Introduction
The most common type of pancreatic cancer is
pancreatic ductal adenocarcinoma (PDA), which presents as lethal
solid tumors and is largely resistant to standard therapeutic
modalities (1). The only curative
treatment for PDA patients is surgical resection, and a limited
number of patients present with resectable tumors at the time of
diagnosis (2). Furthermore, even if
patients with PDA undergo complete surgical resection, the majority
of patients develop early recurrences or metastases, with a 5-year
overall survival (OS) rate of ~5% (3–5). In total,
>60% of surgically resected PDA patients develop recurrences
within 2 years (3). Therefore, novel
prognostic markers associated with early mortality are urgently
required. However, prognostic factors for short-term survival
following surgical resection have not been well characterized
(6–8).
Furthermore, understanding the prognostic factors associated with
early recurrence following surgery can lead to insights into novel
therapeutic targets.
The Wilms' tumor 1 (WT1) gene encodes a protein that
consists of four zinc finger domains at the C terminus and a
glutamine- and proline-rich domain at the N terminus (9). WT1 was initially defined as a tumor
suppressor gene in Wilms' tumors, a childhood kidney neoplasm
(10). However, subsequent research
has revealed that WT1 also serves an oncogenic role in various
other types of tumors through transcriptional regulation in
tumorigenesis (11–13). Knockdown of WT1 can inhibit tumor cell
proliferation (14), and WT1
activates transcription of the interleukin-10 gene, thereby
promoting tumor escape from immune surveillance (15). Furthermore, previous studies have
demonstrated that WT1 serves an important role in the prognosis of
patients with tumors, including ovarian cancer (16,17),
hepatocellular carcinoma (18),
breast cancer (19) and acute
leukemia (20), and WT1 protein
overexpression is associated with a poor prognosis (21). Notably, WT1 was ranked as the top
antigen in a list of 75 tumor-associated antigens by a National
Cancer Institute prioritization project based on several factors,
including the role of the antigen in tumorigenicity and the high
expression level of WT1-positive tumor cells (22). Therefore, WT1 has been used as a
target of cancer vaccines, and results from early clinical trials
have demonstrated that WT1-targeted immunotherapy has the potential
to treat patients with PDA (23–28). As
only a small number of studies (29–31) have
demonstrated WT1 protein expression in 30–70% of patients with PDA,
WT1 expression at the protein level in PDA tissue and its role as a
potential prognostic marker to predict OS and disease-free survival
(DFS) times have not yet been documented.
Tumor-associated systemic inflammation promotes
angiogenesis and tumor cell proliferation, resulting in recurrence
and metastasis through the secretion of various chemokines and
cytokines from tumor cells and proinflammatory cells (32). The systemic inflammatory response,
which serves a crucial role in tumor development and progression,
is reflected by increased levels of C-reactive protein (CRP),
neutrophils, platelets, lymphocytes, leucocytes, monocytes and
lactate dehydrogenase (LDH), and decreased levels of albumin (Alb)
and hemoglobin (Hb) (33). Therefore,
an increased neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte
ratio (PLR), lymphocyte/monocyte ratio (LMR) and CRP/Alb ratio have
been identified as easily accessible tools to assess the systemic
inflammatory response and as indicators of a poor prognosis for
various types of cancer (32,33). These reports led to the hypothesis
that WT1 protein expression in PDA cells may be associated with
specific systemic inflammatory markers that are indicative of
disease progression. If such data were to confirm the prognostic
value of WT1, significant advances may be made in the clinical
translation of biomarkers, as well as the identification of
patients with PDA at high risk for disease progression following
surgical resection and of targets of PDA.
In the present study, the clinical implications of
WT1 protein expression in PDA cells and systemic inflammatory
markers in patients with PDA following surgical resection as
prognostic parameters were investigated. An aim of the present
study was to detect WT1 protein expression in the nuclei and
cytoplasm of PDA cells from all 50 enrolled patients, and to
perform an analysis using a modified immunohistochemical staining
method. Furthermore, WT1 expression was also detected in specific
tumor vessels in PDA stroma. We hypothesized that the
overexpression of WT1 protein in the PDA cytoplasm would be
significantly associated with worse OS and DFS times in PDA
patients following surgical resection. However, further studies are
required to confirm the role of cytoplasmic WT1 overexpression in
PDA in order to identify clinical prognostic factors associated
with early recurrence following surgery.
Materials and methods
PDA patients
Between January 2005 and December 2015, 50
consecutive patients with PDA (mean age, 65.4 years; age range,
33–79 years; sex distribution; 31 males and 19 females) who
underwent macroscopically curative resection at the Jikei
University Kashiwa Hospital (Kashiwa, Chiba, Japan) were enrolled
in the present study. There is an increased incidence of
postoperative mortality and complications among patients over the
age of 80 (34). Furthermore,
surgical resection is not superior to chemotherapy for octogenarian
PDA patients; therefore, only select patients benefit from
pancreatic resection amongst the elderly (35). As a result, PDA patients >80 years
old were excluded from the present study. Information on the
clinical features of the patients, including age, sex and tumor
characteristics (e.g., location, size and pathology), were
prospectively obtained from the patient's medical records. Tumor
stage was assessed according to the American Joint Committee of
Cancer (AJCC) pancreatic cancer Tumor-Node-Metastasis (TNM) staging
system (36). A summary of the
patients' profiles is shown in Table
I. The present study was reviewed and approved by the
Institutional Review Board of Jikei University. The procedures for
the present study were conducted according to the Declaration of
Helsinki. Due to the retrospective and non-interventional nature of
the present study, the IRB waived the requirement for written
informed consent from the participants for their clinical records
and tissues to be used in the present study.
 | Table I.Baseline characteristics of patients
with pancreatic ductal adenocarcinoma. |
Table I.
Baseline characteristics of patients
with pancreatic ductal adenocarcinoma.
| Clinicopathological
characteristics | n | % |
|---|
| Age at surgery,
years |
|
|
|
<65 | 18 | 36 |
|
≥65 | 32 | 64 |
| Sex |
|
|
|
Male | 31 | 62 |
|
Female | 19 | 38 |
| Tumor location |
|
|
|
Head | 39 | 78 |
|
Body-to-tail | 11 | 22 |
| Tumor
differentiation |
|
|
|
Well-to-moderate | 42 | 84 |
|
Poor | 8 | 16 |
| Tumor stage |
|
|
| I and
II | 34 | 68 |
|
III | 16 | 32 |
| Tumor size, cm |
|
|
|
<3 | 15 | 30 |
| ≥3 | 35 | 70 |
OS and DFS times
OS time was defined as the time from diagnosis to
mortality from any cause. DFS time was defined as the time from the
date of surgery to the first radiological evidence of recurrence or
mortality without evidence of recurrence or a second primary
cancer.
Laboratory parameters
All laboratory parameters, including the levels of
leucocytes, lymphocytes, monocytes, neutrophils, Hb, platelets,
CRP, Alb, LDH, amylase (AMY), carcinoembryonic antigen (CEA) and
carbohydrate antigen 19-9 (CA19-9), were measured within the 2
weeks prior to surgery. The systemic inflammatory response markers,
including the NLR, PLR, LMR and CRP/Alb ratio prior to surgery,
were also evaluated. All data were assessed to determine the
prognostic impact on patients with PDA following surgical
resection. Furthermore, the associations between WT1 protein
expression in PDA cells and the various laboratory parameters were
analyzed.
Immunohistochemistry
All PDA surgical tissues were routinely fixed in 10%
formalin for 1 day at room temperature and embedded in paraffin.
Immunohistochemical staining for WT1 was performed on the tissues.
The paraffin sections were cut into 5-µm slices and placed on
slides. Endogenous peroxidase activity was blocked in 10%
H2O2 for 5 min at room temperature. Antigen
retrieval was performed by heating (70°C for 20 min, followed by
110°C for 25 min) in Target Retrieval Solution [Tris/EDTA buffer
(pH 9.0); Dako; Agilent Technologies GmbH, Waldbronn, Germany] to
unmask the WT1 epitopes. The tissue slides were then incubated with
mouse anti-human WT1 monoclonal antibody (clone 6F-H2; dilution
1:100; cat. no. M3561; Dako; Agilent Technologies GmbH) overnight
at 4°C and washed with PBS. Immunostaining was performed for 30 min
at room temperature using the EnVision detection system RUO kit
(cat. no. K500711; Dako; Agilent Technologies GmbH) containing
ready-to-use horseradish peroxidase-conjugated rabbit anti-mouse
IgG and 3,3′-diaminobenzidine-peroxidase solution according to the
manufacturer's protocol. As all cases exhibited diffuse or granular
staining in the cytoplasm of the PDA cells, the intensity of WT1
protein expression in the PDA cells was classified as follows: i)
Negative, no staining was observed in PDA cells; ii) weak, faint
and barely perceptible cytoplasmic staining was observed in PDA
cells under ×200 magnification; iii) moderate, moderate complete
cytoplasmic staining was observed in PDA cells under ×40
magnification; and iv) strong, strong complete cytoplasmic staining
was observed in PDA cells under low magnification. Additionally,
the WT1-expressing tumor vessels (WT1-TV) were counted by light
microscopy in 5 high-power fields (×200 magnification). The
patients with PDA were divided into two groups; high (greater than
the mean number of WT1-positive vessels) and low WT1-TV density
(less than or equal to the mean number of WT1-positive vessels).
Three investigators who were not privy to the requisite clinical
information independently interpreted the WT1 protein expression
results. Negative control staining was applied to all samples using
a mouse immunoglobulin G (IgG) monoclonal antibody (dilution 1:100;
cat. no. X0931; Dako; Agilent Technologies GmbH) overnight at 4°C.
WT1-positive colonic gastrointestinal stromal tumor (GIST)
localized at transverse colon obtained from an unenrolled patient
was used as a positive control (37).
The tissues fixed in 10% formalin for 1 day at room temperature and
embedded in paraffin were cut into 5-µm slices and stained with
hematoxylin (for 4 min at room temperature) and eosin (for 2 min at
room temperature) for histopathological evaluation as previously
described (38).
Statistical analysis
The results of immunohistochemical staining and
laboratory data were assessed to determine their association with
OS and DFS times. All statistical analyses were performed using SAS
version 9.4 software (SAS Institute, Cary, NC, USA). The OS and DFS
times were estimated using the Kaplan-Meier method and were
compared using the log-rank test. Univariate and multivariate
analyses were performed using a Cox proportional hazard model to
evaluate the influence of multiple parameters, including lymphocyte
numbers, NLR and CA19-9 as continuous variables, on independent
predictors of OS and DFS times. Hazard ratios (HRs) with 95%
confidence intervals (CIs) were calculated using Cox proportional
hazard models. Differences in clinical and laboratory findings
between the WT1 protein overexpression and non-overexpression
groups were evaluated using the Mann-Whitney U test. The
χ2 test was used to determine the association between
cytoplasmic WT1 intensity and patient characteristics. P<0.05
was considered to indicate a statistically significant
difference.
Results
PDA patient characteristics
A total of 50 consecutive PDA patients with
histologically confirmed invasive ductal adenocarcinoma who
underwent complete surgical resection at the Jikei University
Kashiwa Hospital between January 2005 and December 2015 were
assessed. The clinical outcome and other characteristics of the PDA
patients are summarized in Table I.
The patient distribution consisted of 31 (62%) males and 19 (38%)
females, with a mean age of 65.4 years (range, 33–79 years). The
number of PDA patients classified as AJCC stage I/II was 34 (68%),
whereas 16 were classified as stage III (32%). The majority of the
PDA lesions (n=39; 78%) were located in the head of the pancreas
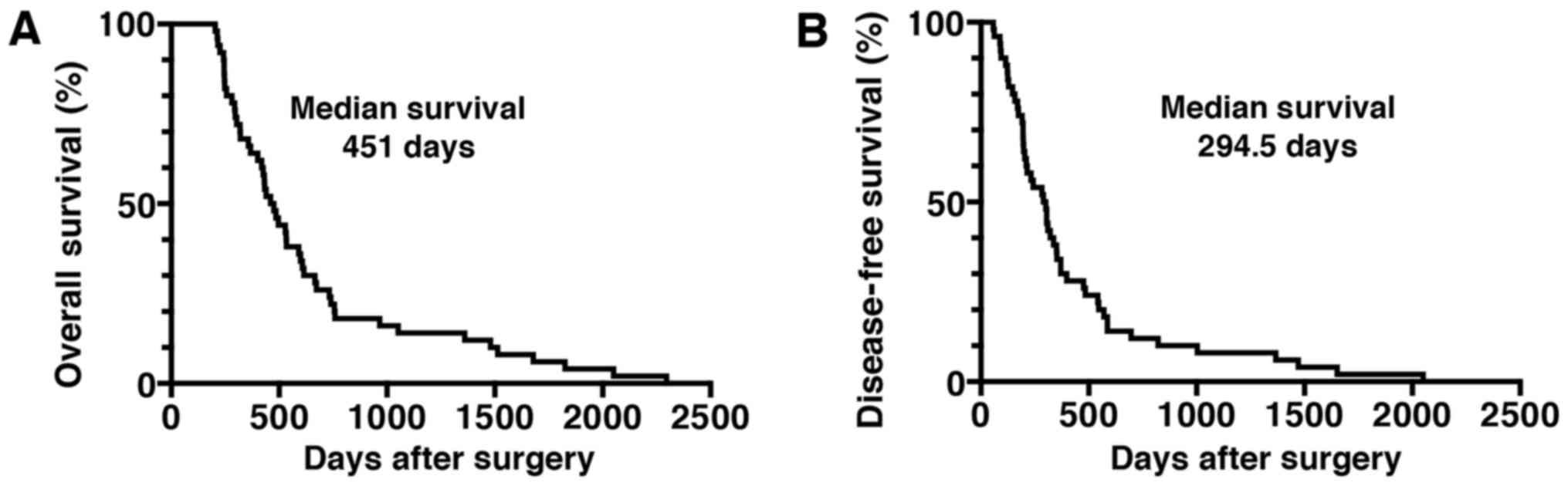
(Table I). The median OS time of the
patients was 451 days, and the median DFS time was 294.5 days
(Fig. 1). The majority of patients
(n=42; 84%) received chemotherapy following surgical resection.
Laboratory parameters, including the levels of leucocytes,
lymphocytes, monocytes, neutrophils, Hb, platelets, CRP, Alb, LDH,
AMY and tumor markers (CEA and CA19-9), obtained within 2 weeks
prior to surgery are shown in Table
II. The systemic inflammatory response markers, such as the
NLR, PLR, LMR and CRP/Alb ratio prior to surgery, are also shown in
Table II.
 | Table II.Baseline characteristics of
laboratory parameters of patients with pancreatic ductal
adenocarcinoma. |
Table II.
Baseline characteristics of
laboratory parameters of patients with pancreatic ductal
adenocarcinoma.
| Laboratory
parameters | Median | Range |
|---|
| Leucocytes,
count/µl | 5,150 | 2,500–12,000 |
| Lymphocytes,
count/µl | 1,400 | 500-2,800 |
| Monocytes,
count/µl | 300 | 100–900 |
| Neutrophils,
count/µl | 3,050 | 1,500–9,000 |
| Hemoglobin,
g/dl | 12.5 | 9.5–16.5 |
| Platelets,
×104 count/µl | 23.2 | 12.1–80.6 |
| C-reactive protein,
mg/dl | 0.1 | 0.1–3.8 |
| Albumin, g/dl | 3.9 | 2.0–4.9 |
| Lactate
dehydrogenase, IU/l | 181.5 | 112–319 |
| Amylase, IU/l | 93 | 15–1556 |
| Carcinoembryonic
antigen, ng/ml | 4.7 | 1.3–43.7 |
| Carbohydrate
antigen 19-9, U/ml | 114 | 1–5739 |
|
Neutrophil/lymphocyte ratio, % | 2.1 | 1.00–7.60 |
| Platelet/lymphocyte
ratio, % | 174.4 | 61.8–424.2 |
| Lymphocyte/monocyte
ratio, % | 5.12 | 0.6–11.0 |
| C-reactive
protein/albumin, % | 0.031 | 0.02–1.07 |
WT1 protein expression in PDA
Regarding the immunohistochemical staining of WT1,
granular or diffuse nucleo-cytoplasmic staining in PDA cells is
considered positive (29,30). In the present study, WT1 protein
expression was detected in the nuclei and cytoplasm of PDA samples
from all patients, whereas no expression was detected in
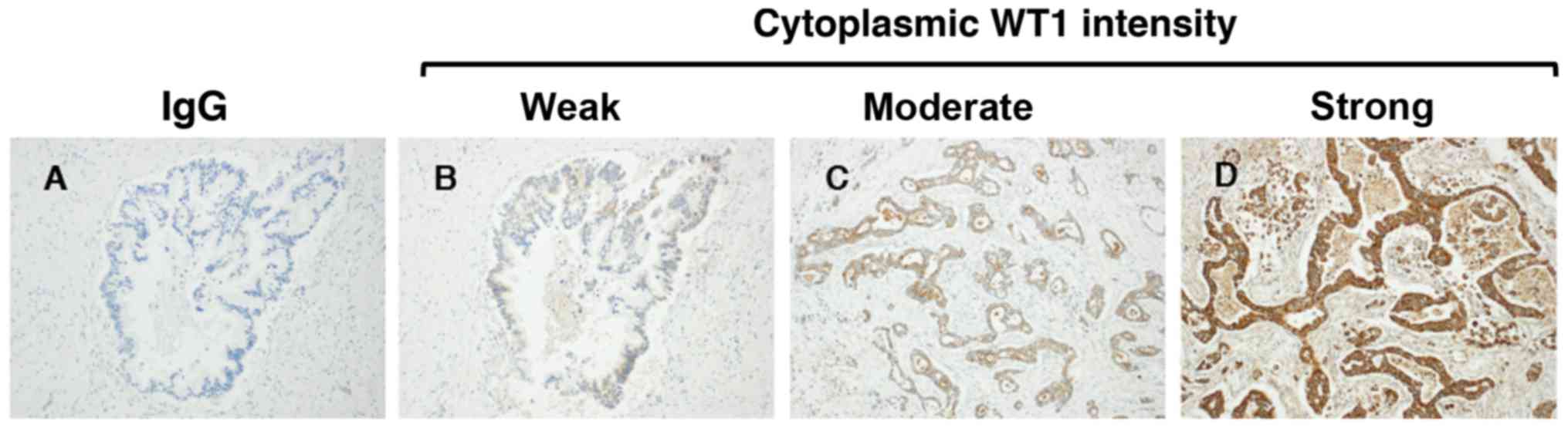
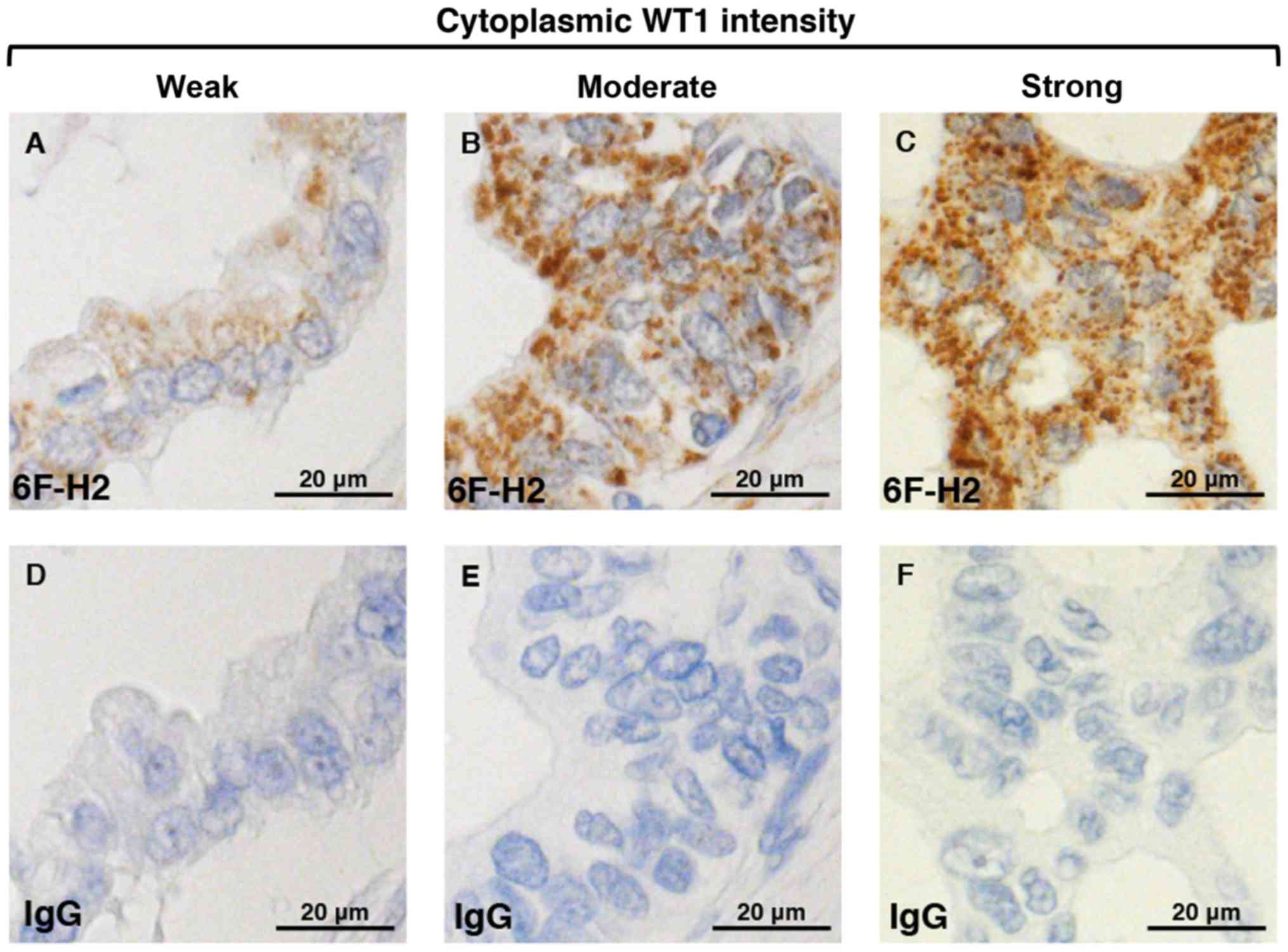
corresponding normal pancreatic ductal cells (Figs. 2 and 3).
There was weak nuclear immunostaining using WT1-specific monoclonal
antibodies; however, WT1 proteins were predominantly localized to
the cytoplasm in all cases. Therefore, the patients with PDA were
subdivided into four groups based on the cytoplasmic WT1 staining
intensity: Negative (0%), weak (38%), moderate (46%) and strong
(16%) (Fig. 2). In addition, no
staining was observed in any samples stained with the non-reactive
mouse IgG (negative control). In the present study, in order to
assess the prognostic significance of WT1 expression in patients
with PDA, patients were classified as having either a weak or
moderate-to-strong cytoplasmic WT1 intensity. The associations
between cytoplasmic WT1 intensity and the various
clinicopathological parameters of PDA are illustrated in Table III. There were no statistically
significant associations between cytoplasmic WT1 intensity and
clinicopathological parameters, including age at surgical
resection, sex or tumor characteristics (e.g., location, pathology,
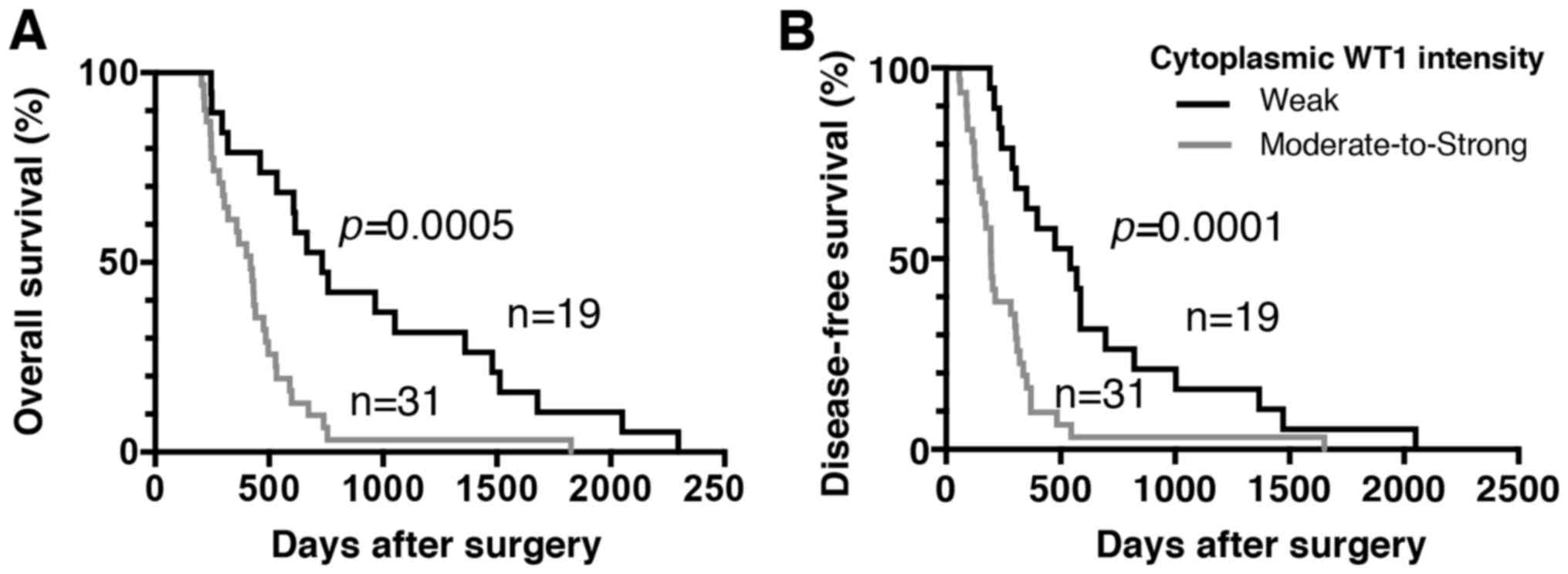
size and stage). The median survival time for patients with PDA
with weak and moderate-to-strong cytoplasmic WT1 expression was 732
and 419 days, respectively. Furthermore, the median DFS time for
patients with PDA with weak and moderate-to-strong cytoplasmic WT1
expression was 543 and 196 days, respectively. Importantly, the OS
and DFS times of PDA patients with weak cytoplasmic WT1 expression
were significantly prolonged compared with those of patients with
moderate-to-strong cytoplasmic WT1 expression (P=0.0005 and
P=0.0001, respectively) (Fig. 4).
Univariate and multivariate analyses of OS and DFS times according
to the cytoplasmic WT1 intensity and various clinicopathological
parameters were also performed. The cytoplasmic WT1 intensity in
PDA, which was revealed to be significant by univariate analysis,
was further analyzed by multivariate analysis (Tables IV and V). Multivariate analysis also revealed that
the cytoplasmic WT1 intensity in PDA cells was a significant
prognostic factor independent of the other prognostic factors
(Tables IV and V). Furthermore, the HRs of the cytoplasmic
WT1 intensity in relation to OS and DFS times based on the
multivariate analysis (3.972 and 4.117, respectively) were greater
than those observed in the univariate analysis (2.992 and 2.952,
respectively). These data suggest that moderate-to-strong WT1
immunostaining in the cytoplasm of PDA cells may serve as a
surrogate marker for the poor prognosis of patients with PDA
following surgical resection. Furthermore, WT1 expression was also
detected in certain tumor vessels in the PDA stroma. Therefore, the
PDA patients were divided into two groups according to WT1-TV
density. The mean WT1-TV density was 4.03 vessels, confirming the
stroma-rich and hypovascular features of PDA. The mean WT1-TV count
was used as the cutoff value between low and high density. An
association between a high density of WT1-TV (>4.03 vessels) and
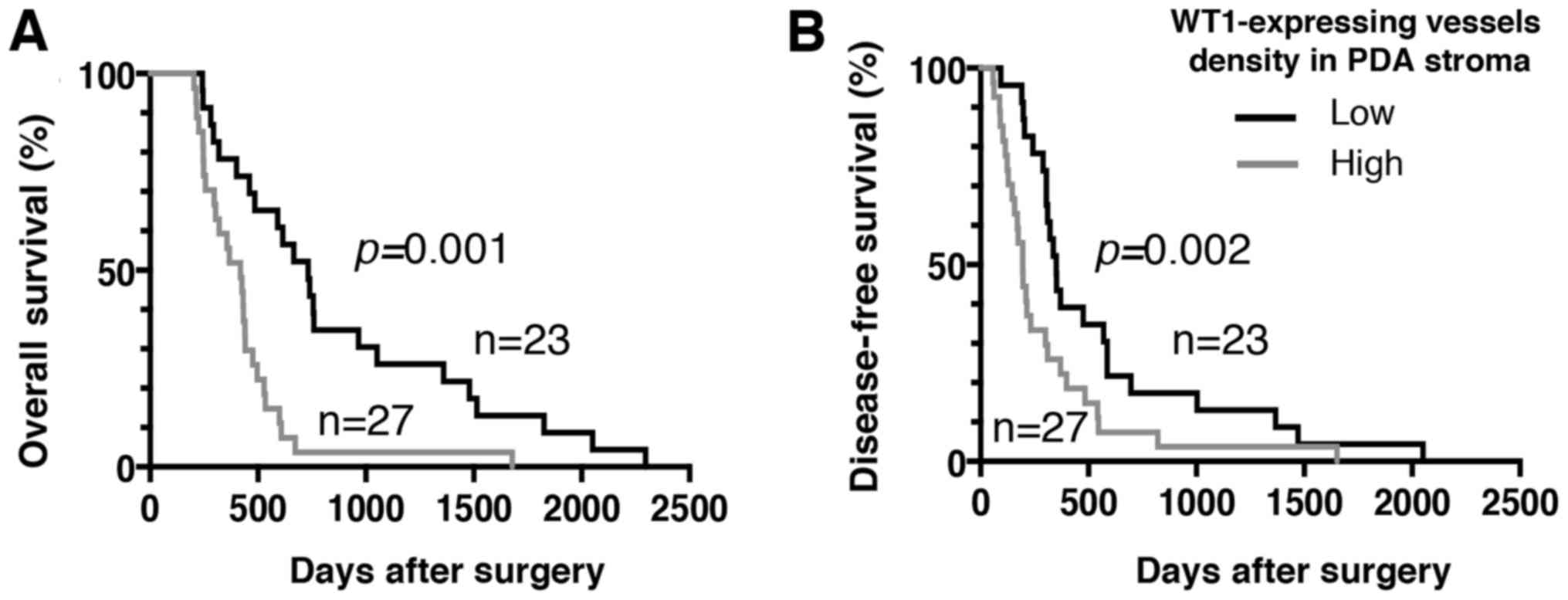
worse OS or DFS times was also demonstrated (Fig. 5).
 | Table III.Associations between cytoplasmic WT1
intensity and patient characteristics. |
Table III.
Associations between cytoplasmic WT1
intensity and patient characteristics.
|
| Cytoplasmic WT1
intensity, n (%) |
|
|---|
|
|
|
|
|---|
| Clinicopathological
characteristics | Weak |
Moderate-to-strong | P-value |
|---|
| Age at surgery,
years |
|
| 0.764 |
|
≥65 | 13 (68.4) | 19 (61.3) |
|
|
<65 | 6 (31.6) | 12 (38.7) |
|
| Sex |
|
| 0.556 |
|
Male | 13 (68.4) | 18 (58.1) |
|
|
Female | 6 (31.6) | 13 (41.9) |
|
| Tumor location |
|
| 0.293 |
|
Body-to-tail | 6 (31.6) | 5 (16.1) |
|
|
Head | 13 (68.4) | 26 (83.9) |
|
| Tumor
differentiation |
|
| 0.659 |
|
Poor | 2 (10.5) | 6 (19.4) |
|
|
Well-to-moderate | 17 (89.5) | 25 (80.6) |
|
| Tumor stage |
|
| 0.549 |
|
I/II | 14 (73.7) | 20 (64.5) |
|
|
III/IV | 5 (26.3) | 11 (35.5) |
|
| Tumor size, cm |
|
| 0.205 |
|
<3 | 8 (42.1) | 7 (22.6) |
|
| ≥3 | 11 (57.9) | 24 (77.4) |
|
 | Table IV.Univariate and multivariate analysis
of overall survival. |
Table IV.
Univariate and multivariate analysis
of overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Clinicopathological
characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age at surgery (≥65
vs. <65 years) | 0.855 | 0.482–1.565 | 0.601 | 0.990 | 0.460–2.130 | 0.980 |
| Tumor location
(body-tail vs. head) | 0.762 | 0.366–1.465 | 0.438 | 0.559 | 0.219–1.428 | 0.224 |
| Pathology (poor vs.
well-to-moderate) | 1.863 | 0.789–3.922 | 0.123 | 0.923 | 0.378–2.254 | 0.860 |
| Tumor stage (III/IV
vs. I/II) | 0.928 | 0.491–1.680 | 0.809 | 1.041 | 0.469–2.311 | 0.921 |
| Lymphocyte (per 100
cells/µl) | 1.001 | 0.943–1.061 | 0.961 | 0.938 | 0.861–1.021 | 0.141 |
| NLR (per unit) | 0.962 | 0.790–1.143 | 0.680 | 0.923 | 0.670–1.272 | 0.625 |
| CA19-9 (per 10
U/ml) | 1.001 | 0.997–1.003 | 0.574 | 1.002 | 0.999–1.005 | 0.230 |
| Cytoplasmic WT1
intensity (moderate-to-strong vs. weak) | 2.992 | 1.609–5.749 | <0.001 | 3.972 | 1.877–8.404 | <0.001 |
 | Table V.Univariate and multivariate analysis
of disease-free survival. |
Table V.
Univariate and multivariate analysis
of disease-free survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Clinicopathological
characteristics | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age at surgery (≥65
vs. <65 years) | 0.859 | 0.482–1.574 | 0.614 | 0.968 | 0.477–1.964 | 0.927 |
| Tumor location
(body-tail vs. head) | 0.790 | 0.382–1.504 | 0.496 | 0.797 | 0.339–1.877 | 0.604 |
| Pathology (poor vs.
well-to-moderate) | 2.204 | 0.927–4.690 | 0.053 | 1.350 | 0.560–3.252 | 0.504 |
| Tumor stage (III/IV
vs. I/II) | 0.946 | 0.503–1.704 | 0.856 | 0.833 | 0.387–1.794 | 0.641 |
| Lymphocyte (per 100
cells/µl) | 0.992 | 0.933–1.052 | 0.781 | 0.939 | 0.866–1.018 | 0.129 |
| NLR (per unit) | 0.995 | 0.813–1.187 | 0.962 | 1.017 | 0.751–1.378 | 0.912 |
| CA19-9 (per 10
U/ml) | 1.000 | 0.997–1.002 | 0.879 | 1.001 | 0.998–1.004 | 0.471 |
| Cytoplasmic WT1
intensity (moderate-to-strong vs. weak) | 2.952 | 1.610–5.580 | <0.001 | 4.117 | 1.922–8.817 | <0.001 |
Association of laboratory data with OS
and DFS times in PDA cells
The association between prognostic and laboratory
data [e.g., leucocytes, lymphocytes, monocytes, neutrophils, Hb,
platelets, CRP, Alb, LDH, AMY and tumor markers (CEA and CA19-9)]
and systemic inflammatory response markers (e.g., NLR, PLR, LMR and
CRP/Alb ratio) obtained within 2 weeks prior to surgery were also
analyzed in the present study (Tables
III and IV). The OS and DFS
times were estimated using the Kaplan-Meier method. All laboratory
data were not found to be significantly associated with a poor
prognosis (data not shown). Univariate and multivariate analysis
also revealed that lymphocyte numbers, NLR and CA19-9, all of which
have been reported as prognostic markers, were not significant
prognostic factors in the patient cohort of the present study
following surgical resection (Tables
IV and V).
Associations between cytoplasmic WT1
intensity and laboratory parameters
The associations between WT1 staining intensity in
the cytoplasm and laboratory parameters were then assessed in
patients with PDA. Table VI shows
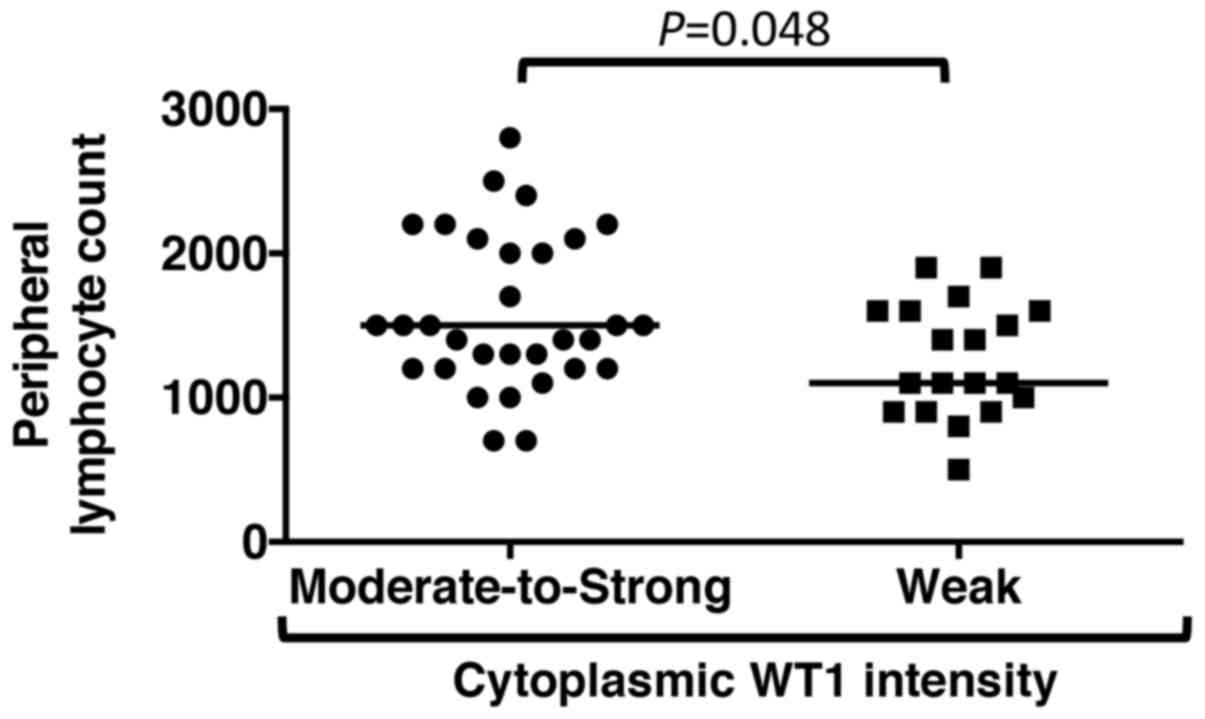
that laboratory data, with the exception of peripheral lymphocyte
numbers, demonstrated no associations with the WT1 reaction
intensity in PDA cytoplasm (Table
VI); PDA patients with moderate-to-strong WT1 staining in the
cytoplasm showed high peripheral blood lymphocyte numbers obtained
within 2 weeks prior to surgery compared with those with weak WT1
staining (P=0.048) (Fig. 6).
 | Table VI.Associations between cytoplasmic WT1
intensity and laboratory parameters in patients with pancreatic
duct adenocarcinoma. |
Table VI.
Associations between cytoplasmic WT1
intensity and laboratory parameters in patients with pancreatic
duct adenocarcinoma.
|
| WT1 intensity |
|
|---|
|
|
|
|
|---|
| Laboratory
parameters | Weak |
Moderate-to-strong | P-value |
|---|
| Leucocytes,
count/µl | 5,100
(2,880–7,140) | 5,200
(3,300–8,800) | 0.246 |
| Lymphocytes,
count/µl | 1,100
(770–1,900) | 1,500
(850–2,450) | 0.048 |
| Monocytes,
count/µl | 300 (100–450) | 300 (200–450) | 0.238 |
| Neutrophils,
count/µl | 3,100
(1,590–5,670) | 3,000
(1,650–5,800) | 0.764 |
| Hemoglobin,
g/dl | 12.5
(10.2–14.3) | 12.4
(10.4–15.0) | 0.548 |
| Platelets
×104 count/µl | 20.4
(12.9–35.3) | 24.9
(13.4–45.8) | 0.259 |
| C-reactive protein,
mg/dl | 0.1 (0.1–1.7) | 0.1 (0.1–2.8) | 0.851 |
| Albumin, g/dl | 3.9 (2.9–4.5) | 3.8 (3.0–4.5) | 0.873 |
| Lactate
dehydrogenase, IU/l | 168 (117–233) | 185 (145–257) | 0.171 |
| Amylase, IU/l | 100 (29–381) | 93 (24–862) | 0.852 |
| Carcinoembryonic
antigen, ng/ml | 3.9 (1.9–24.1) | 6.1 (2.2–24.3) | 0.224 |
| Carbohydrate
antigen 19-9, U/ml | 93 (20–1759) | 139 (1–743) | 0.479 |
|
Neutrophil/lymphocyte ratio, % | 2.0 (1.3–6.1) | 2.1 (1.0–4.3) | 0.569 |
| Platelet/lymphocyte
ratio, % | 170 (107–302) | 176 (73–307) | 0.484 |
| Lymphocyte/monocyte
ratio, % | 5.3 (2.5–9.2) | 5.0 (3.0–11.0) | 0.96 |
| C-reactive
protein/albumin, % | 0.05
(0.02–0.47) | 0.03
(0.02–0.78) | 0.936 |
Discussion
The data from the current study showed that
nucleo-cytoplasmic expression of the WT1 protein was present in all
50 examined patients with PDA. Notably, the cytoplasmic
overexpression of WT1 protein was significantly associated with
worse OS and DFS times in patients with PDA undergoing complete
surgical resection. Furthermore, an association was detected
between a high density of tumor vessels expressing WT1 proteins and
worse OS and DFS times.
Currently, few studies are available regarding WT1
protein expression in patients with PDA (29–31).
Therefore, the present study attempted to assess whether WT1
protein expression could be detected in PDA cells from
formalin-fixed and paraffin-embedded tissues. In order to identify
the epitopes, antigen retrieval was performed using a combination
of high-temperature heating and a Tris/EDTA buffer (pH 9.0), which
are well suited for use on formalin-fixed, paraffin-embedded tissue
sections mounted on glass slides. Previous studies have reported
that WT1 proteins were detected in 30 of 40 patients (75%) using a
polyclonal (C-19) antibody raised against the C terminus (amino
acids 431–450) of the WT1 protein (29) and in 10 of 15 samples (66.7%) using a
monoclonal (6F-H2) antibody targeting the N terminus (amino acids
1–181) (30). Another study also
detected WT1 expression using the monoclonal (6F-H2) antibody in 19
out of 63 (30.2%) PDA cells (31). In
the present study, the WT1 monoclonal (6F-H2) antibody was selected
due to its increased specificity for the WT1 protein compared with
a polyclonal (C-19) antibody (29,30).
Although citrate buffer (pH 6.0) was previously used to expose the
WT1 epitopes on tissue sections mounted on glass slides (29,30), the
present study implemented a combination method using Tris/EDTA
buffer (pH 9.0) at a high temperature. According to the
manufacturer, compared with citrate buffer (pH 6.0), Tris/EDTA
buffer (pH 9.0) can significantly improve the staining results for
numerous antigens. Furthermore, immunostaining was performed using
a newly developed immunohistochemical detection system, EnVision,
which has an extremely high sensitivity and was recently made
available to the Institute of Clinical Medicine and Research, The
Jikei University School of Medicine (Chiba, Japan). The modified
antigen retrieval and highly sensitive detection methods are, at
least in part, why the nucleo-cytoplasmic expression of WT1
proteins in all 50 patients with PDA were detected using the 6F-H2
antibody. It is an urgent requirement to establish a uniform
validated method for the analysis of WT1 reactions in PDA
cells.
WT1 was detected in the granular or diffuse
nucleo-cytoplasmic staining of all the tested PDA cells. Although
the cytoplasmic staining of 6F-H2 antibodies in certain
adenocarcinomas and glioblastomas (30,39) has
been reported, specific nuclear immunoreactivity to 6F-H2 in
leukemia blast cells has also been demonstrated (40). The nucleo-cytoplasmic staining of WT1
in PDA cells observed in the present study suggests that in
tumorigenesis, WT1 has complex functions outside its traditional
role as a transcription factor, such as functions as a tumor
suppressor or oncogene. Although nuclear immunostaining was weak in
all the PDA cells, moderate-to-strong cytoplasmic WT1 expression in
PDA cells (62%) was detected in the present study. It has been
reported that the WT1 protein shuttles between the nucleus and the
cytoplasm in several types of tumors, including rhabdomyosarcomas,
certain breast cancer types and colorectal adenocarcinomas, through
the alternative splicing of WT1 mRNA (41,42).
Although the WT1 protein is predominantly localized to the nucleus
in several tumors, this expression pattern is altered upon protein
kinase A phosphorylation of the DNA-binding domain of WT1,
resulting in the inhibition of DNA binding and the cytoplasmic
sequestration of WT1 (43).
Furthermore, WT1 can also undergo nucleo-cytoplasmic shuttling in
PDA cells following exposure to the standard chemotherapeutic agent
gemcitabine via activation of nuclear factor κB (44). It is hypothesized that WT1 protein
localization may depend on tumor cell conditions, suggesting the
complexity of the role of WT1 in tumorigenesis through
transcriptional and post-transcriptional regulation (45). As WT1 has multiple isoforms,
alternative WT1 transcripts may primarily localize in the PDA
cytoplasm depending on the cellular context. The results of the
present study demonstrated nucleo-cytoplasmic WT1 staining patterns
in all patients with PDA, which also indicates a complex role of
WT1 in tumorigenesis. However, the clinical implications of the
localization of WT1 expression in PDA cells remain unknown.
The present study also sought to investigate the
associations between WT1 expression and the prognosis of PDA
patients following complete surgical resection, and the cytoplasmic
WT1 intensity was thus graded as negative, weak, moderate or
strong. Of the 8 PDA patients with an OS time of >1,000 days, 7
(87.5%) exhibited weak cytoplasmic WT1 expression. At the Jikei
University School of Medicine, Kashiwa Hospital (Chiba, Japan), the
median OS time is 451 days for patients with PDA following surgical
resection, which indicates that an OS time of >1,000 days
represents significantly extended survival. To clarify the
association between cytoplasmic WT1 expression and prognostic
significance, PDA patients were divided into 2 groups: Weak and
moderate-to-strong. Patients with PDA with weak cytoplasmic WT1
expression presented with significantly longer OS and DFS times
compared with those patients with moderate-to-strong cytoplasmic
WT1 expression. Furthermore, patients with strong cytoplasmic WT1
overexpression exhibited shorter OS and DFS times than those with
weak-to-moderate WT1 expression (data not shown). Therefore,
cytoplasmic WT1 overexpression may be involved in PDA pathogenesis
and have a potential prognostic impact on patients with PDA. The
association between WT1 overexpression and worse OS and DFS times
has been previously demonstrated in solid tumors, including serous
epithelial ovarian carcinoma, endometrial cancer, non-small cell
lung cancer, colorectal cancer, osteogenic sarcoma, uterine
sarcoma, glioblastoma, melanomas, malignant pleural mesothelioma,
prostate cancer, breast cancer, hepatocellular carcinoma,
soft-tissue sarcoma and astrocytoma (21,46–48).
Despite the clinical development of WT1-targeted therapies, no
report has demonstrated an association between WT1 protein
expression and the prognosis of PDA patients following surgical
resection. The results of the present study, to the best of our
knowledge, are the first to suggest that strong immunostaining of
WT1 in the PDA cytoplasm may be a potentially useful marker to
predict the risk of relapse and progression in patients with PDA.
Recently, it was reported that the overexpression of WT1-associated
protein (WTAP), a ubiquitously expressed nuclear protein, reduced
the OS time of PDA patients via a WTAP-WT1 axis (49). This study supports the present
findings that cytoplasmic WT1 overexpression in PDA is associated
with a worse prognosis. WTAP and WT1 have recently been reported as
oncogenic factors associated with the regulation of tumor migration
and invasion (50). However, the
function of the WTAP-WT1 axis is not yet clear, and the mechanism
by which WT1 is regulated by WTAP requires further study. PDA
patients with cytoplasmic overexpression of WT1 may require
standard chemotherapy combined with a WT1-targeted therapy rapidly
following surgical resection in order to overcome early mortality.
Previous studies have produced WT1-based cancer vaccines, including
WT1 peptide vaccines (25,51) and dendritic cells pulsed with WT1
peptides (24,52), to extend the survival time of patients
with advanced PDA (53). The results
from these clinical trials demonstrated that patients with PDA who
received WT1-based cancer vaccines exhibited delayed type
hypersensitivity and significantly improved OS time compared with
the negative control patients. These results also support the
development of WT1-targeted cancer therapies for patients either
with inoperable PDA or following surgical resection to improve
their prognosis.
It has been reported that WT1 serves an important
role in tumorigenesis via expansion of tumor vessels and metastasis
formation (54,55). Therefore, the present study also
examined WT1-TV in the PDA stroma. According to the density of
WT1-TV, patients with PDA were classified into 2 groups: High
density (greater than the mean number of vessels) and low density
(less than or equal to the mean number of vessels). An association
between the high-density group and worse OS/DFS times was observed.
These results support previous findings that WT1 acts as a critical
regulator of tumor progression via tumor vascularization (55). Therefore, the overexpression of
cytoplasmic WT1 proteins in PDA cells may be associated with a
higher density of WT1-TV. Inhibition of tumor angiogenesis has been
suggested as a therapeutic option for cancer therapy (55,56). WT1
may have the potential to act as a therapeutic target in patients
with PDA by regulating WT1-expressing PDA cells and tumor vessels
implicated in tumor progression (55,56).
Previous studies have reported that laboratory data
and systemic inflammatory response markers (e.g., NLR, PLR, LMR and
CRP/Alb ratio) can serve as prognostic factors for patients with
several types of cancer (57–59). Therefore, the association of the
prognosis of patients with PDA who received surgical resection and
laboratory data obtained within 2 weeks prior to surgery were
analyzed in the present study. However, in this experimental
setting, no biomarkers associated with the prognosis of PDA
patients following surgical resection were detected. A total of 31
patients with moderate-to-strong cytoplasmic WT1 overexpression in
their PDA cells showed high peripheral blood lymphocyte numbers
obtained within 2 weeks prior to surgery compared with that in 19
PDA patients with weak WT1 staining (P=0.048). However, the PDA
sample number collected following surgical resection is relatively
low and may be too small to assess the laboratory data and identify
potential prognostic markers. Therefore, further studies are
required to assess the associations between peripheral lymphocyte
numbers and WT1 expression in PDA cytoplasm. Although previous
studies have indicated that the WT1 protein is involved in tumor
cell growth (14,29), no associations were observed between
WT1 intensity and clinicopathological characteristics in patients
with PDA who received surgery in the present study. Multiple
factors, including KRAS proto-oncogene GTPase wild-type, human
epidermal growth factor receptor 2 amplification, BRCA2 DNA
repair-associated mutation and ATM serine/threonine kinase mutation
may be involved in PDA tumorigenesis (60). In certain patients with PDA who
exhibit weak cytoplasmic expression of WT1 proteins, the WT1 gene
may not be associated with PDA cell growth (29), which may be the reason why the WT1
protein staining intensity and clinicopathological characteristics
were not significantly associated in the present study.
In conclusion, the results of the present study
suggest that immunostaining of WT1 in PDA cytoplasm and tumor
vessels may serve as a marker to predict the risk of relapse and
progression in patients with PDA following complete surgical
resection. A limitation of the present study is the relatively
small sample size used to evaluate the indicative value of WT1 for
PDA-specific mortality. Furthermore, the present study analyzed
retrospective data collected from a single institution. Therefore,
a large prospective cohort study to evaluate the specific
association between WT1 expression and prognosis in patients with
PDA is strongly recommended. Future results may expand the body of
knowledge presently available on the clinical implications of
WT1-targeting cancer vaccines for all patients with PDA.
Acknowledgements
The authors would like to thank clinical
technologist Mrs Tsuuse Bito (Division of Gastroenterology and
Hepatology, Department of Internal Medicine, The Jikei University
School of Medicine, Kashiwa Hospital) for providing technical
support.
Funding
The present study was supported, in part, by Grants
in Aid for Scientific Research (C) from the Ministry of Education,
Cultures, Sports, Science and Technology of Japan (grant no.
15K09050).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YO, SN, SS, MO, HS and SK were responsible for study
conception and design and developed the methodology. The
acquisition, analysis, and interpretation of data was undertaken by
TK, ZI, YO, MSu, KT, MK, MSa, SF, TM, TA, HY and SK, and
administrative, technical or material support was provided by TK,
YO, SN, SF, TM, TA and SK. The writing, review and/or revision of
the manuscript was the responsibility of TK and SK. All authors
approved the final version of this manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Institutional Review Board (IRB) of the Jikei University [IRB no:
27–071 (7956)].
Patient consent for publication
Due to the retrospective and non-interventional
nature of the present study, the IRB waived the requirement for
written informed consent from the participants for their clinical
records and tissue to be used in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:1039–1049. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zuckerman DS and Ryan DP: Adjuvant therapy
for pancreatic cancer: A review. Cancer. 112:243–249. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hsu CC, Herman JM, Corsini MM, Winter JM,
Callister MD, Haddock MG, Cameron JL, Pawlik TM, Schulick RD,
Wolfgang CL, et al: Adjuvant chemoradiation for pancreatic
adenocarcinoma: The Johns Hopkins Hospital-Mayo Clinic
collaborative study. Ann Surg Oncol. 17:981–990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adham M, Jaeck D, Le Borgne J,
Oussoultzouglou E, Chenard-Neu MP, Mosnier JF, Scoazec JY, Mornex F
and Partensky C: Long-term survival (5–20 years) after
pancreatectomy for pancreatic ductal adenocarcinoma: A series of 30
patients collected from 3 institutions. Pancreas. 37:352–357. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cleary SP, Gryfe R, Guindi M, Greig P,
Smith L, Mackenzie R, Strasberg S, Hanna S, Taylor B, Langer B and
Gallinger S: Prognostic factors in resected pancreatic
adenocarcinoma: Analysis of actual 5-year survivors. J Am Coll
Surg. 198:722–731. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimada K, Sakamoto Y, Nara S, Esaki M,
Kosuge T and Hiraoka N: Analysis of 5-year survivors after a
macroscopic curative pancreatectomy for invasive ductal
adenocarcinoma. World J Surg. 34:1908–1915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huff V: Wilms' tumours: About tumour
suppressor genes, an oncogene and a chameleon gene. Nat Rev Cancer.
11:111–121. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Call KM, Glaser T, Ito CY, Buckler AJ,
Pelletier J, Haber DA, Rose EA, Kral A, Yeger H, Lewis WH, et al:
Isolation and characterization of a zinc finger polypeptide gene at
the human chromosome 11 Wilms' tumor locus. Cell. 60:509–520. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Osaka M, Koami K and Sugiyama T: WT1
contributes to leukemogenesis: Expression patterns in
7,12-dimethylbenz[a]anthracene (DMBA)-induced leukemia. Int J
Cancer. 72:696–699. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang L, Han Y, Saiz Suarez F and Minden
MD: A tumor suppressor and oncogene: The WT1 story. Leukemia.
21:868–876. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sugiyama H: WT1 (Wilms' Tumor Gene 1):
Biology and cancer immunotherapy. Jap J Clin Oncol. 40:377–387.
2010. View Article : Google Scholar
|
|
14
|
Tatsumi N, Oji Y, Tsuji N, Tsuda A,
Higashio M, Aoyagi S, Fukuda I, Ito K, Nakamura J, Takashima S, et
al: Wilms' tumor gene WT1-shRNA as a potent apoptosis-inducing
agent for solid tumors. Int J Oncol. 32:701–711. 2008.PubMed/NCBI
|
|
15
|
Sciesielski LK, Kirschner KM, Scholz H and
Persson AB: Wilms' tumor protein Wt1 regulates the Interleukin-10
(IL-10) gene. FEBS Lett. 584:4665–4671. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taube ET, Denkert C, Sehouli J, Kunze CA,
Dietel M, Braicu I, Letsch A and Darb-Esfahani S: Wilms tumor
protein 1 (WT1)-not only a diagnostic but also a prognostic marker
in high-grade serous ovarian carcinoma. Gynecol Oncol. 140:494–502.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Köbel M, Kalloger SE, Boyd N, McKinney S,
Mehl E, Palmer C, Leung S, Bowen NJ, Ionescu DN, Rajput A, et al:
Ovarian carcinoma subtypes are different diseases: Implications for
biomarker studies. PLoS Med. 5:e2322008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sera T, Hiasa Y, Mashiba T, Tokumoto Y,
Hirooka M, Konishi I, Matsuura B, Michitaka K, Udaka K and Onji M:
Wilms' tumour 1 gene expression is increased in hepatocellular
carcinoma and associated with poor prognosis. Eur J Cancer.
44:600–608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi XW, Zhang F, Yang XH, Fan LJ, Zhang Y,
Liang Y, Ren L, Zhong L, Chen QQ, Zhang KY, et al: High Wilms'
tumor 1 mRNA expression correlates with basal-like and ERBB2
molecular subtypes and poor prognosis of breast cancer. Oncol Rep.
28:1231–1236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lapillonne H, Renneville A, Auvrignon A,
Flamant C, Blaise A, Perot C, Lai JL, Ballerini P, Mazingue F,
Fasola S, et al: High WT1 expression after induction therapy
predicts high risk of relapse and death in pediatric acute myeloid
leukemia. J Clin Oncol. 24:1507–1515. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qi XW, Zhang F, Wu H, Liu JL, Zong BG, Xu
C and Jiang J: Wilms' tumor 1 (WT1) expression and prognosis in
solid cancer patients: A systematic review and meta-analysis. Sci
Rep. 5:89242015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheever MA, Allison JP, Ferris AS, Finn
OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL,
Weiner LM and Matrisian LM: The prioritization of cancer antigens:
A national cancer institute pilot project for the acceleration of
translational research. Clin Cancer Res. 15:5323–5337. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaida M, Morita-Hoshi Y, Soeda A, Wakeda
T, Yamaki Y, Kojima Y, Ueno H, Kondo S, Morizane C, Ikeda M, et al:
Phase 1 trial of Wilms tumor 1 (WT1) peptide vaccine and
gemcitabine combination therapy in patients with advanced
pancreatic or biliary tract cancer. J Immunother. 34:92–99. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kimura Y, Tsukada J, Tomoda T, Takahashi
H, Imai K, Shimamura K, Sunamura M, Yonemitsu Y, Shimodaira S,
Koido S, et al: Clinical and immunologic evaluation of dendritic
cell-based immunotherapy in combination with gemcitabine and/or S-1
in patients with advanced pancreatic carcinoma. Pancreas.
41:195–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishida S, Koido S, Takeda Y, Homma S,
Komita H, Takahara A, Morita S, Ito T, Morimoto S, Hara K, et al:
Wilms tumor gene (WT1) peptide-based cancer vaccine combined with
gemcitabine for patients with advanced pancreatic cancer. J
Immunother. 37:105–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kobayashi M, Shimodaira S, Nagai K,
Ogasawara M, Takahashi H, Abe H, Tanii M, Okamoto M, Tsujitani S,
Yusa S, et al: Prognostic factors related to add-on dendritic cell
vaccines on patients with inoperable pancreatic cancer receiving
chemotherapy: A multicenter analysis. Cancer Immunol Immunother.
63:797–806. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koido S, Homma S, Okamoto M, Takakura K,
Mori M, Yoshizaki S, Tsukinaga S, Odahara S, Koyama S, Imazu H, et
al: Treatment with chemotherapy and dendritic cells pulsed with
multiple Wilms' tumor 1 (WT1)-specific MHC class I/II-restricted
epitopes for pancreatic cancer. Clin Cancer Res. 20:4228–4239.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mayanagi S, Kitago M, Sakurai T, Matsuda
T, Fujita T, Higuchi H, Taguchi J, Takeuchi H, Itano O, Aiura K, et
al: Phase I pilot study of Wilms tumor gene 1 peptide-pulsed
dendritic cell vaccination combined with gemcitabine in pancreatic
cancer. Cancer Sci. 106:397–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oji Y, Nakamori S, Fujikawa M, Nakatsuka
S, Yokota A, Tatsumi N, Abeno S, Ikeba A, Takashima S, Tsujie M, et
al: Overexpression of the Wilms' tumor gene WT1 in pancreatic
ductal adenocarcinoma. Cancer Sci. 95:583–587. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakatsuka S, Oji Y, Horiuchi T, Kanda T,
Kitagawa M, Takeuchi T, Kawano K, Kuwae Y, Yamauchi A, Okumura M,
et al: Immunohistochemical detection of WT1 protein in a variety of
cancer cells. Mod Pathol. 19:804–814. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Naitoh K, Kamigaki T, Matsuda E, Ibe H,
Okada S, Oguma E, Kinoshita Y, Takimoto R, Makita K, Ogasawara S
and Goto S: Immunohistochemical analysis of WT1 antigen expression
in various solid cancer cells. Anticancer Res. 36:3715–3724.
2016.PubMed/NCBI
|
|
32
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gu L, Li H, Chen L, Ma X, Li X, Gao Y,
Zhang Y, Xie Y and Zhang X: Prognostic role of lymphocyte to
monocyte ratio for patients with cancer: Evidence from a systematic
review and meta-analysis. Oncotarget. 7:31926–31942. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sukharamwala P, Thoens J, Szuchmacher M,
Smith J and DeVito P: Advanced age is a risk factor for
post-operative complications and mortality after a
pancreaticoduodenectomy: A meta-analysis and systematic review. HPB
(Oxford). 14:649–657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kinoshita S, Sho M, Yanagimoto H, Satoi S,
Akahori T, Nagai M, Nishiwada S, Yamamoto T, Hirooka S, Yamaki S,
et al: Potential role of surgical resection for pancreatic cancer
in the very elderly. Pancreatology. 15:240–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bing Z, Pasha TL, Acs G and Zhang PJ:
Cytoplasmic overexpression of WT-1 in gastrointestinal stromal
tumor and other soft tissue tumors. Appl immunohistochem Mol
Morphol. 16:316–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Martina JD, Simmons C and Jukic DM:
High-definition hematoxylin and eosin staining in a transition to
digital pathology. J Pathol Inform. 2:452011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oji Y, Suzuki T, Nakano Y, Maruno M,
Nakatsuka S, Jomgeow T, Abeno S, Tatsumi N, Yokota A, Aoyagi S, et
al: Overexpression of the Wilms' tumor gene W T1 in primary
astrocytic tumors. Cancer Sci. 95:822–827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Menssen HD, Renkl HJ, Rodeck U, Kari C,
Schwartz S and Thiel E: Detection by monoclonal antibodies of the
Wilms' tumor (WT1) nuclear protein in patients with acute leukemia.
Int J Cancer. 70:518–523. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vajjhala PR, Macmillan E, Gonda T and
Little M: The Wilms' tumour suppressor protein, WT1, undergoes
CRM1-independent nucleocytoplasmic shuttling. FEBS Lett.
554:143–148. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Niksic M, Slight J, Sanford JR, Caceres JF
and Hastie ND: The Wilms' tumour protein (WT1) shuttles between
nucleus and cytoplasm and is present in functional polysomes. Hum
Mol Genet. 13:463–471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ye Y, Raychaudhuri B, Gurney A, Campbell
CE and Williams BR: Regulation of WT1 by phosphorylation:
Inhibition of DNA binding, alteration of transcriptional activity
and cellular translocation. EMBO J. 15:5606–5615. 1996.PubMed/NCBI
|
|
44
|
Takahara A, Koido S, Ito M, Nagasaki E,
Sagawa Y, Iwamoto T, Komita H, Ochi T, Fujiwara H, Yasukawa M, et
al: Gemcitabine enhances Wilms' tumor gene WT1 expression and
sensitizes human pancreatic cancer cells with WT1-specific
T-cell-mediated antitumor immune response. Cancer Immunol
Immunother. 60:1289–1297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Magro G, Salvatorelli L, Vecchio GM,
Musumeci G, Rita A and Parenti R: Cytoplasmic expression of Wilms
tumor transcription factor-1 (WT1): A useful immunomarker for
young-type fibromatoses and infantile fibrosarcoma. Acta Histochem.
116:1134–1140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ito K, Oji Y, Tatsumi N, Shimizu S, Kanai
Y, Nakazawa T, Asada M, Jomgeow T, Aoyagi S, Nakano Y, et al:
Antiapoptotic function of 17AA(+)WT1 (Wilms' tumor gene) isoforms
on the intrinsic apoptosis pathway. Oncogene. 25:4217–4229. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jomgeow T, Oji Y, Tsuji N, Ikeda Y, Ito K,
Tsuda A, Nakazawa T, Tatsumi N, Sakaguchi N, Takashima S, et al:
Wilms' tumor gene WT1 17AA(−)/KTS(−) isoform induces morphological
changes and promotes cell migration and invasion in vitro. Cancer
Sci. 97:259–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Oji Y, Tatsumi N, Kobayashi J, Fukuda M,
Ueda T, Nakano E, Saito C, Shibata S, Sumikawa M, Fukushima H, et
al: Wilms' tumor gene WT1 promotes homologous
recombination-mediated DNA damage repair. Mol Carcinog.
54:1758–1771. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li BQ, Huang S, Shao QQ, Sun J, Zhou L,
You L, Zhang TP, Liao Q, Guo JC and Zhao YP: WT1-associated protein
is a novel prognostic factor in pancreatic ductal adenocarcinoma.
Oncol Lett. 13:2531–2538. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jin DI, Lee SW, Han ME, Kim HJ, Seo SA,
Hur GY, Jung S, Kim BS and Oh SO: Expression and roles of Wilms'
tumor 1-associating protein in glioblastoma. Cancer Sci.
103:2102–2109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nishida S, Ishikawa T, Egawa S, Koido S,
Yanagimoto H, Ishii J, Kanno Y, Kokura S, Yasuda H, Oba MS, et al:
Combination gemcitabine and WT1 peptide vaccination improves
progression-free survival in advanced pancreatic ductal
adenocarcinoma: A phase II randomized study. Cancer Immunol Res.
2018 Jan 22;Doi: 10.1158/2326-6066.CIR-17-0386. View Article : Google Scholar
|
|
52
|
Koido S, Homma S, Okamoto M, Takakura K,
Gong J, Sugiyama H, Ohkusa T and Tajiri H: Chemoimmunotherapy
targeting Wilms' tumor 1 (WT1)-specific cytotoxic T lymphocyte and
helper T cell responses for patients with pancreatic cancer.
Oncoimmunology. 3:e9589502014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Koido S, Okamoto M, Shimodaira S and
Sugiyama H: Wilms' tumor 1 (WT1)-targeted cancer vaccines to extend
survival for patients with pancreatic cancer. Immunotherapy.
8:1309–1320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wagner N, Michiels JF, Schedl A and Wagner
KD: The Wilms' tumour suppressor WT1 is involved in endothelial
cell proliferation and migration: Expression in tumour vessels in
vivo. Oncogene. 27:3662–3672. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wagner KD, Cherfils-Vicini J, Hosen N,
Hohenstein P, Gilson E, Hastie ND, Michiels JF and Wagner N: The
Wilms' tumour suppressor Wt1 is a major regulator of tumour
angiogenesis and progression. Nat Commun. 5:58522014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. New Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yang JJ, Hu ZG, Shi WX, Deng T, He SQ and
Yuan SG: Prognostic significance of neutrophil to lymphocyte ratio
in pancreatic cancer: A meta-analysis. World J Gastroenterol.
21:2807–2815. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Qi Q, Geng Y, Sun M, Wang P and Chen Z:
Clinical implications of systemic inflammatory response markers as
independent prognostic factors for advanced pancreatic cancer.
Pancreatology. 15:145–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang P, Zou M, Wen X, Gu F, Li J, Liu G,
Dong J, Deng X, Gao J, Li X, et al: Development of serum parameters
panels for the early detection of pancreatic cancer. Int J Cancer.
134:2646–2655. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chantrill LA, Nagrial AM, Watson C, Johns
AL, Martyn-Smith M, Simpson S, Mead S, Jones MD, Samra JS, Gill AJ,
et al: Precision medicine for advanced pancreas cancer: The
individualized molecular pancreatic cancer therapy (IMPaCT) trial.
Clinical Cancer Res. 21:2029–2037. 2015. View Article : Google Scholar
|