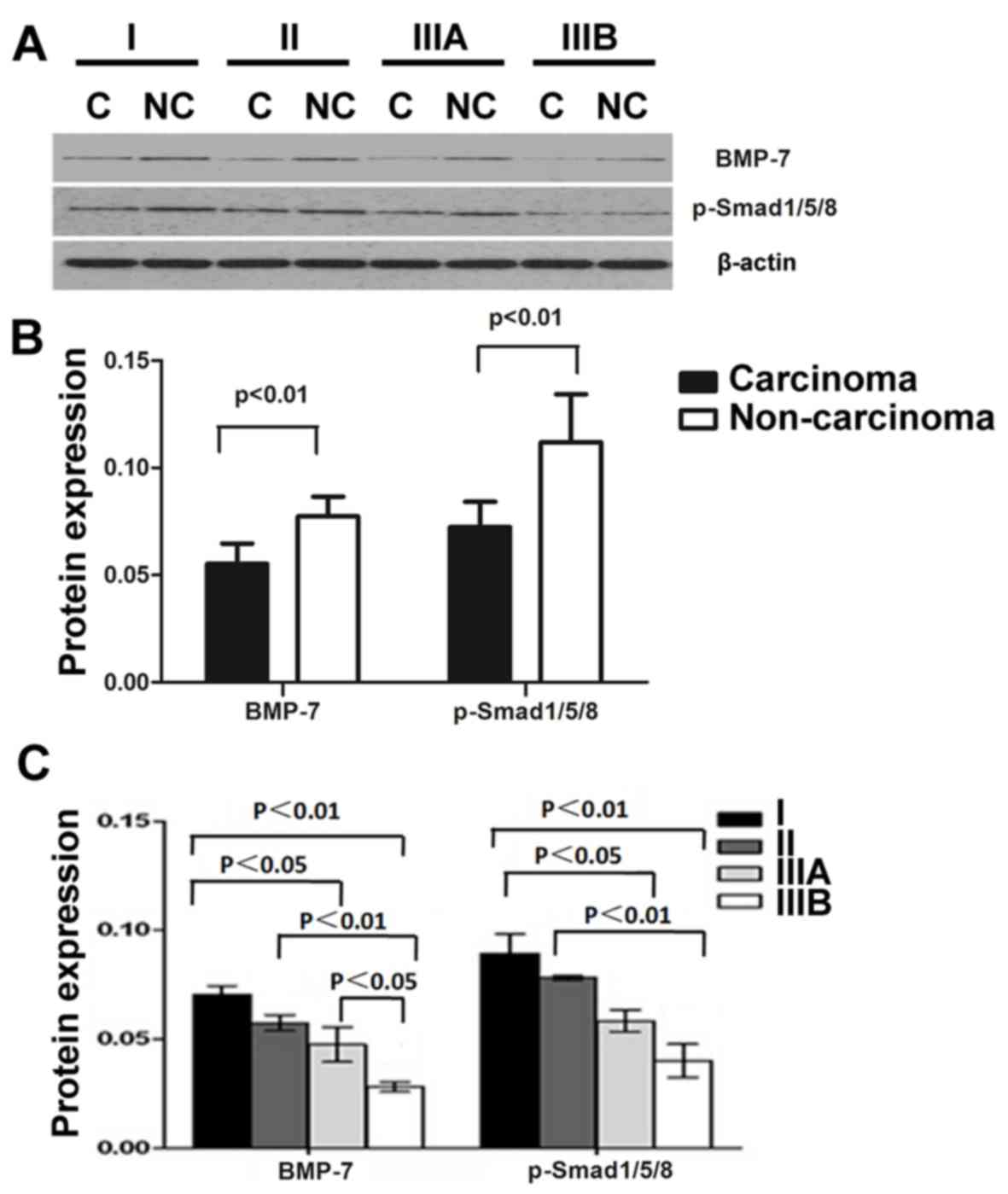
(F=18.45, P<0.01; Fig. 4). Western
blot analysis indicates that BMP-7 protein expression was
downregulated in carcinoma tissues compared with non-carcinoma
tissues (Fig. 5A and B), which is
consistent with the results of PCR and histological analysis
(Figs. 1 and 2). Furthermore, BMP-7 levels were decreased
with advanced disease progression, since a lower level of BMP-7
expression was detected in samples from patients with stage IIIB
HCC compared with stage I HCC samples (Fig. 5A-C). In addition, the expression
pattern of p-Smad1/5/8 was similar to that of BMP-7. The level of
p-Smad1/5/8 was downregulated in carcinoma tissues, and it was
significantly downregulated in advanced stage HCC (P<0.01;
Fig. 5C).
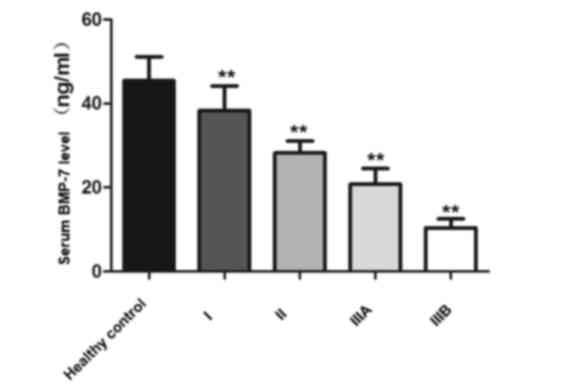
Serum levels of BMP-7
To investigate the potential use of serum BMP-7
levels for the prediction of HCC progression, serum BMP-7 levels in
patients with HCC and healthy subjects were compared. Serum BMP-7
levels were relatively high in healthy subjects (45.41±5.73 ng/ml),
whereas the levels of BMP-7 in patients with HCC were decreased and
the level of BMP-7 decreased to varying degrees depending on the
stage of the disease. The reduction in serum BMP-7 levels was
observed to be associated with disease progression since patients
with more advanced stages of HCC generally exhibited lower serum
level of BMP-7 (stage I, 38.35±5.81; stage II, 28.24±2.82; stage
IIIA, 20.79±3.72; stage IIIB, 10.40±2.15; F=74.467; P<0.01;
Fig. 6).
Discussion
HCC is a severe hepatic disorder associated with a
high mortality rate (19). Patient
prognosis is typically poor as the majority of cases are diagnosed
at an advanced stage (19).
Understanding the molecular mechanisms that underlie
hepatocarcinogenesis may help facilitate therapeutic
decision-making in clinical settings. In the present study, it was
demonstrated that the BMP-7-Smad signaling pathway may have an
anti-tumorigenic function in the progression of HCC.
In the present study, it was observed that there was
a lower level of BMP-7 expression in HCC tumor tissues compared
with non-carcinoma tissues. BMP-7 expression appeared to be
directly associated with the extent of tumor differentiation and
was inversely associated with the clinical stage. Therefore, these
results indicate that BMP-7 may act as a tumor suppressor, and that
the loss of BMP-7 may accelerate the progression of HCC.
Consistent with the findings in the present study,
Yang et al (20) demonstrated
that BMP-7 was able to block the development of hepatic fibrosis
via inhibition of TGF-β1 production and increase in gremlin
generation. Similarly, Wang et al (21) reported that BMP-7 mediated the
prevention of liver fibrosis and hepatic stellate cell activation
in mice injected with carbon tetrachloride (21). In addition, exogenous administration
of BMP-7 was demonstrated to suppress hepatic fibrosis in rodents
via regulation of the Smad signaling pathway (22).
In the present study, protein expression of
p-Smad1/5/8 was observed to be downregulated in HCC tissues
compared with non-carcinoma tissues. The reduced level of
p-Smad1/5/8 was associated with advanced stages of HCC, which
indicates that BMP-7/Smad signaling pathway may act as a
self-defense mechanism against carcinogenesis and progression of
HCC. BMP-7/Smad signals may have anti-fibrogenic and
anti-hepatocarcinogenic effect during the pathogenesis of HCC
(8,9,14,23). By contrast, Li et al (24) reported that the mRNA and protein
expression of BMP-7 was upregulated in HCC cells compared with
normal hepatic cells. Furthermore, increased BMP-7 levels were
associated with poor prognosis in patients with HCC, which implies
that BMP-7 may serve as an oncogene for HCC. This discrepancy may
have been caused by the small sample size. Therefore, future
studies are required which should include larger sample sizes of
patients with HCC. Although the molecular mechanisms involved in
BMP-7/Smad-regulated HCC development remain unclear, it is possible
that BMP-7 counteracts the actions of TGF-β, which is known to
contribute to epithelial-mesenchymal transition (EMT) of
hepatocytes (25). In addition,
efforts should be made to investigate the regulatory role of
BMP-7/Smad signaling pathway in the EMT process in HCC using cell
culture and rodent models.
Notably, serum BMP-7 levels were also reduced in
patients with HCC, particularly in patients with advanced stages of
HCC, as compared with healthy subjects. This particular finding
indicates a potential clinical relevance of serum BMP-7 level for
the prediction of HCC stage. In agreement with the present
findings, Aktug et al (26)
demonstrated an association between serum BMP-7 levels with hepatic
fibrosis. It was reported that higher BMP-7 serum levels were
observed in patients with grades I, II, III and IV liver fibrosis,
while the levels were significantly lower in patients with severe
hepatic failure (grade V) compared with healthy subjects (26). These findings suggest an
anti-inflammatory and anti-fibrogenic role of BMP-7. Plasma levels
of BMP-7 in patients with chronic liver disease were shown to be
significantly higher than those in healthy subjects (27). These findings indicate a potential use
of serum BMP-7 levels for prediction of the extent of liver
fibrosis and carcinogenesis.
The current study has several limitations. Firstly,
the sample size is relatively small, and therefore confirmation of
the inferences drawn requires evidence from larger studies.
Secondly, the patients recruited for the present study were
predominantly male. The effect of gender bias on the present
results cannot be ruled out. Thirdly, in the diagnosis of
hepatocellular carcinoma, α-fetoprotein (AFP) is a marker of liver
cancer, although its sensitivity is not high (28). Fourthly, negative and positive
controls of BMP-7 and gremlin proteins were not included.
Therefore, future studies will be conducted with a more robust
design. Finally, correlation analysis between the expression level
of BMP-7 and the characteristics of the patients has not yet been
conducted.
In summary, the present study demonstrated that the
levels of BMP-7 and p-Smad1/5/8 are decreased, while the level of
gremlin is increased in HCC, compared with non-carcinoma tissues.
These findings suggest a potential involvement of BMP-7/Smad
signaling pathway in the carcinogenesis and progression of HCC. In
addition, serum BMP-7 levels may have potential diagnostic value as
a marker for hepatic carcinogenesis.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural
Science Foundation of China (grant no. 81371867) and Educational
Commission of Jiangsu Province of China (grant no.
08KJD320012).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW prepared samples, performed experiments and was a
major contributor in writing the manuscript. QD contribution to
conception and design. LZ, YP and XY performed analysis and
interpretation of data. ZS and YQ participated in acquisition of
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
subjects prior to their enrollment in the present study. The
present study was approved by the Ethics Committee at the
Affiliated Hospital of Xuzhou Medical University (Xuzhou,
China).
Consent for publication
Patients provided written consent for the
publication of the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
London WT and McGlynn KA: Liver cancer.
Cancer Epidemiology and Prevention. Schottenfeld D and Fraumeni J
Jr: 3rd ed. Oxford University Press; New York, NY; pp. 763–786.
2006, View Article : Google Scholar
|
|
3
|
Wang X, Zhang A and Sun H: Power of
metabolomics in diagnosis and biomarker discovery of hepatocellular
carcinoma. Hepatology. 57:2072–2077. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ye L and Jiang WG: Bone morphogenetic
proteins in tumour associated angiogenesis and implication in
cancer therapies. Cancer Lett. 380:586–597. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naber HP, Wiercinska E, Pardali E, van
Laar T, Nirmala E, Sundqvist A, van Dam H, van der Horst G, van der
Pluijm G, Heckmann B, et al: BMP-7 inhibits TGF-beta-induced
invasion of breast cancer cells through inhibition of integrin
beta(3) expression. Cell Oncol (Dordr). 35:19–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buijs JT, van der Horst G, van den Hoogen
C, Cheung H, de Rooij B, Kroon J, Petersen M, van Overveld PG,
Pelger RC and van der Pluijm G: The BMP2/7 heterodimer inhibits the
human breast cancer stem cell subpopulation and bone metastases
formation. Oncogene. 31:2164–2174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tate CM, Pallini R, Ricci-Vitiani L,
Dowless M, Shiyanova T, D'Alessandris GQ, Morgante L, Giannetti S,
Larocca LM, di Martino S, et al: A BMP7 variant inhibits the
tumorigenic potential of glioblastoma stem-like cells. Cell Death
Differ. 19:1644–1654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou F, Liu R, Liu X, Cui L, Wen Y, Yan S
and Yin C: Attenuation of liver fibrosis by herbal compound 861 via
upregulation of BMP-7/Smad signaling in the bile duct ligation
model rat. Mol Med Rep. 13:4335–4342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ji X, Jin S, Qu X, Li K, Wang H, He H, He
H, Guo F and Dong L: Lysine-specific demethylase 5C promotes
hepatocellular carcinoma cell invasion through inhibition BMP7
expression. BMC Cancer. 15:8012015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu J, Saito K, Maruya Y, Nakamura T,
Yamada A, Fukumoto E, Ishikawa M, Iwamoto T, Miyazaki K, Yoshizaki
K, et al: Mutant GDF5 enhances ameloblast differentiation via
accelerated BMP2-induced Smad1/5/8 phosphorylation. Sci Rep.
6:236702016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lopez-Coviella I, Mellott TM, Kovacheva
VP, Berse B, Slack BE, Zemelko V, Schnitzler A and Blusztajn JK:
Developmental pattern of expression of BMP receptors and Smads and
activation of Smad1 and Smad5 by BMP9 in mouse basal forebrain.
Brain Res. 1088:49–56. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brazil DP, Church RH, Surae S, Godson C
and Martin F: BMP signalling: Agony and antagony in the family.
Trends Cell Biol. 25:249–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boers W, Aarrass S, Linthorst C, Pinzani
M, Elferink RO and Bosma P: Transcriptional profiling reveals novel
markers of liver fibrogenesis: Gremlin and insulin-like growth
factor-binding proteins. J Biol Chem. 281:16289–16295. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guimei M, Baddour N, Elkaffash D, Abdou L
and Taher Y: Gremlin in the pathogenesis of hepatocellular
carcinoma complicating chronic hepatitis C: An immunohistochemical
and PCR study of human liver biopsies. BMC Res Notes. 5:3902012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Professional Committee of Chinese
Anti-Cancer Association of liver cancer: Primary liver cancer
diagnosis and staging criteria. Zhonghua Hepatology. 9:3242001.
|
|
16
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miao Y, Zong M, Jiang T, Yuan X, Guan S,
Wang Y and Zhou D: A comparative analysis of ESM-1 and vascular
endothelial cell marker (CD34/CD105) expression on pituitary
adenoma invasion. Pituitary. 19:194–201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tejeda-Maldonado J, Garcia-Juarez I,
Aguirre-Valadez J, Gonzalez-Aguirre A, Vilatoba-Chapa M,
Armengol-Alonso A, Escobar-Penagos F, Torre A, Sánchez-Ávila JF and
Carrillo-Pérez DL: Diagnosis and treatment of hepatocellular
carcinoma: An update. World J Hepatol. 7:362–376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang T, Chen SL, Lu XJ, Shen CY, Liu Y and
Chen YP: Bone morphogenetic protein 7 suppresses the progression of
hepatic fibrosis and regulates the expression of gremlin and
transforming growth factor beta1. Mol Med Rep. 6:246–252.
2012.PubMed/NCBI
|
|
21
|
Wang LP, Dong JZ, Xiong LJ, Shi KQ, Zou
ZL, Zhang SN, Cao ST, Lin Z and Chen YP: BMP-7 attenuates liver
fibrosis via regulation of epidermal growth factor receptor. Int J
Clin Exp Pathol. 7:3537–3547. 2014.PubMed/NCBI
|
|
22
|
Chen BL, Peng J, Li QF, Yang M, Wang Y and
Chen W: Exogenous bone morphogenetic protein-7 reduces hepatic
fibrosis in Schistosoma japonicum-infected mice via transforming
growth factor-beta/Smad signaling. World J Gastroenterol.
19:1405–1415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jones CN, Tuleuova N, Lee JY, Ramanculov
E, Reddi AH, Zern MA and Revzin A: Cultivating hepatocytes on
printed arrays of HGF and BMP7 to characterize protective effects
of these growth factors during in vitro alcohol injury.
Biomaterials. 31:5936–5944. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li W, Cai HX, Ge XM, Li K, Xu WD and Shi
WH: Prognostic significance of BMP7 as an oncogene in
hepatocellular carcinoma. Tumour Biol. 34:669–674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeisberg M, Yang C, Martino M, Duncan MB,
Rieder F, Tanjore H and Kalluri R: Fibroblasts derive from
hepatocytes in liver fibrosis via epithelial to mesenchymal
transition. J Biol Chem. 282:23337–23347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aktug Demir N, Kolgelier S, Inkaya AC,
Sumer S, Demir LS, Pehlivan FS, Arslan M and Arpaci A: Are bone
morphogenetic protein-7 (BMP-7) serum levels correlated with
development of hepatic fibrosis? J Infect Dev Ctries. 8:605–610.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tacke F, Gabele E, Bataille F, Schwabe RF,
Hellerbrand C, Klebl F, Straub RH, Luedde T, Manns MP, Trautwein C,
et al: Bone morphogenetic protein 7 is elevated in patients with
chronic liver disease and exerts fibrogenic effects on human
hepatic stellate cells. Dig Dis Sci. 52:3404–3415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Daniele B, Bencivenga A, Megna AS and
Tinessa V: Alpha-fetoprotein and ultrasonography screening for
hepatocellular carcinoma. Gastroenterology. 127(Suppl 1):
S108–S112. 2004. View Article : Google Scholar : PubMed/NCBI
|