Introduction
Hepatocellular carcinoma (HCC) has become the second
leading cause of cancer-associated mortality in men and sixth in
women globally; and in 2012, of the total number of new cases of
HCC globally, 50% of the cases originated from China (1). Although the correct identification of
high-risk groups for liver cancer is improving and screening
methods are becoming more accurate, the onset and rapid progression
of HCC means that the majority of patients are diagnosed at an
advanced stage of the disease, the effect of treatment is
unsatisfactory and the median overall survival time has remained at
<12 months (2). Therefore, further
examination of HCC is required to identify novel therapeutic
targets for treatment.
Histone deacetylase (HDAC) is mainly involved in
histone acetylation, and serves a vital role in the structural
modification of chromosomes and regulation of gene expression
(3,4).
HDAC6 belongs to class II of the HDAC family, has two homologous
catalytic domains and is mainly localized in the cytoplasm
(5). Studies have confirmed that
HDAC6 serves an essential role in transcriptional regulation, cell
cycle progression, autophagy and apoptosis (6–8). Wnt
signaling is essential for organogeny and tumorigenesis (9,10). The
aberrant activation of Wnt signaling is involved in
hepatocarcinogenesis and metastasis (11,12).
β-catenin, regarded as the key protein of the canonical
Wnt/β-catenin signaling pathway, promotes the activation of Wnt
signaling through translocating to the nucleus and attenuating Wnt
signaling by degrading itself (13).
In addition, the post-transcriptional modifications of β-catenin,
including phosphorylation and acetylation, serve key roles in the
regulation of β-catenin stability, its endocellular location and
transcriptional activity (14,15). It
has been revealed that β-catenin may be deacetylated by HDAC6 and
associated with the activation of interferon regulatory factor 3
signaling (16). However, the
function of HDAC6 in the canonical Wnt/β-catenin signaling pathway
and its underlying mechanism of action in HCC cells remain
unclear.
To date, there are only a few articles on HDAC6 in
HCC (17–21), and the results are conflicting.
Therefore, the role of HDAC6 in HCC cells requires further
investigation. Consequently, the present study aimed to uncover the
function of HDAC6 in HCC cells, and further understanding of its
function in HCC.
Materials and methods
Cell culture and plasmid
transfection
Human HCC cell lines Huh-7 and Hep3B were obtained
from the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China) and cultured in Dulbecco's modified Eagle's
medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
mixed with 10% fetal bovine serum (Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd., Hangzhou, China), 100 µg/ml
streptomycin and 100 µg/ml penicillin, in a humidified incubator
with 5% CO2. The HDAC6 overexpression plasmid (Shanghai
GenePharma Co. Ltd., Shanghai, China) was named P3-HDAC6. HCC cells
were transfected with P3-HDAC6 (4 ng) using Lipofectamine 2000 for
20 min at room temperature (Invitrogen; Thermo Fisher Scientific,
Inc.) when the cells were cultured to 80–90% confluence.
Patient information and
immunohistochemical staining
In the present study, the pathological tissue
sections of 7 male and 3 female (n=10) patients (range, 34–65;
mean, 47.5 years of age) with HCC were collected from the
Department of Hepatobiliary Surgery, the Affiliated Hospital of
Xuzhou Medical University (Xuzhou, China) between October 2015 and
May 2016. All patients had a confirmed diagnosis of hepatocellular
carcinoma by pathology. The pathological diagnosis of these
patients was divided into histological grade I, grade II and grade
III. The present study was ethically approved by the Ethics
Committee of Xuzhou Medical University. All experiments were
performed consistent with the principles of the Declaration of
Helsinki. Written informed consent was obtained from the patients
prior to the study.
Tissues were fixed with 10% neutral formalin for 6 h
at room temperature, fixed and washed for 10 min and dipped in
xylene for 5 min, gradient ethanol solution (80, 90, 95 and 100%)
for 5 min at room temperature. Subsequently they were infiltrated
with paraffin at 56°C. Tissue sections were cut into a thickness of
~5 µm. The sections were deparaffinized at 65°C for 2 h, and then
soaked with xylene and rehydrated using a descending ethanol (80,
90, 95 and 100%) series. Tissue antigen retrieval was performed
using a citrate buffer (Beyotime Institute of Biotechnology,
Nantong, China). Sections were covered with 3%
H2O2 for 20 min to block endogenous
peroxidase activity at room temperature. Antigens were repaired by
placing the sections in citrate buffer for 10 min at room
temperature. The tissue sections were then incubated with HDAC6
monoclonal antibody (dilution, 1:100; cat. no. ab133539; Abcam,
Shanghai, China) overnight at 4°C, treated with secondary antibody
goat anti-rabbit IgG horseradish peroxidase (dilution, 1:10,000;
cat. no. C10224; Anbo Biotechnology Co., Ltd. San Francisco, CA,
USA) for 10 min at room temperature, stained with diaminobenzidine
(OriGene Technologies, Inc., Beijing, China) diluent for 30 sec and
counterstained with hematoxylin for 2 min at room temperature.
Finally, sections were dehydrated using a graded alcohol series
(80, 90, 95 and 100%) for ~1–2 min and mounted with a neutral
balsam. Images (magnification, ×200) were captured using a Nikon
DS-Ri1 microscope.
Western blot analysis
Each cell line was divided into two groups, a mock
group (transfected with a blank plasmid) and a P3-HDAC6 group
(transfected with P3-HDAC6 plasmid). Each group of cells were
washed with cold phosphate buffered saline and lysed in 20 mM Tris
(pH 7.5), 150 mM NaCl, 1% Triton X-100, sodium pyrophosphate (PBS),
β-glycerophosphate, EDTA, Na3VO4, leupeptin and 1 mM
phenylmethyl-sulfonyl fluoride (Beyotime Institute of
Biotechnology). Lysates were centrifuged at 4°C at 12,000 × g for 5
min and the total protein was quantified using a bicinchoninic acid
kit (Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. Total protein (50 ng) were separated by
electrophoresis on SDS-PAGE (8–12% gels) and then transferred on to
nitrocellulose membranes (GE Healthcare, Chicago, IL, USA). HDAC6
(dilution, 1:10,000; cat. no. ab133539), E-cadherin (dilution,
1:10,000; cat. no. ab76319), N-cadherin (dilution, 1:10,000; cat.
no. ab76011), vimentin (dilution, 1:1,000; cat. no. ab92547) and
matrix metalloproteinase-9 (MMP-9; dilution, 1:1,000; cat. no. ab
137867) rabbit monoclonal antibodies were purchased from Abcam
(dilution, 1:10,000; cat. no. ab6721; Shanghai, China) and
β-catenin (dilution, 1:10,000; cat. no. C16402), cyclin-D1
(dilution, 1:10,000; cat. no. C12333), cleaved caspase 3 (dilution,
1:10,000; cat. no. C18514) and GAPDH rabbit antibodies (dilution,
1:100,000; cat. no. AB0037) were purchased from Anbo Biotechnology
Co., Ltd. (San Francisco, CA, USA). The membrane was blocked with
5% high calcium nonfat milk for >2 h at room temperature and
incubated with the primary antibodies at 4°C overnight. The
following day, the membrane was incubated with secondary rabbit
antibody (dilution, 1: 10,000; cat. no. C10224; Anbo Biotechnology
Co., Ltd. San Francisco, CA, USA) for 2 h at room temperature and
rinsed with 0.05% Tris-buffered saline with Tween 20 three times
for 10 min. The protein bands were visualized using DAB coloring
solution (A:B 1:1) in a dark condition on ice (cat. no. AR1025;
BIOSS, Beijing, China) according to the manufacturer's protocols.
Membranes were then exposed to an Odyssey infrared imaging system
(Odyssey; LI-COR Biosciences, Lincoln, NE, USA).
Cell proliferation assay
Each group of cells were diluted into
5×103/100 µl cell suspensions and cultured in 96-well
plates, and then the cell proliferation was measured at 24, 48, 72
and 96 h using a Cell counting kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) assay, according to the
manufacturer's protocol.
Apoptosis assay
Huh-7 cells were divided into three groups
(~1×105 cells per group), the blank group (no
treatment), the negative control (NC) group (transfected with the
blank plasmid) and the P3-HDAC6 group (transfected with P3-HDAC6
plasmid) and used to detect the apoptosis levels with Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit I (BD
Biosciences, San Jose, CA, USA), according to the manufacturer's
protocols. Each group of cells were washed with cold PBS,
trypsinized and resuspended. Each cell suspension was added to a
detecting tube and 5 µl annexin V-FITC and 5 µl propidium iodide
(PI) were subsequently added in sequence. The cell suspension was
incubated in the dark for 15 min at room temperature, and then 400
µl 1X binding solution was added to each tube. Finally, levels of
apoptosis were detected using a flow cytometer (BD Biosciences) for
1 h and subsequently analyzed with ModFit 3.0 software (Verity
Software House Inc., Topsham, ME, USA).
Flow cytometric analysis of cell
cycle
The Huh-7 cell line was divided into three groups as
previously described. Each group of cells (~1×105 cells
per group) were harvested by trypsinization and washed with cold
PBS, then fixed in 70% ethanol overnight at −20°C. On the following
day, the cells were washed with cold PBS twice, incubated with 100
µl RNase A at 37°C for 30 min. Subsequently, 400 µl propidium
iodide (PI) was added at 4°C in the dark for 30 min and detected
using a flow cytometer (BD Biosciences). The data was analyzed by
ModFit 3.0 software analysis software (Verity Software House, Inc.,
Topsham, ME, USA).
Cell migration and invasion assay
Migration and invasion assays were performed with
24-Transwell chambers (EMD Millipore, Billerica, MA, USA). In
short, Huh-7 and Hep3B (5×103 cells) were harvested and
resuspended in 150 µl DMEM without serum and then loaded in the
upper chamber. The lower chamber of the Transwell was filled with
600 µl DMEM supplemented with 10% FBS. The chamber contained an 8
µm pore polycarbonate membrane filter covered either without
Matrigel (for the migration assay) or with Matrigel (for the
invasion assay). Each cell suspension was loaded in the chamber
without or with Matrigel (migration or invasion assay,
respectively) and incubated at 37°C for 24 h (migration assay) or
48 h (invasion assay). The cells passed through the membrane
without or with Matrigel, the upper chamber cells were removed with
cotton swabs and the filtered cells were fixed with 70% methanol 20
min and stained with 0.1% crystal violet for 25 min at room
temperature. They were subsequently washed with PBS and then
counted under a florescent microscope (magnification, ×200, Nikon
Corporation, Tokyo, Japan).
Statistical analysis
The cell proliferation assay, relative protein
levels, apoptosis and cell cycle results were presented as the mean
± standard deviation from three independent experiments and
analyzed using the statistical software SPSS16.0 (SPSS, Inc.,
Chicago, IL, USA). Differences between groups were assessed using a
two-tailed Student's t-test or a one-way analysis of variance and
the Least-significant Difference and Student-Neuman-Keuls post hoc
test was performed by GraphPad Prism Software version 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
HDAC6 is downregulated in HCC cell
lines and tissues
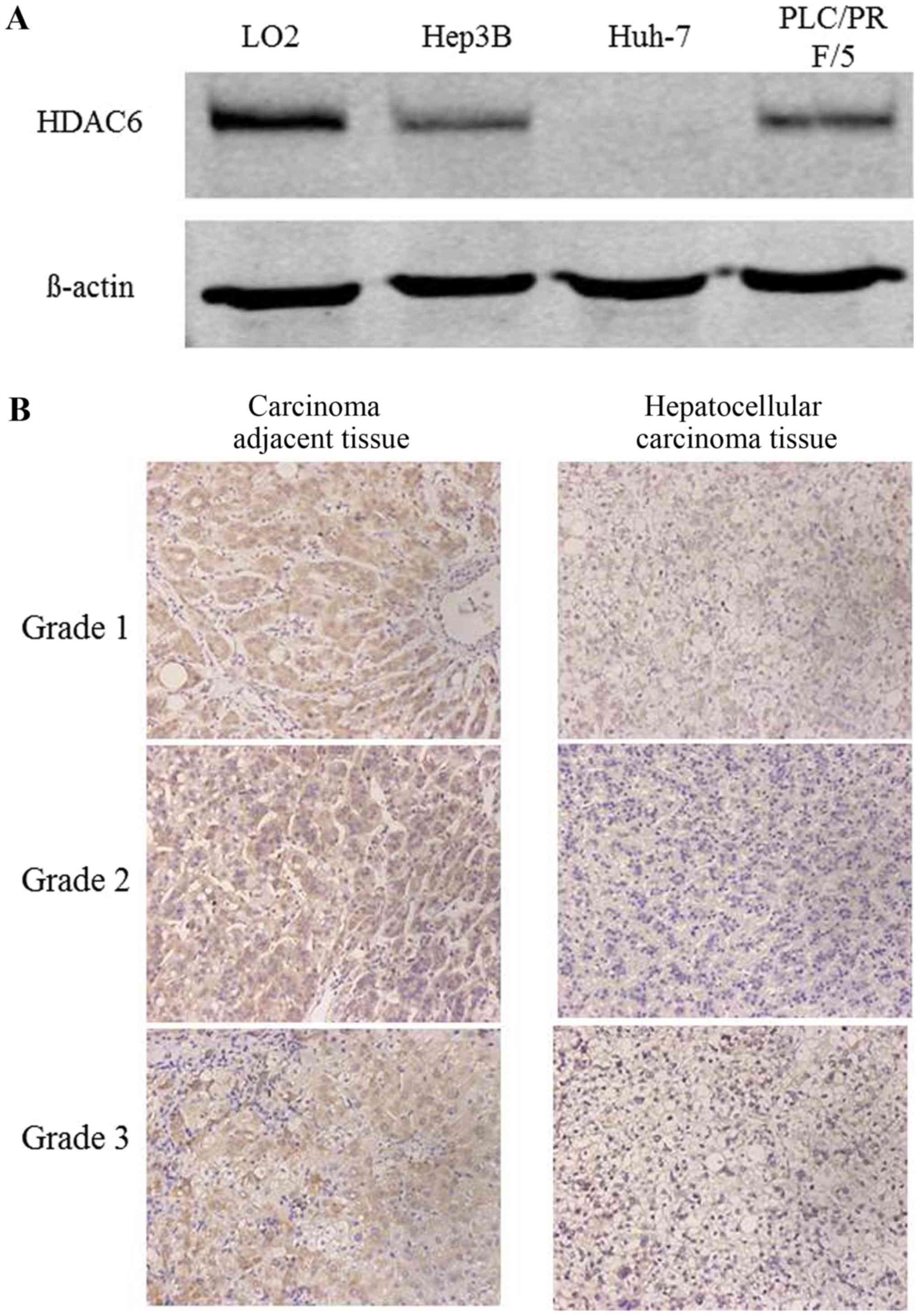
Initially, the protein expression of HDAC6 was
detected in two HCC cell lines using western blot analysis and the
pathological tissue sections of 10 patients with HCC using
immunohistochemical staining. The protein expression of HDAC6 was
downregulated in Huh-7 and Hep3B cells compared with the normal
hepatic cell line Lo2 (Fig. 1A). The
results of the immunohistochemical staining additionally indicated
that HDAC6 was decreased in cancer tissues compared with
tumor-adjacent tissues (Fig. 1B).
Therefore, these results revealed that HDAC6 was downregulated in
HCC cell lines and tissues.
Overexpression of HDAC6 suppresses the
proliferation of HCC cells via inhibiting the canonical
Wnt/β-catenin signaling cascade
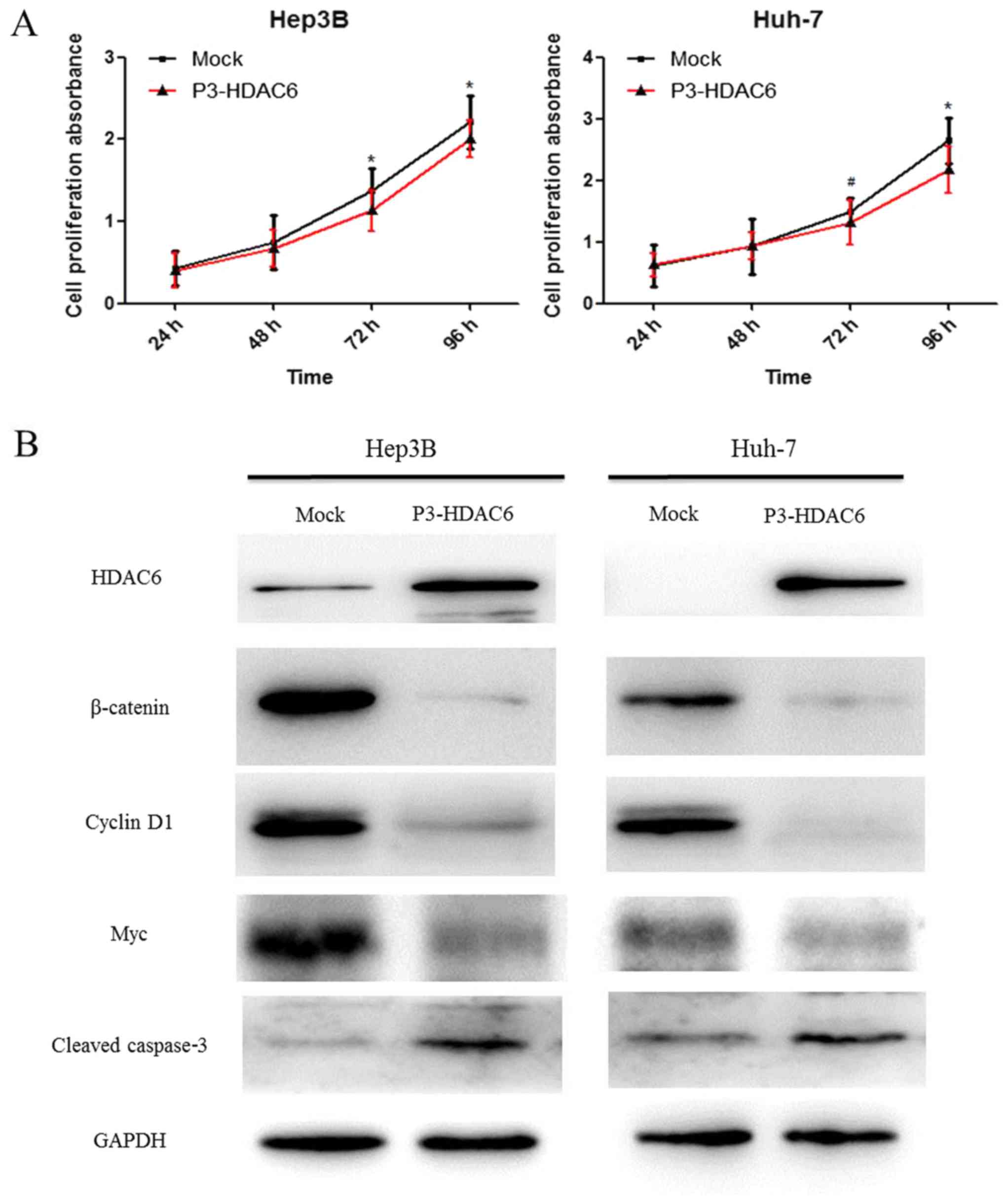
In order to identify the function of HDAC6, Huh-7
and Hep3B cell lines were used to investigate the effects on
proliferation. It was revealed that the proliferation ability of
the two types of HCC cells, Hep3B (P<0.01) and Huh-7 (P<0.05
and P<0.01), was significantly suppressed via the upregulated
expression of HDAC6 using a CCK-8 assay (Fig. 2A). However, the exact mechanism of
action remains unclear. The activation of the canonical
Wnt/β-catenin signaling pathway regulates numerous cellular
processes including tumor formation, cell proliferation and
metastasis (22). A number of studies
have reported that the activity of HDAC6 is associated with the
acetylation, degradation and nuclear localization of β-catenin
(23,24). In the present study, the protein
expression of HDAC6 was upregulated following transfection with
P3-HDAC6 plasmid and the β-catenin degradation was increased
compared with the mock group as determined by western blot analysis
(Fig. 2B). The result indicated that
HDAC6 may be considered to function as a tumor suppressor through
inhibiting the canonical Wnt/β-catenin signaling pathway.
Subsequently, the downstream target genes Myc and Cyclin D1 were
analyzed. As expected, these target genes were notably decreased in
P3-HDAC6 plasmid-transfected cells (Fig.
2B). Altogether, the results indicated that the overexpression
of HADC6 suppressed the proliferation of HCC cells through
inhibiting the canonical Wnt/β-catenin signaling cascade.
Upregulation of HDAC6 attenuates the
metastasis of HCC cells via the inhibition of
epithelial-mesenchymal transition (EMT)
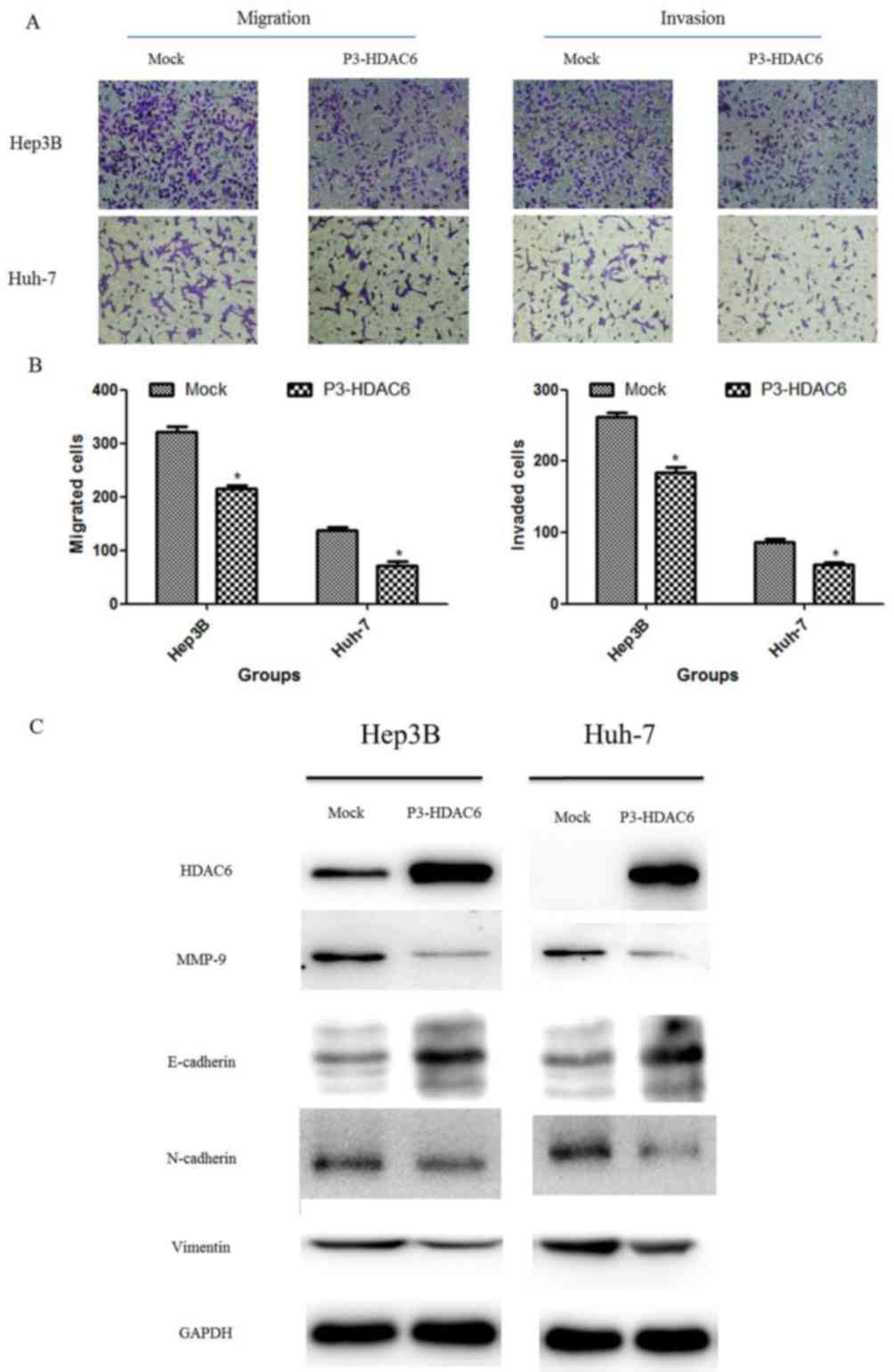
Based on former results that the degradation of
β-catenin was increased following HDAC6 overexpression, the present
study determined the effect of HDAC6 on the metastasis of HCC cells
using invasion and migration assays. The P3-HDAC6 plasmid was
transfected into HCC cell lines (Hep3B and Huh-7) for 48 h and the
invasion and migration of the cell population was observed and
compared with a mock group. Concurrent with the results of the cell
proliferation assay, the metastasis ability of the HCC cells was
significantly attenuated in the P3-HDAC6 group compared with the
mock group (P<0.01; Fig. 3A and
B). The process of EMT is essential for the metastasis of a
malignant tumor (25,26). Thus, the protein expression of a
number of EMT markers was examined, including E-cadherin,
N-cadherin, vimentin and MMP-9 (Fig.
3C). E-cadherin expression was substantially increased in the
P3-HDAC6 group accompanied with the reduction of the expression of
N-cadherin, vimentin and MMP-9 compared with the mock group. These
results revealed that HDAC6 functioned as a tumor suppressor via
inhibiting the EMT process in HCC cells.
Overexpression of HDAC6 is associated
with cell cycle arrest and the induction of apoptosis in the Huh7
cell line
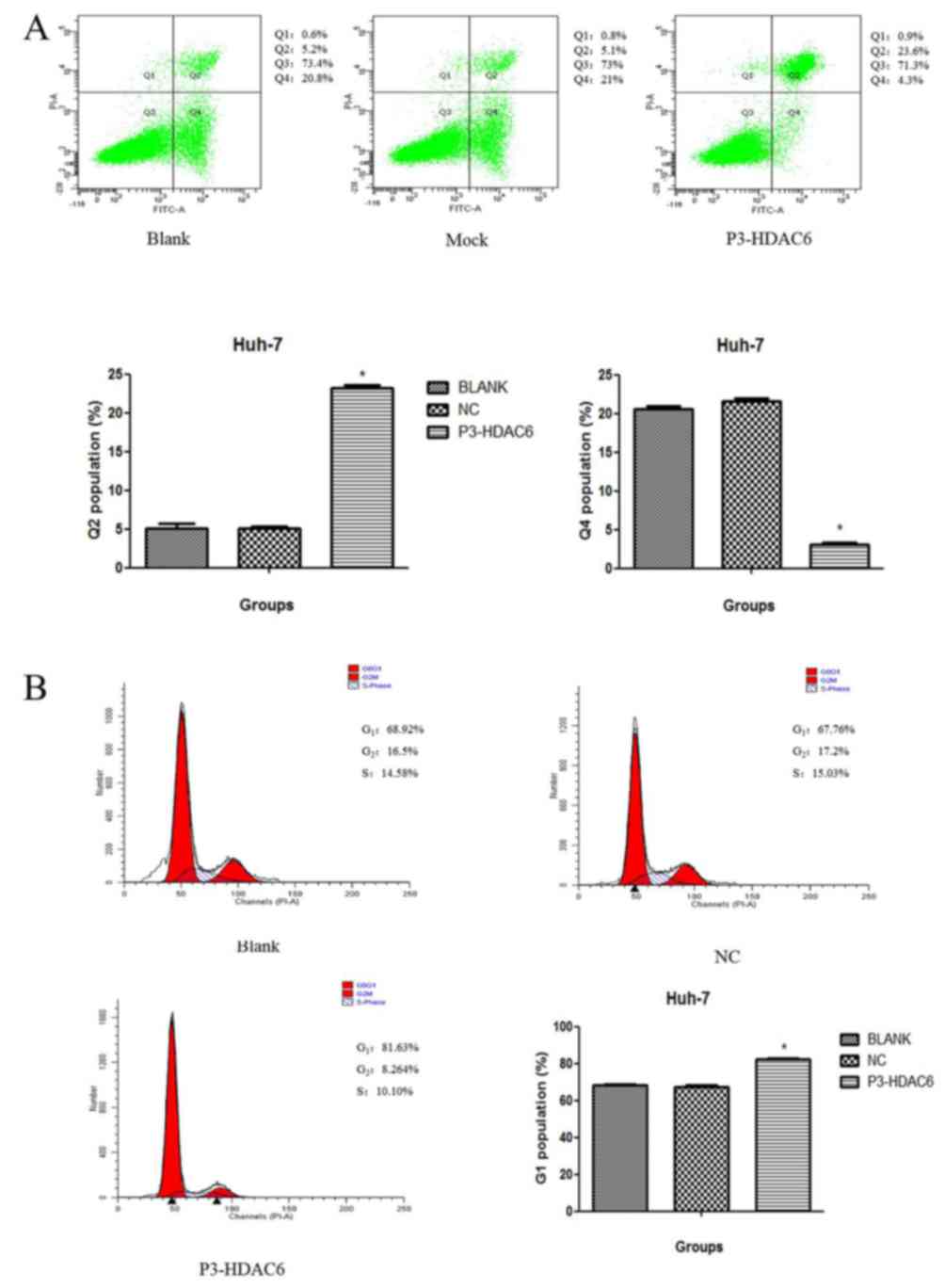
As presented in Fig.
2B, cyclin D1 was decreased and cell proliferation was
suppressed in the P3-HDAC6 group compared with the mock group.
Accordingly, the Huh-7 cell line was used to perform a cell cycle
and apoptosis assay using flow cytometry. The HDAC6 overexpression
group (P3-HDAC6) produced an increased number of cells at the G1
phase by 10% (Fig. 4A), and a
statistically significant compared with other two groups (group NC
and Blank, P<0.05). Moreover, once Huh7 cells were transfected
with the P3-HDAC6 plasmid and stained using PI, a significant
change in cell apoptosis rate was observed compared with the
control cells (P<0.01; Fig. 4B).
In addition, an increase in cleaved caspase-3 expression in the
P3-HDAC6 groups of Huh-7 and Hep3B cells was observed compared with
the control cells (Fig. 2B). Thus,
the overexpression of was HDAC6 revealed to affect cell cycle
arrest and the induction of apoptosis.
Discussion
In the present study, it was revealed that HDAC6, a
class II histone deacetylase mainly located in the cytoplasm,
serves as a tumor suppressor through regulating the activation of
the canonical Wnt/β-catenin signaling pathway in HCC. The protein
expression of HDAC6 was downregulated in HCC cell lines (Huh-7 and
Hep3B) and human liver tumor tissues. It was revealed that the
overexpression of HDAC6 suppressed the proliferation and metastasis
of cells in vitro by inhibiting the activity of the
canonical Wnt/β-catenin signaling pathway and the transcription of
downstream target genes. Upregulation of HDAC6 also suppressed the
cell migratory and invasive abilities via attenuating the process
of EMT in liver cancer. These results reveal a vital role for HDAC6
in hepatocarcinogenesis and progression, and provide a novel
treatment method for HCC.
Acetylation modification serves an essential role in
chromosome conformation and gene expression. The balance between
the activity of the HDAC enzyme and histone acetyltransferase
determines the degree of histone acetylation and is associated with
physiological and pathological processes (27,28). A
number of HDAC family members are abnormally expressed in certain
tumor types and have specific functions in controlling the cell
characteristics (29). Aberrant HDAC
activity is involved in tumorigenesis and progression. Thus, it is
difficult to research the function of HDACs. It has been suggested
that HDAC6, a cytoplasmic deacetylase, is associated with the
acetylation regulation of α-tubulin, Hsp90 and β-catenin and serves
a key role in gene expression, and the transcription and
translation processes of the aforementioned proteins (5,6,8). In addition, HDAC6 has been suggested to
serve an important role in tumorigenesis and tumor progression as
well as maintaining the phenotype of malignant cancer types
(5,6,8). However,
further investigation of the aforementioned roles of HDAC6 is
required. Additionally, the expression of HDAC6 has been reported
to be upregulated in human breast cancer (30), lung cancer (31) and primary acute myeloid leukemia
blasts (32). However, previous
studies (17–21) on the function of HDAC6 in human HCC
are widely divergent as they contradict each other.
Concurrent with the results of a study by Jung et
al (19), HDAC6 expression was
detected to be markedly decreased in HCC cell lines and
significantly (P<0.05) decreased in patients with HCC compared
with their respective controls. This was revealed in a set of
histopathological slides from patients with HCC and human HCC cell
lines (Fig. 1A and B). These results
differ from those produced in a study by Kanno et al
(18). Accordingly, from these
results it was hypothesized that HDAC6 functioned as a tumor
suppressor during the formation of liver cancer and development.
Subsequently, it was elucidated that the overexpression of HDAC6
inhibited the proliferation and metastasis of liver cancer cells
(Figs. 2 and 3). Ultimately, the results of the present
study suggest that HDAC6 functions as a tumor inhibitor in liver
cancer.
The abnormal activation of the canonical
Wnt/β-catenin signaling pathway is essential for tumorigenesis and
progression in a number of different tumor types, including liver
cancer (22). β-catenin, the key
protein of the canonical Wnt/β-catenin signaling pathway, was
deacetylated during the progression of breast cancer by HDAC6, and
subsequently the protein levels of acetylated β-catenin were
significantly decreased (33).
Stimulated by epidermal growth factor (EGF), HDAC6 is associated
with the translocation of β-catenin to the caveolae membrane
(23). β-catenin may be deacetylated
at lysine 49 and inhibits phosphorylation at serine 45 by HDAC6
(23). HDAC6 is also involved in
hindering EGF induced β-catenin nucleus localization, decreases Myc
expression and suppresses tumor cell proliferation (23). Mak et al (24) reported that HDAC6, cluster of
differentiation 133 (CD133) and β-catenin formed a ternary complex,
and that the reduction of HDAC6 or CD133 results in raised
β-catenin acetylation and degradation. Therefore, the present study
assessed whether HDAC6 overexpression affects the protein levels of
β-catenin in HCC cell lines. As presented in Fig. 2B, the total β-catenin levels were
substantially decreased compared with the healthy controls.
Furthermore, the present study detected that the canonical
Wnt/β-catenin signaling pathway downstream target genes cyclin D1
and Myc were significantly (P<0.05) reduced in HCC compared with
the healthy controls (Fig. 2B) and
the proliferation of HCC cells was substantially suppressed
compared with the controls (Fig. 2A).
These results indicate that HDAC6 overexpression attenuates the
activity of the canonical Wnt/β-catenin signaling via the
downregulation of β-catenin in liver cancer.
EMT is essential for tumor dissemination and
invasion (25). The aberrant
activation of the canonical Wnt/β-catenin signaling pathway
increases β-catenin and N-cadherin, decreases E-cadherin
expression, enhances EMT and promotes tumor metastasis in HCC
(34). In the canonical Wnt/β-catenin
pathway, the degradation complex is composed of adenomatous
polyposis coli, glycogen synthase kinase 3β and axin, which is
associated with the phosphorylation and ubiquitin-dependent
degradation of β-catenin (33,34). The
accumulation of β-catenin increases nucleus localization and
combines with lymphoid enhancer factor (LEF)/T-cell factor (TCF) as
a transcription factor to regulate proliferation and metastasis
(35). However, its effects on EMT
have been gradually expounded (36).
Slug or Snail, functioning as E-cadherin repressors, are also
regulated by the β-catenin-LEF/TCF complex transcriptional activity
(37,38). Activating the canonical Wnt/β-catenin
signaling pathway is associated with EMT in a number of other tumor
types (39,40). Hence, it was hypothesized that HDAC6
may modulate EMT via regulating the activity of the canonical
Wnt/β-catenin signaling pathway in HCC. The association between EMT
and the canonical Wnt/β-catenin signaling pathway was determined,
and it was demonstrated that HDAC6 overexpression induced the
degradation of β-catenin and attenuated the activity of the
canonical Wnt/β-catenin signaling pathway, inhibited EMT by
decreasing N-cadherin, vimentin and MMP-9 and increasing E-cadherin
in HCC compared with the healthy controls (Fig. 3C). These results revealed a potential
mechanism for the regulation of β-catenin expression in the
HDAC6-induced inhibition of EMT in HCC. Although the specific
mechanism of HDAC6-mediated degradation of β-catenin is unclear, it
is overt that HDAC6 overexpression causes the suppression of the
proliferation and metastasis of tumor cells via inhibition of the
canonical Wnt/β-catenin signaling pathway in HCC.
In previous studies on HDAC6 in HCC, the results
differ substantially between the studies. In a study by Jung et
al (19), it was elucidated that
HDAC6 activated the c-Jun N-terminal kinases-mediated Beclin-1
depended autophagic cell death process in HCC. It was additionally
revealed that the downregulation of HDAC6 indicated the poor
prognosis of patients with liver cancer. However, in a study by
Kanno et al (18), it was
revealed that HDAC6 was overexpressed and increased the activity of
cell migration and invasion in HCC. The data of 70 patients with
HCC were collected in that previous study. In the present study, it
was revealed that the expression of HDAC6 was decreased in Hep3B
and Huh-7 cell lines by western blot analysis, and verified in
liver cancer tissues by immunohistochemical staining. Therefore, it
was hypothesized that HDAC6 functioned as a tumor suppresser in
HCC, although the results of the present study may differ from some
previous reports. This may be caused by a difference in the cell
states. In addition, it was detected that HDAC6 overexpression
induced cell cycle arrest in the late G1 phase and apoptosis in
Huh-7 cells (Fig. 4), decreased the
protein cyclin D1 and increased cleaved casepase-3 expression
(Fig. 2B). Altogether, the present
study revealed that the expression of HDAC6 is downregulated in
HCC, and that the aberrant expression of HDAC6 suppresses tumor
cell growth and metastasis in vitro by inhibiting the
activity of canonical Wnt/β-catenin signaling pathway. A further
in-depth study is requires to investigate how HDAC6 induces
β-catenin degradation. The present study provided evidence to
identify that the HDAC6 functions as a tumor inhibitor by
attenuating the activity of the canonical Wnt/β-catenin signaling
pathway. Finally, the results of the present study may provide
potential support for the clinical treatment of liver cancer.
Acknowledgements
The authors would like to thank Professor Wei Xu,
Junnian Zheng, Hao Xu and Yuming Gu for their guidance and for
assisting colleagues in the research group.
Funding
The present study was supported by the Health
Department Foundation of Jiangsu province (grant no. H201322).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZY is responsible for experimental design,
experimental operation, data collation and thesis writing. WX, HX,
JZ and YG are responsible for the collection of clinical
pathological tissues, experimental quality control, revision and
publication of research topics and papers. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was ethically approved by the
Ethics Committee of Xuzhou Medical University (Xuzhou, China). All
experiments were performed consistent with the principles of the
Declaration of Helsinki. Written informed consent was obtained from
the patients prior to the study.
Patient consent for publication
All patients gave informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grozinger CM, Hassig CA and Schreiber SL:
Three proteins define a class of human histone deacetylases related
to yeast Hda1p. Proc Natl Acad Sci USA. 96:4868–4873. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marks P, Rifkind RA, Richon VM, Breslow R,
Miller T and Kelly WK: Histone deacetylases and cancer: Causes and
therapies. Nat Rev Cancer. 1:194–202. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seidel C, Schnekenburger M, Dicato M and
Diederich M: Histone deacetylase 6 in health and disease.
Epigenomics. 7:103–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valenzuela-Fernández A, Cabrero JR,
Serrador JM and Sánchez-Madrid F: HDAC6: A key regulator of
cytoskeleton, cell migration and cell-cell interactions. Trends
Cell Biol. 18:291–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng Q and Wang X: Autophagy and the
ubiquitin-proteasome system in cardiac dysfunction. Panminerva Med.
52:9–25. 2010.PubMed/NCBI
|
|
8
|
Aldana-Masangkay GI and Sakamoto KM: The
role of HDAC6 in cancer. J Biomed Biotechnol. 2011:8758242011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bienz M and Clevers H: Linking colorectal
cancer to Wnt signaling. Cell. 103:311–320. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen C, Xue Y, Zhang D, Xu W, Xu H, Yao H,
Pei D and Gu Y: Short hairpin RNA silencing of TGF-βRII and FZD-7
synergistically suppresses proliferation and metastasis of
hepatocellular carcinoma cells. Oncol Lett. 11:2039–2046. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian Y, Mok MT, Yang P and Cheng AS:
Epigenetic activation of Wnt/β-catenin signaling in
NAFLD-associated hepatocarcinogenesis. Cancers (Basel). 8:E762016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nelson WJ and Nusse R: Convergence of Wnt,
beta-catenin, and cadherin pathways. Science. 303:1483–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu C, Li Y, Semenov M, Han C, Baeg GH,
Tan Y, Zhang Z, Lin X and He X: Control of beta-catenin
phosphorylation/degradation by a dual-kinase mechanism. Cell.
108:837–847. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ikeda S, Kishida S, Yamamoto H, Murai H,
Koyama S and Kikuchi A: Axin, a negative regulator of the Wnt
signaling pathway, forms a complex with GSK-3beta and beta-catenin
and promotes GSK-3beta-dependent phosphorylation of beta-catenin.
EMBO J. 17:1371–1384. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu J, Coyne CB and Sarkar SN: PKC alpha
regulates Sendai virus-mediated interferon induction through HDAC6
and β-catenin. EMBO J. 30:4838–4849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ding G, Liu HD, Huang Q, Liang HX, Ding
ZH, Liao ZJ and Huang G: HDAC6 promotes hepatocellular carcinoma
progression by inhibiting P53 transcriptional activity. FEBS Lett.
587:880–886. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanno K, Kanno S, Nitta H, Uesugi N, Sugai
T, Masuda T, Wakabayashi G and Maesawa C: Overexpression of histone
deacetylase 6 contributes to accelerated migration and invasion
activity of hepatocellular carcinoma cells. Oncol Rep. 28:867–873.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jung KH, Noh JH, Kim JK, Eun JW, Bae HJ,
Chang YG, Kim MG, Park WS, Lee JY, Lee SY, et al: Histone
deacetylase 6 functions as a tumor suppressor by activating c-Jun
NH2-terminal kinase-mediated beclin 1-dependent autophagic cell
death in liver cancer. Hepatology. 56:644–657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bae HJ, Jung KH, Eun JW, Shen Q, Kim HS,
Park SJ, Shin WC, Yang HD, Park WS, Lee JY and Nam SW: MicroRNA-221
governs tumor suppressor HDAC6 to potentiate malignant progression
of liver cancer. J Hepatol. 63:408–419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lv Z, Weng X, Du C, Zhang C, Xiao H, Cai
X, Ye S, Cheng J, Ding C, Xie H, et al: Downregulation of HDAC6
promotes angiogenesis in hepatocellular carcinoma cells and
predicts poor prognosis in liver transplantation patients. Mol
Carcinog. 55:1024–1033. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Y, Zhang X, Polakiewicz RD, Yao TP and
Comb MJ: HDAC6 is required for epidermal growth factor-induced
beta-catenin nuclear localization. J Biol Chem. 283:12686–12690.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mak AB, Nixon AM, Kittanakom S, Stewart
JM, Chen GI, Curak J, Gingras AC, Mazitschek R, Neel BG, Stagljar I
and Moffat J: Regulation of CD133 by HDAC6 promotes β-catenin
signaling to suppress cancer cell differentiation. Cell Rep.
2:951–963. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vasko V, Espinosa AV, Scouten W, He H,
Auer H, Liyanarachchi S, Larin A, Savchenko V, Francis GL, de la
Chapelle A, et al: Gene expression and functional evidence of
epithelial-to-mesenchymal transition in papillary thyroid carcinoma
invasion. Proc Natl Acad Sci USA. 104:2803–2088. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Todaro M, Iovino F, Eterno V, Cammareri P,
Gambara G, Espina V, Gulotta G, Dieli F, Giordano S, De Maria R and
Stassi G: Tumorigenic and metastatic activity of human thyroid
cancer stem cells. Cancer Res. 70:8874–8885. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salminen A, Kauppinen A and Kaarniranta K:
AMPK/Snf1 signaling regulates histone acetylation: Impact on gene
expression and epigenetic functions. Cell Signal. 28:887–895. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park G, Tan J, Garcia G, Kang Y, Salvesen
G and Zhang Z: Regulation of histone acetylation by autophagy in
Parkinson disease. J Biol Chem. 291:3531–3540. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Witt O, Deubzer HE, Milde T and Oehme I:
HDAC family: What are the cancer relevant targets? Cancer Lett.
277:8–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JY, Kuo CW, Tsai SL, Cheng SM, Chen
SH, Chan HH, Lin CH, Lin KY, Li CF, Kanwar JR, et al: Inhibition of
HDAC3- and HDAC6-promoted survivin expression plays an important
role in SAHA-induced autophagy and viability reduction in breast
cancer cells. Front Pharmacol. 7:812016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lim JA and Juhnn YS: Isoproterenol
increases histone deacetylase 6 expression and cell migration by
inhibiting ERK signaling via PKA and Epac pathways in human lung
cancer cells. Exp Mol Med. 48:e2042016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hackanson B, Rimmele L, Benkißer M,
Abdelkarim M, Fliegauf M, Jung M and Lübbert M: HDAC6 as a target
for antileukemic drugs in acute myeloid leukemia. Leuk Res.
36:1055–1062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang SH, Li N, Wei Y, Li QR and Yu ZP:
β-catenin deacetylation is essential for WNT-induced proliferation
of breast cancer cells. Mol Med Rep. 9:973–978. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ye Y, Long X, Zhang L, Chen J, Liu P, Li
H, Wei F, Yu W, Ren X and Yu J: NTS/NTR1 co-expression enhances
epithelial-to-mesenchymal transition and promotes tumor metastasis
by activating the Wnt/β-catenin signaling pathway in hepatocellular
carcinoma. Oncotarget. 7:70303–70322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu
LX, Zhang SH, Huang DD, Tang L, Kong XN, et al: Wnt/beta-catenin
signaling contributes to activation of normal and tumorigenic liver
progenitor cells. Cancer Res. 68:4287–4295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuan Z, Yu X, Ni B, Chen D, Yang Z, Huang
J, Wang J, Chen D and Wang L: Overexpression of long non-coding
RNA-CTD903 inhibits colorectal cancer invasion and migration by
repressing Wnt/β-catenin signaling and predicts favorable
prognosis. Int J Oncol. 48:2675–2685. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Baldwin LA, Hoff JT, Lefringhouse J, Zhang
M, Jia C, Liu Z, Erfani S, Jin H, Xu M, She QB, et al: CD151-α3β1
integrin complexes suppress ovarian tumor growth by repressing
slug-mediated EMT and canonical Wnt signaling. Oncotarget.
5:12203–12217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zou W, Zou Y, Zhao Z, Li B and Ran P:
Nicotine-induced epithelial-mesenchymal transition via
Wnt/β-catenin signaling in human airway epithelial cells. Am J
Physiol Lung Cell Mol Physiol. 304:L199–L209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu CC, Cai DL, Sun F, Wu ZH, Yue B, Zhao
SL, Wu XS, Zhang M, Zhu XW, Peng ZH and Yan DW: FERMT1 mediates
epithelial-mesenchymal transition to promote colon cancer
metastasis via modulation of β-catenin transcriptional activity.
Oncogene. 36:1779–1792. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu ZJ, Liu HL, Zhou HC and Wang GC: TIPE2
inhibits hypoxia-induced Wnt/β-catenin pathway activation and EMT
in glioma cells. Oncol Res. 24:255–261. 2016. View Article : Google Scholar : PubMed/NCBI
|