Introduction
Early metastasis to the lymph nodes is a
characteristic feature of human pancreatic ductal adenocarcinoma
(PDAC). These lymph-node metastases and the disseminated tumor
cells in the lymphatic vessels of peri-pancreatic tissues may be
the source of local tumor recurrence, which frequently occurs in
patients following PDAC resection (1). Chemokines, a superfamily of small,
secreted peptides characterized by their ability to induce
leukocyte migration, together with their receptors, have been
revealed to be involved in the migration of cells of the lymphatic
system (2). In several types of
tumor, chemokine receptors in tumor cells and those secreted by
lymphatic endothelial cells (LECs) have been demonstrated to serve
a role in the extravasation and homing of circulating tumor cells
(3,4).
C-C motif chemokine receptor 7 (CCR7), the receptor
for the C-C motif chemokine ligand 21 (CCL21), is expressed on
naive T cells, memory T cells, B cells, mature dendritic cells and
certain tumor cells, and is considered to be important in
lymphocyte cell trafficking and homing to the lymph nodes (5). In addition, the expression of CCR7 has
been reported to promote cancer cell metastasis to lymph nodes in
several types of cancer (6–9). Therefore, CCR7 and its ligand may
participate in the metastasis of cancer cells of various organ
origins. CCR7 was also revealed to be involved in the development
and progression of PDAC (10).
Various studies have confirmed the roles of
CCL21/CCR7 in tumor development and progression: CCL21/CCR7 is able
to upregulate matrix metalloproteinase-9 (MMP-9) in human colon
cancer metastasis (11), mediate
transforming growth factor β1-induced epithelial-mesenchymal
transition via crosstalk with nuclear factor-κB signaling in
gastric cancer (12), induce janus
kinase 2/signal transducer and activator of transcription 3
phosphorylation in metastatic squamous cell carcinoma of the head
and neck (13), upregulate Twist via
extracellular signal-regulated kinase (ERK) and phosphoinositide
3-kinase/protein kinase B signaling in PDAC (14) and so on.
The family of anoctamins (ANOs) consists of ten
different proteins (TMEM16 genes A-J), ANO1-ANO10. However, there
are certain controversies regarding the molecular functions of ANO6
and whether it is a chloride channel, a cation channel or a
phospholipid scramblase (15).
Notably, the expression of ANO proteins is known to be upregulated
in cancer and to be associated with poor patient prognosis
(16). Jacobsen et al
(17) suggested that ANO6 had an
important function in Ehrlich-Lettre ascites (ELA) migration, as
part of the migratory ‘engine’ that determines the speed of
cellular migration.
The present study aimed to determine whether ANO6
also contributes to the migration of PDAC cells via the ERK pathway
induced by the CCL21/CCR7 axis.
Materials and methods
Cell line and reagents
Three human PDAC cell lines, BxPC-3, AsPC-1 and
PANC-1, were obtained from American Type Culture Collection
(Manassas, VA, USA). Recombinant human CCL21 was obtained from
Cyagen Biosciences, Inc. (Santa Clara, CA, USA). The ERK inhibitor
U0126 was obtained from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). The following antibodies were purchased from various
sources: Anti-CCR7 (cat no. ab32527; Abcam, Cambridge, UK),
anti-ANO6 (cat no. ab156409; Abcam), anti-phosphorylated ERK (pERK)
(cat no. KGYT1625; Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China) and GAPDH (cat no. ab8245; Abcam). The reverse
transcription-quantitative polymerase chain reaction primers for
CCR7, ANO6, ERK1/2 and GAPDH were synthesized commercially by
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). A cell
proliferation kit was obtained from Beijing Dingguo Changsheng
Biotechnology Co., Ltd. (Beijing, China). The Migration kit (cat
no. #3422) was obtained from Corning Incorporated (Corning, NY,
USA).
Cell culture and transfection
All cells used in the present study were immediately
cryopreserved in liquid nitrogen. They were cultured under standard
conditions in Dulbecco's Modified Eagle's Medium (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (FBS; Hyclone; GE Healthcare Life Sciences), 100
U/ml ampicillin (Hyclone; GE Healthcare Life Sciences) and 100
µg/ml streptomycin (Hyclone; GE Healthcare Life Sciences). The
cultures were incubated at 37°C in a humidified atmosphere
containing 5% CO2.
Human CCR7 complementary DNA reverse-transcribed
from the longest transcript NM_001838 was cloned into the
recombined lentiviral vector, recombined by GV358
(pGC-FU-3FLAG-SV40-EGFP-IRES-puromycin; Shanghai GeneChem Co.,
Ltd., Shanghai, China), pHelper 1.0 (Shanghai GeneChem Co., Ltd.)
and pHelper 2.0 (Shanghai GeneChem Co., Ltd.), to create a complete
functional overexpression plasmid named LV-CCR7-OE. The empty
lentiviral vector was named as LV-GFP. BxPC-3 cells were seeded
onto 6-well plates. At 24 h after seeding, the cells were treated
with 5E+8 titration units of lentivirus and harvested at 72 h for
transfection at 37°C. The transfected BxPC-3 cells were selected if
the positive rate of green fluorescent protein (GFP) expression
reached >80%, evaluated by a fluorescence microscope (×200
magnification, Olympus Corporation, Tokyo, Japan). The expression
of CCR7 was confirmed using western blotting and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) at
96 h after transfection in BxPC-3 CCR7-overexpressing cells
(BxPC-3-CCR7-GFP cells; OE), blank-vector-transfected negative
control (BxPC-3-GFP cells; NC) and untransfected control (BxPC-3
cells; CON).
Cell proliferation assay
BxPC-3-CCR7-GFP and BxPC-3-GFP cells were plated at
a density of 2×103 cells/well in a final assay volume of
100 µl per well into 96-well plates. The cells were incubated for
various times (24, 48, 72, 96 and 120 h) under these conditions. At
4 h prior to the designed time point, the cells were incubated with
MTT. The purple formazan deposits were solubilized in dimethyl
sulfoxide. An automated fluorescence plate reader was used to
measure the proliferating cell population at an emission wavelength
of 490 nm. BxPC-3-CCR7-GFP and BxPC-3-GFP cells were pre-incubated
for 16 h in the presence or absence of 100 ng/ml CCL21, which is a
chemo-attractant (18), in a
humidified, 37°C, 5% CO2 chamber. To examine the effect
of inhibitors, the BxPC-3-CCR7-GFP cells in the absence of CCL21
were pretreated with 10 µmol/l U0126 for 2 h.
Cell migration assay
All cell migration assays were performed using a
24-well migration chamber with an 8-mm pore polycarbonate membrane,
based on the Boyden chamber principle. Briefly, BxPC-3-CCR7-GFP and
BxPC-3-GFP cells were re-suspended in serum-free RPMI 1640, and
1×105 cells were added to the interior of the Transwell
inserts in the upper chamber. CCL21 (0 and 100 ng/ml) were added to
RPMI 1640 (500 ml) containing 10% FBS in the lower chamber
individually. Following migration for 24 h at 37°C, the cells from
the top of the membrane were wiped off using cotton swabs whereas
the migrated cells (in the bottom chamber) were stained with Giesma
for 20 min at room temperature and washed with distilled water
three times. A fluoresence Direct microscopic (Olympus Corporation,
Tokyo, Japan) was used at a magnification of ×40 to observe the
cells that had migrated to the lower side of the membrane. The
absorption value was measured at a wavelength of 570 nm. To examine
the effect of inhibitors, the BxPC-3-CCR7-GFP cells in the absence
of CCL21 were pretreated with 10 µmol/l U0126 for 2 h.
RT-qPCR
Total cellular mRNA, purified using TRIzol (Shanghai
Pufei Biotechnology Co., Ltd., Shanghai, China), was subsequently
reverse transcribed to cDNA for 60 min at 37°C using a Promega
M-MLV kit (Promega Corporation, Madison, WI, USA). For quantitative
analysis of gene expression, RT-qPCR was conducted using a SYBR
premix Ex Taq II kit (Takara Biotechnology Co., Ltd., Dalian,
China) according to the manufacturer's protocols. The reaction
mixture was subjected to RT-qPCR to detect levels of CCR7, ANO6,
ERK1/2 and GAPDH. GAPDH was used as an endogenous reference. All
primer sequences used for the amplification are listed in Table I. The PCR thermocycling conditions
were as follows: Initial denaturation for 30 sec at 95°C, followed
by 45 cycles of denaturation for 5 sec at 95°C and annealing for 30
sec at 60°C. Following the last cycle, a final extension of 10 sec
at 60°C was completed and thereafter the samples were maintained at
4°C. The relative expression level of the genes was calculated
using the 2−ΔΔCq method (19).
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer,
5′-3′ | Reverse primer,
5′-3′ | Product size, bp |
|---|
| CCR7 |
TGGTGGTGGCTCTCCTTG |
CTGTGGTGTTGTCTCCGATG | 84 |
| ANO6 |
GAACAAGCCCGACCAGAATAC |
CCCAATAACTGAAGCGATGATC | 171 |
| ERK1 |
ATGTCATCGGCATCCGAGAC |
GGATCTGGTAGAGGAAGTAGCA | 156 |
| ERK2 |
TTACGACCCGAGTGACGA |
CTGTATCCTGGCTGGAATCT | 129 |
| GAPDH |
TGACTTCAACAGCGACACCCA |
CACCCTGTTGCTGTAGCCAAA | 121 |
Western blot analysis
BxPC-3-CCR7-GFP and BxPC-3-GFP cells were stimulated
or not with CCL21 for 16 h at 37°C and the BxPC-3-CCR7-GFP cells in
the absence of CCL21 were pretreated with 10 µmol/l U0126 for 2 h
at 37°C. Then the whole-cell protein and nuclear protein extracts
from cells were prepared in an ice-cold lysis buffer (50 mM Tris,
pH 6.8, 0.2% SDS, 10% glycerol and 1% mercaptoethanol) for western
blot analysis according to the manufacturers' protocols. Total
protein was determined using BCA assay (Beyotime Institute of
Biotechnology, Haimen, China). A total of 15 µg protein were
subjected to 10% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes. Following blocking for 1 h at room temperature
with 5% skimmed milk in TBST, filters were incubated with the
following primary antibodies for 2 h at room temperature: CCR7
(dilution, 1:5,000), ANO6 (dilution, 1:500), pERK antibody
(dilution, 1:500) and GAPDH (dilution, 1:500), and were visualized
with a horseradish peroxidase-conjugated secondary antibody rabbit
IgG for 1.5 h at room temperature (cat no. sc-2004; dilution,
1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and an
ECL substrate detection ECL-PLUS/kit (Thermo Fisher Scientific,
Inc.). Protein bands were quantified using ImageJ software version
1.49 (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Each experiment was performed in triplicate and
repeated as three independent experiments. Error bars represent the
standard deviation. Statistical significance was determined using
Student's t-test or one-way analysis of variance followed by
Turkey's post-hoc test, P<0.05 was considered to indicate a
statistically significant difference.
Results
CCR7 overexpression promotes BxPC-3
cell migration
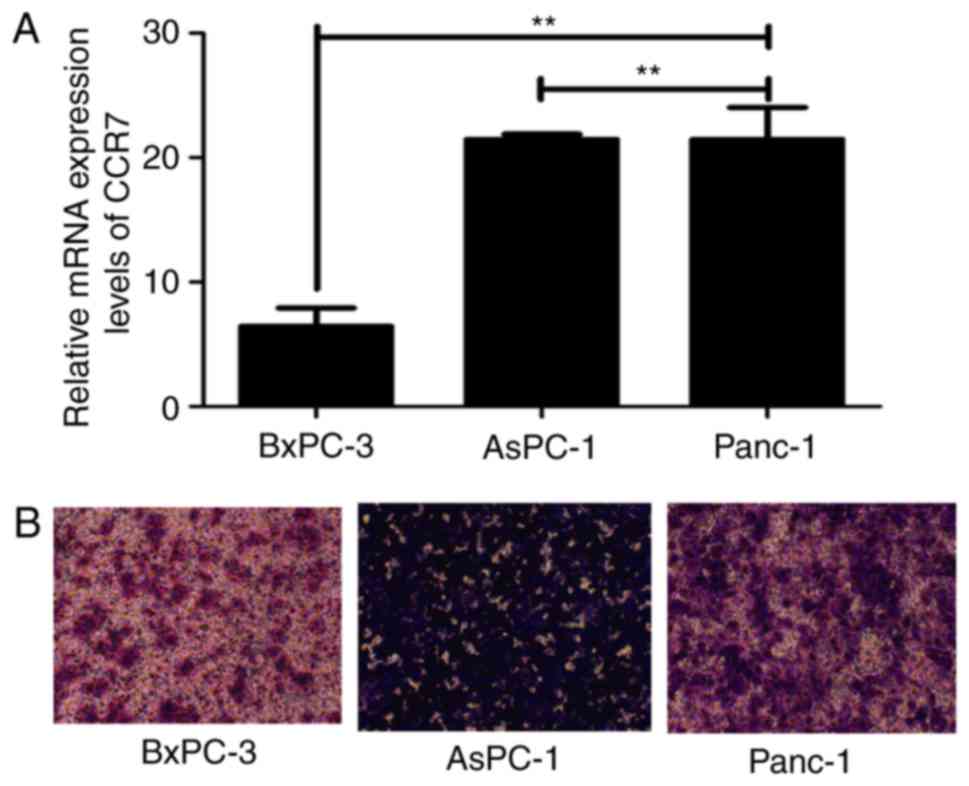
The present study evaluated the mRNA expression
levels of CCR7 and the migratory capacities of three PDAC cell
lines, BxPC-3, AsPC-1 and Panc-1. The results revealed that CCR7
was expressed in all the analyzed PDAC cell lines at the mRNA level
and a positive association was observed between CCR7 expression and
migratory capacities (P<0.01; Fig.
1).
To investigate the involvement of CCR7 in regulating
the migration phenotype of PDAC cells in vitro, exogenous
CCR7 was expressed in BxPC-3 cells via lentivirus transfection, as
these cells exhibit relatively low levels of CCR7 expression and
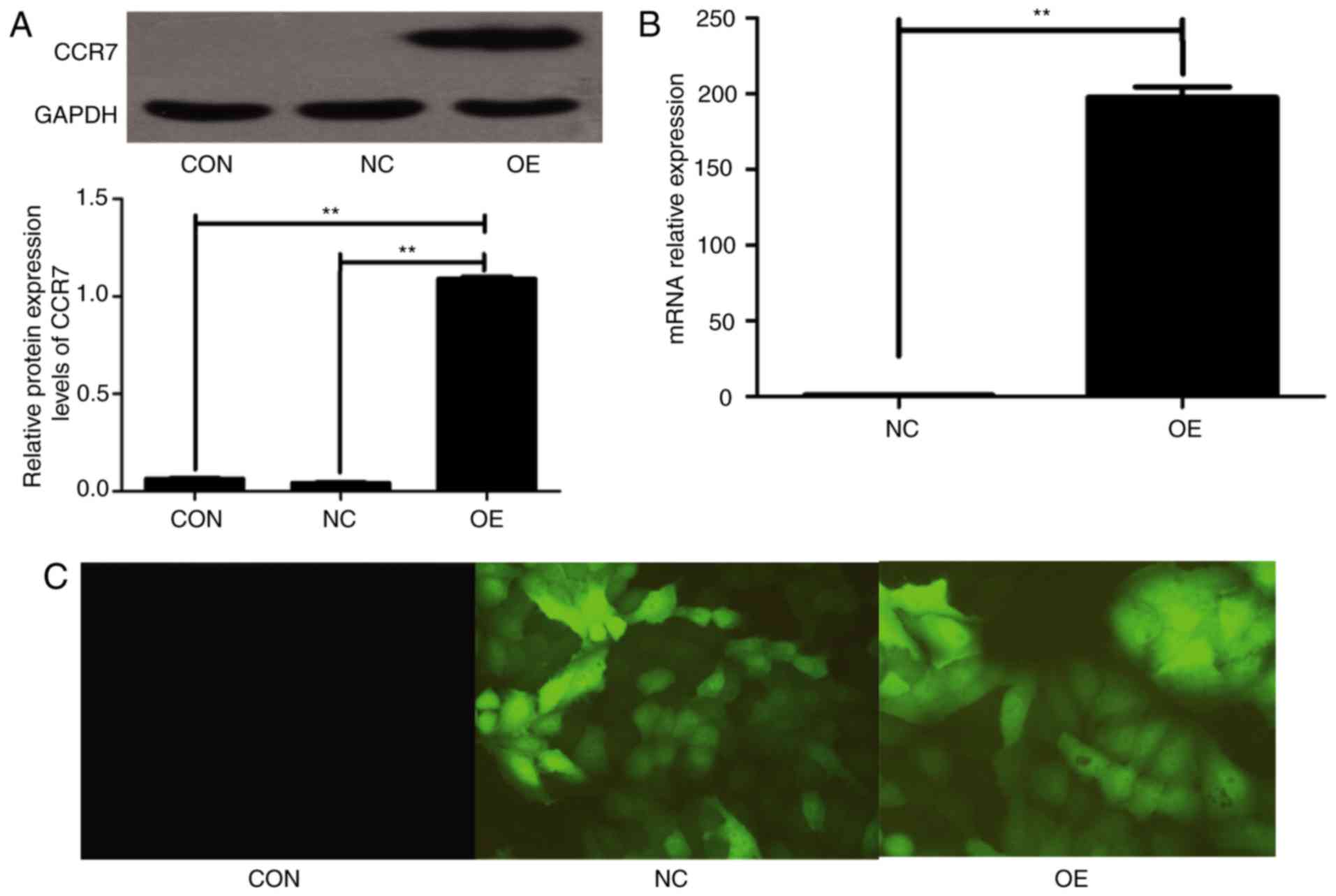
relatively poor migration ability. The lentivirus plasmid
LV-CCR7-GFP contained a GFP reporter gene. The high mRNA and
protein expression of CCR7 was detectable in BxPC-3-CCR7-GFP cells,
compared with BxPC-3-GFP cells and BxPC-3 cells (P<0.001;
Fig. 2). BxPC-3-CCR7-GFP cells
exhibited a markedly increased migration ability compared with the
BxPC-3-GFP cells (P<0.001; Fig.
3). These data indicated that CCR7 overexpression may promote
migration in BxPC-3 cells.
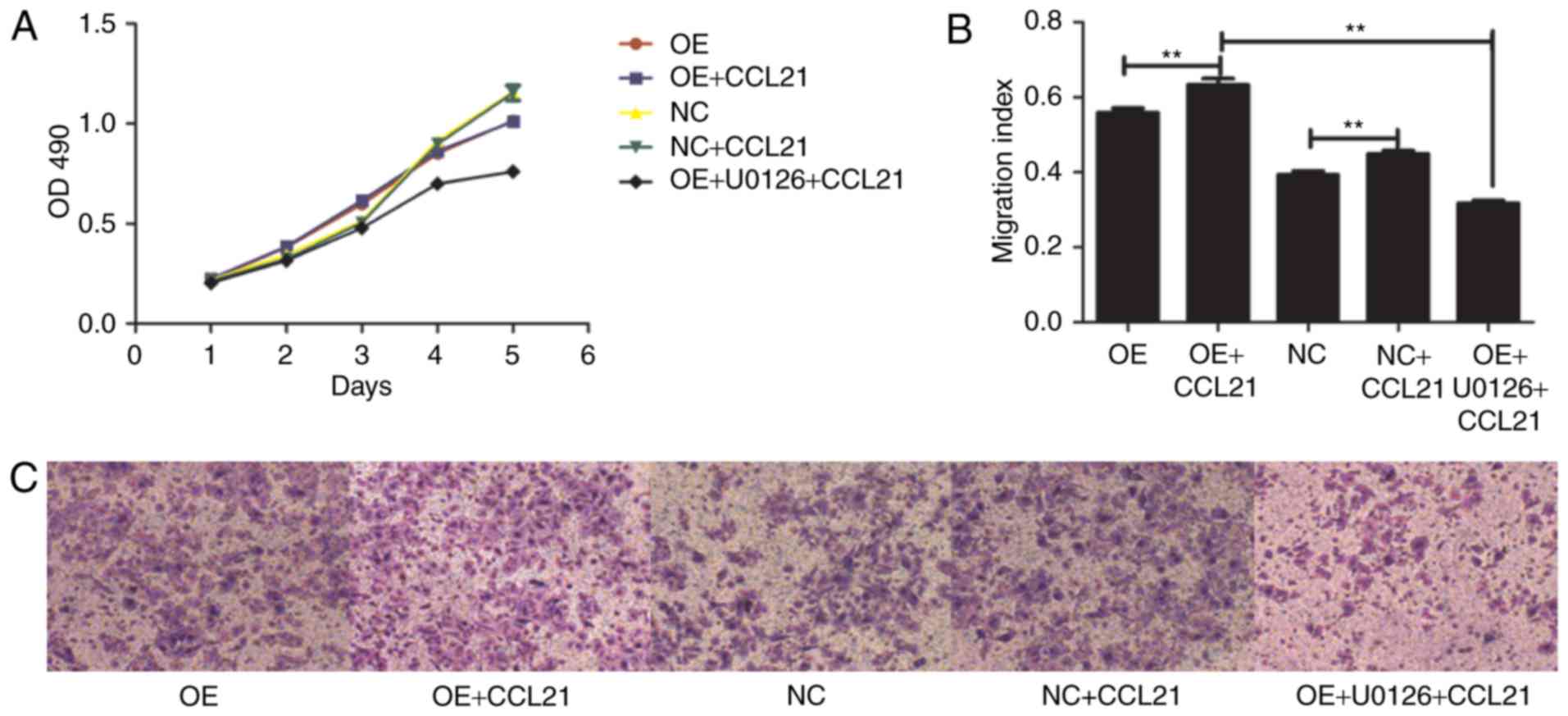 | Figure 3.Effects of CCL21/CCR7 axis and the
extracellular signal-regulated kinase inhibitor, U0126, on
proliferation and migration. OE cells were pretreated with or
without U0126 for 2 h, followed by stimulation with CCL21 for 16 h.
(A) Proliferative ability of OE cells and NC cells pretreated with
or without CCL21 and U0126. (B) Migratory ability of OE and NC
cells pretreated with or without CCL21 and U0126. (C) Transwell
migration assay was used to evaluate the migration capacity of
different cells (magnification, ×100). **P<0.01. CCL21, C-C
motif chemokine ligand 21; CCR7, C-C motif chemokine receptor 7;
OD, optical density; CON, untransfected control; NC,
blank-vector-transfected negative control; OE, CCR7-overexpressing
cells. |
CCR7 ligand-binding function affects
BxPC-3 cell migration
To determine whether the ligand-binding ability of
CCR7 affected BxPC-3 cell migration, BxPC-3-CCR7-GFP and BxPC-3-GFP
cells were pretreated with 100 ng/ml CCL21 for 16 h. The binding of
ligand and receptor resulted in an increase in migration,
indicating that ligand-binding to CCR7 was positively associated
with the migration of BxPC-3 cells (P<0.01; Fig. 3).
In addition, the effect of the CCL21/CCR7 axis on
the proliferation of BxPC-3 cells was also examined. The
proliferation change of different pretreated BxPC-3 cells was
determined using an MTT cell proliferation assay. The results
demonstrated that the rate of proliferation of BxPC-3-CCR7-GFP
cells may increase from the third day of the experiment compared
with that of the BxPC-3-GFP cells (P<0.001; Fig. 3). However, exogenous CCL21 did not
significantly alter the cellular proliferation of either cell type.
These results indicated that ligand binding to CCR7 is not
associated with BxPC-3 cell proliferation.
CCR7 overexpression promotes BxPC-3
cell migration by inducing ERK pathway activity
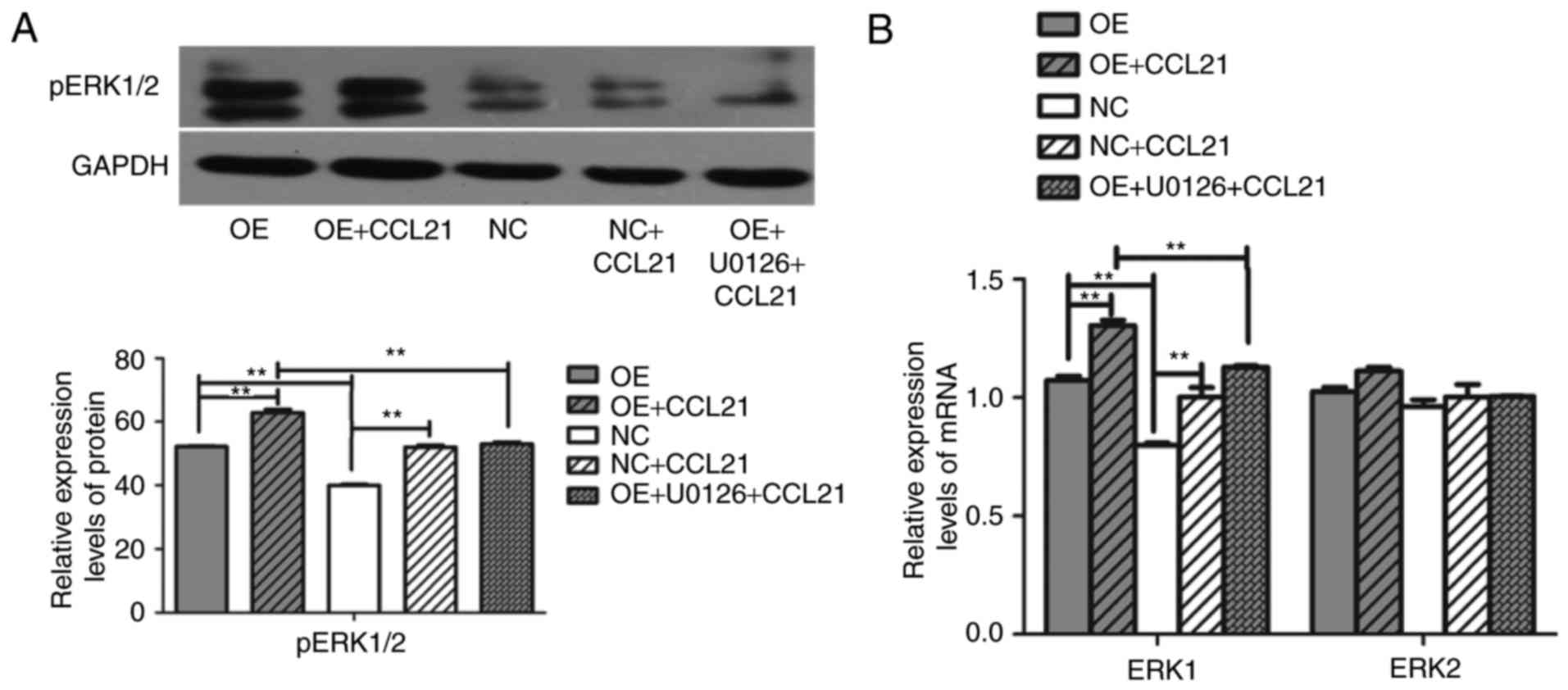
A previous study reported that CCR7 pathway
upregulates Twist expression via ERK signaling to manage the
epithelial-mesencymal transition of PDAC (20). To investigate whether the ERK1/2
pathway is downstream activated by CCL21-CCR7 binding, the
phosphorylation of ERK1/2 was observed for the entire duration of
stimulation with CCL21. To assess whether the phosphorylation of
ERK1/2 was dependent on the activation of CCR7, the cells were
pre-incubated with the ERK1/2 specific inhibitor U0126.
Furthermore, BxPC-3-CCR7-GFP cells underwent migration assays
adding CCL21 as a chemoattractant into the lower chamber of the
transwell chamber in the presence or absence of U0126. As
demonstrated in Fig. 3, according to
the transwell assay, the number of migrated cells was significantly
decreased following pre-incubation with U0126 (P<0.001). The
level of cellular proliferation was also inhibited by U0126
significantly (P<0.001; Fig. 3).
These results indicate that ERK serves a notable role in the
response of BxPC-3 cells to CCR7. To confirm further whether CCR7
regulates the migration of BxPC-3 cells by modulating ERK
expression, the mRNA and protein levels of ERK in different cell
lines were analyzed. It was revealed that overexpression of CCR7
and the binding of CCL21 to CCR7 upregulated the gene expression
level of ERK1 and the level of pERK protein in BxPC-3-CCR7-GFP
cells compared with BxPC-3-GFP cells (P<0.001; Fig. 4), but this had no influence on the
gene expression level of ERK2 (P>0.05). Following the
pre-incubation of BxPC-3-CCR7-GFP cells with U0126 for 2 h, the
ERK1 gene and levels of pERK protein decreased, as determined by
RT-qPCR and western blot analysis (P<0.001; Fig. 4). Pre-incubation with U0126 inhibited
the influence of CCL21/CCR7 on the expression of ERK. These
findings indicated that CCL21 drives increases in BxPC-3 cell
migration through the ERK pathway.
Involvement of the ERK signaling
pathway in CCR7 ligand-mediated upregulation of ANO6
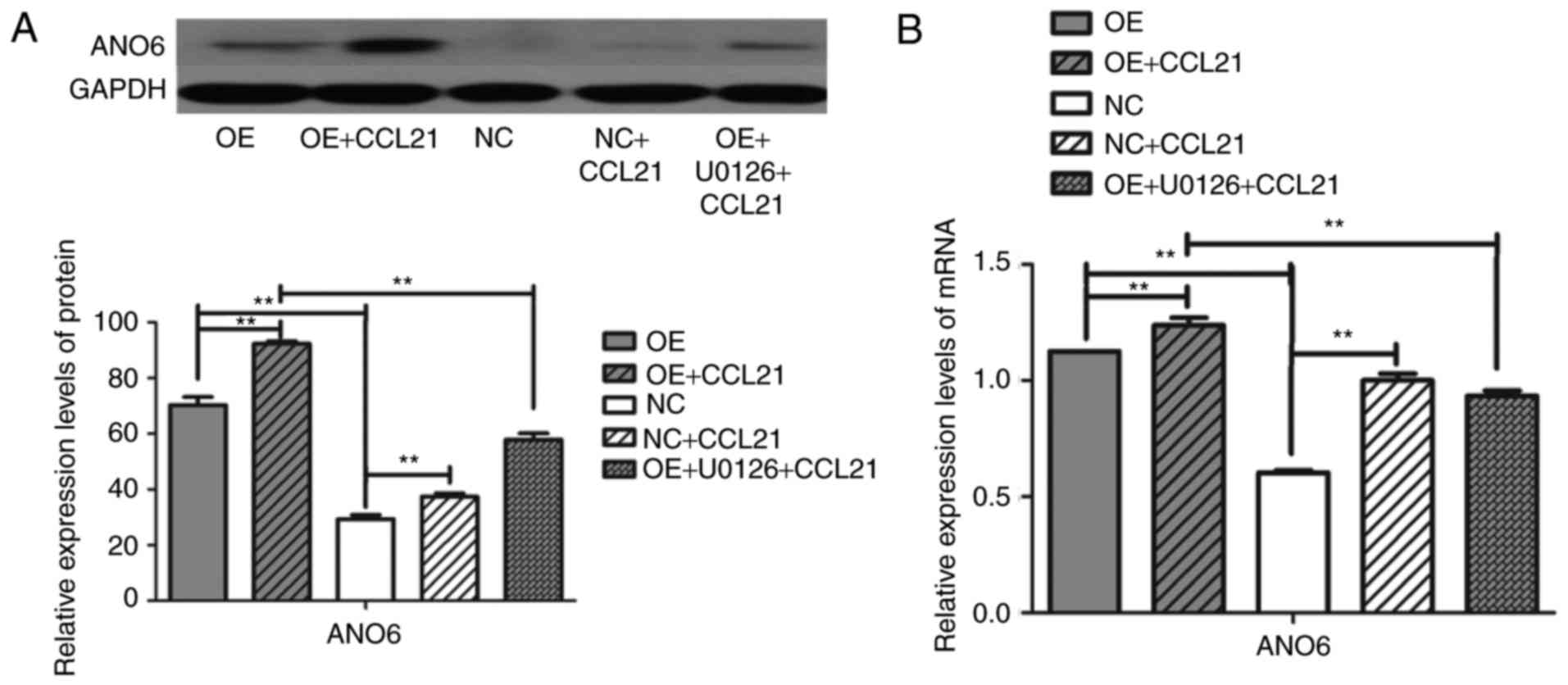
To investigate the role of CCL21/CCR7 binding in
ANO6 production, the constitutive expression of CCR7 was screened
in different PDAC cell lines and a gain-of-function model was
developed. A high level of CCR7 expression was associated with the
significant upregulation of ANO6 mRNA and protein expression
(Fig. 5; P<0.001). To determine
whether CCL21/CCR7 interaction regulated the expression of ANO6,
BxPC-3 cells were incubated with or without human CCL21 for 16 h.
These results demonstrated that ANO6 expression mRNA and protein
levels were significantly higher in the CCL21-treated cells than in
the untreated cells. Overall, these findings indicated that
CCL21/CCR7 binding has the potential to regulate ANO6
expression.
Finally, to assess the functional roles of the
ERK1/2 pathway in CCL21-induced ANO6 secretion, BxPC-3 cells were
pre-treated with the ERK1/2 inhibitor U0126 prior to treatment with
CCL21 for 16 h. Pre-treatment with the ERK1/2 inhibitor yielded a
significantly reduced ANO6 expression when compared with CCL21
treatment alone (Fig. 5; P<0.001).
Therefore, it can be concluded that activation of the ERK1/2
signaling pathway downstream of CCR7 is involved in the regulation
of ANO6 expression.
Discussion
Metastasis is the leading cause of cancer-associated
mortality; this is particularly true for PDAC, owing to its highly
invasive and metastatic behavior (21). Further understanding of the mechanisms
underlying aggressive cancer cell phenotypes, including knowledge
of this invasive, metastatic behavior, is required if patient
outcomes are to be improved (22).
The metastasis of tumor cells to the lymph nodes is
a complex process. Previous studies have indicated that the
CCL21/CCR7 axis serves a pivotal role in triggering lymphatic
metastasis of PDAC (1,10,23). The
aim of the present study was to determine whether the CCL21/CCR7
axis is associated with BxPC-3 cell proliferation and migration by
upregulating the expression of ANO6 via the ERK signaling
pathway.
To investigate the mechanism of CCR7 promotion on
PDAC cell lines, the expression of CCR7 in different PDAC cell
types was examined and a gain-of-function model comprising
BxPC-3-CCR7-GFP cells was generated. Stable CCR7 overexpression led
to an enhancement of the migratory ability of BxPC-3 cells, but
elicited no effect on BxPC-3 cell proliferation. These results
further supported the hypothesis that CCR7 may serve a role in
pancreatic cancer metastasis. However, the molecular mechanisms by
which CCR7 regulates cell migration remain unclear. To determine
how CCR7 promotes BxPC-3 cell migration, the present study focused
on delineating the association between CCR7 and ANO6.
Autocrine CCR7 stimulation by endogenous CCL21 may
present a possible mechanism to enhance migration in PDAC cells, a
finding that was consistent with the findings of Sperveslage et
al (24). Additionally, following
blocking with a CCR7 antibody, a significant decrease in bladder
cancer cells was observed (25).
However, the interactions between chemokines and their receptors
are fundamental to tumor cell motility and migration. Therefore,
CCL21 may activate CCR7 to enhance BxPC-3 cell migration.
The ANO family members are accessible cell surface
proteins that are upregulated in tumors and are therefore potential
targets for therapeutic antibodies and as biomarkers for tumors
(26). ANO6 was activated upon the
interaction of CCL21 with CCR7 and serves an important role in
chemokine-induced dendritic cell migration. In a transwell
migration assay, the chemokine-induced migration of immature and
mature dendritic cells was markedly impaired by treatment with a
small interfering RNA against ANO6 (27). However, the effect of ANO6 on PDAC
cells remains unknown. Whether ANO6 mediates the migration of human
PDAC cells and whether the transcription expression levels are
promoted by CCR7 requires further investigation. RT-qPCR and
western blot analysis confirmed that the ANO6 mRNA and protein
expression levels were upregulated, which was coupled with
increased ANO6 activity in BxPC-3-CCR7-GFP cells, compared with
that in the NC cells. Additionally, it was revealed that CCR7
promoted the expression of ANO6 mRNA and protein when BxPC-3 cells
were incubated with CCL21 for 16 h.
It is known that ERK, as a mitogen-activated protein
kinase signal transduction pathway, is modulated by the activity of
CCR7 (28–32). The present study demonstrated that
overexpression of CCR7 significantly increased the gene and protein
expression levels of ERK. When BxPC-3 cells were pretreated with
the ERK inhibitor U0126, the migration of BxPC-3 cells was reduced
and the mRNA transcription levels and protein expression levels of
ANO6 and pERK in BxPC-3 cells were decreased. Therefore, it is
likely that the ERK signaling pathway is associated with
CCR7-dependent cell motility, including regulation of ANO6
transcription.
In conclusion, to the best of our knowledge, the
present study is the first to indicate that CCL21/CCR7 upregulates
ANO6 via ERK to promote BxPC-3 cell migration, which indicates that
CCL21/CCR7 and ANO6 may serve crucial roles in the lymphatic
metastasis of PDAC. Further animal studies are required to
elucidate the sequence of events leading to the CCR7-mediated
metastatic phenotype, and will enable the development of
therapeutic strategies aimed at blocking these carcinogenic and
metastatic effects.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shanghai
Municipal Commission of Health and Family Planning Grant of China
(grant no. 955), the Shanghai Jiao Tong University Affiliated
Shanghai Sixth People's Hospital Grant of China (grant no. 1581)
and the Science and Technology Innovation Special Fund of Shanghai
Jiao Tong University Shanghai Jiao Tong University (grant no.
YG2016QN18).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LW performed the cellular functional experiments and
was a major contributor in writing the manuscript. XYZ performed
the cell culture. JSZ and NWC provided advice about the conception
and design, directed the experiment and revised the manuscript
critically for important intellectual content. HNF performed the
reverse transcription quantitative polymerase chain reaction. WY
performed the western blotting. JHG performed transfection of the
cells, data analysis, writing the manuscript and was committed to
funding the experiment. All authors have read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Neoptolemos JP, Stocken DD, Friess H,
Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C,
Lacaine F, et al: A randomized trial of chemoradiotherapy and
chemotherapy after resection of pancreatic cancer. N Engl J Med.
350:1200–1210. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zlotnik A and Yoshie O: Chemokines: A new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ben-Baruch A: The multifaceted roles of
chemokines in malignancy. Cancer Metastasis Rev. 25:357–371. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zlotnik A: Chemokines and cancer. Int J
Cancer. 119:2026–2029. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ding Y, Shimada Y, Maeda M, Kawabe A,
Kaganoi J, Komoto I, Hashimoto Y, Miyake M, Hashida H and Imamura
M: Association of CC chemokine receptor 7 with lymph node
metastasis of esophageal squamous cell carcinoma. Clin Cancer Res.
9:3406–2412. 2003.PubMed/NCBI
|
|
6
|
Tutunea-Fatan E, Majumder M, Xin X and
Lala PK: The role of CCL21/CCR7 chemokine axis in breast
cancer-induced lymphangiogenesis. Mol Cancer. 14:352015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Irino T, Takeuchi H, Matsuda S, Saikawa Y,
Kawakubo H, Wada N, Takahashi T, Nakamura R, Fukuda K, Omori T and
Kitagawa Y: CC-Chemokine receptor CCR7: A key molecule for lymph
node metastasis in esophageal squamous cell carcinoma. BMC Cancer.
14:2912014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oliveira-Neto HH, de Souza PP, da Silva
MR, Mendonça EF, Silva TA and Batista AC: The expression of
chemokines CCL19, CCL21 and their receptor CCR7 in oral squamous
cell carcinoma and its relevance to cervical lymph node metastasis.
Tumor Biol. 34:65–70. 2013. View Article : Google Scholar
|
|
9
|
Al-Shareef H, Hiraoka SI, Tanaka N, Shogen
Y, Lee AD, Bakhshishayan S and Kogo M: Use of NRP1, a novel
biomarker, along with VEGF-C, VEGFR-3, CCR7 and SEMA3E, to predict
lymph node metastasis in squamous cell carcinoma of the tongue.
Oncol Rep. 36:2444–2454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo J, Lou W, Ji Y and Zhang S: Effect of
CCR7, CXCR4 and VEGF-C on the lymph node metastasis of human
pancreatic ductal adenocarcinoma. Oncol Lett. 5:1572–1578. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Sun R, Tao K and Wang G: The
CCL21/CCR7 pathway plays a key role in human colon cancer
metastasis through regulation of matrix metalloproteinase-9. Dig
Liver Dis. 43:40–47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma H, Gao L, Li S, Qin J, Chen L, Liu X,
Xu P, Wang F, Xiao H, Zhou S, et al: CCR7 enhances TGF-β1-induced
epithelial-mesenchymal transition and is associated with lymph node
metastasis and poor overall survival in gastric cancer. Oncotarget.
6:24348–24360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu FY, Safdar J, Li ZN, Fang QG, Zhang X,
Xu ZF and Sun CF: CCR7 regulates cell migration and invasion
through JAK2/STAT3 in metastatic squamous cell carcinoma of the
head and neck. Biomed Res Int. 2014:4153752014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu FY, Safdar J, Li ZN, Fang QG, Zhang X,
Xu ZF and Sun CF: CCR7 regulates cell migration and invasion
through MAPKs in metastatic squamous cell carcinoma of head and
neck. Int J Oncol. 45:2502–2510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kunzelmann K, Nilius B, Owsianik G,
Schreiber R, Ousingsawat J, Sirianant L, Wanitchakool P, Bevers EM
and Heemskerk JW: Molecular functions of anoctamin 6 (TMEM16F): A
chloride channel, cation channel, or phospholipid scramblase?
Pflugers Arch. 466:407–414. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miettinen M: Immunohistochemistry of soft
tissue tumours-review with emphasis on 10 markers. Histopathology.
64:101–118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jacobsen KS, Zeeberg K, Sauter DR, Poulsen
KA, Hoffmann EK and Schwab A: The role of TMEM16A (ANO1) and
TMEM16F (ANO6) in cell migration. Pflugers Arch. 465:1753–1762.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu S, Xing W, Peng J, Yuan X, Zhao X, Lei
P, Li W, Wang M, Zhu H, Huang B, et al: Tumor transfected with
CCL21 enhanced reactivity and apoptosis resistance of human
monocyte-derived dendritic cells. Immunobiology. 213:417–426. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li K, Xu B, Xu G and Liu R: CCR7 regulates
Twist to induce the epithelial-mesenchymal transition in pancreatic
ductal adenocarcinoma. Tumour Biol. 37:419–424. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giovannetti E, van der Borden CL, Frampton
AE, Ali A, Firuzi O and Peters GJ: Never let it go: Stopping key
mechanisms underlying metastasis to fight pancreatic cancer. Semin
Cancer Biol. 44:43–59. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakata B, Fukunaga S, Noda E, Amano R,
Yamada N and Hirakawa K: Chemokine receptor CCR7 expression
correlates with lymph node metastasis in pancreatic cancer.
Oncology. 74:69–75. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sperveslage J, Frank S, Heneweer C,
Egberts J, Schniewind B, Buchholz M, Bergmann F, Giese N, Munding
J, Hahn SA, et al: Lack of CCR7 expression is rate limiting for
lymphatic spread of pancreatic ductal adenocarcinoma. Int J Cancer.
131:E371–E381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mo M, Zhou M, Wang L, Qi L, Zhou K, Liu
LF, Chen Z and Zu XB: CCL21/CCR7 enhances the proliferation,
migration, and invasion of human bladder cancer T24 cells. PLoS
One. 10:e01195062015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shang L, Hao JJ, Zhao XK, He JZ, Shi ZZ,
Liu HJ, Wu LF, Jiang YY, Shi F, Yang H, et al: ANO1 protein as a
potential biomarker for esophageal cancer prognosis and
precancerous lesion development prediction. Oncotarget.
7:24374–24382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Szteyn K, Schmid E, Nurbaeva MK, Yang W,
Münzer P, Kunzelmann K, Lang F and Shumilina E: Expression and
functional significance of the Ca(2+)-activated Cl(−) channel ANO6
in dendritic cells. Cell Physiol Biochem. 30:1319–1332. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng S, Guo J, Yang Q and Yang X:
Crk-like adapter protein regulates CCL19/CCR7-mediated
epithelial-to-mesenchymal transition via ERK signaling pathway in
epithelial ovarian carcinomas. Med Oncol. 32:472015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J, Zhou Y and Yang Y: CCR7 pathway
induces epithelial-mesenchymal transition through up-regulation of
Snail signaling in gastric cancer. Med Oncol. 32:4672015.PubMed/NCBI
|
|
30
|
Li G, Yang Y, Xu S, Ma L, He M and Zhang
Z: Slug signaling is up-regulated by CCL21/CCR7 [corrected] to
induce EMT in human chondrosarcoma. Med Oncol. 32:4782015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su ML, Chang TM, Chiang CH, Chang HC, Hou
MF, Li WS and Hung WC: Inhibition of chemokine (C-C motif) receptor
7 sialylation suppresses CCL19-stimulated proliferation, invasion
and anti-anoikis. PLoS One. 9:e988232014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang L, Wang D, Li Y, Liu Y, Xie X, Wu Y,
Zhou Y, Ren J, Zhang J, Zhu H and Su Z: CCL21/CCR7 axis contributed
to CD133+ pancreatic cancer stem-like cell metastasis via EMT and
Erk/NF-κB pathway. PLoS One. 11:e01585292016. View Article : Google Scholar : PubMed/NCBI
|