Introduction
Anaplastic lymphoma kinase (ALK) is a tyrosine
kinase receptor frequently rearranged, mutated, or amplified in
specific neoplastic diseases, including lymphoma, neuroblastoma,
non-small cell lung cancer, and to a lesser extent in melanoma
(1). In addition, ALK-specific mRNA
and protein have been described in several cell lines from solid
tumors of ectodermal origin, including melanoma (2). ALK break points have been
identified in four acral cases (6.9%) of acral/mucosal melanomas
from southern China (3). More
recently, a novel ALK isoform derived from a de novo
alternative transcription initiation (ATI) site in ALK intron 19
(ALKATI) has been described by Wiesner et al
(4). Using the RNA-seq dataset of the
TCGA project, these authors found ALKATI expression in
11% of melanoma patients (38/334) and sporadically in other human
cancer types, but not in normal tissues (4). Thereafter, the same group identified
ALKATI expression in 3% of 303 metastatic melanoma
patients (5).
In the present study, we investigated the prognostic
significance of ALK expression, as detected by immunohistochemistry
and reverse transcription-quantitative polymerase chain reaction
(RT-qPCR), in a cohort of metastatic melanomas characterized by
BRAF, KRAS, NRAS and PIK3CA mutational status.
Materials and methods
Patients
A retrospective series of 71 metastatic melanoma
patients with complete clinico-pathological information underwent
mutational analyses at the Pathology Unit and were followed-up at
the Dermatologic Clinic of ‘Città della Salute e della Scienza’
University Hospital (Torino, Italy). The study was conducted in
accordance with The Code of Ethics of the World Medical Association
(Declaration of Helsinki) for experiments involving humans and
within guidelines and regulations by the Research Ethics Committee
of the University of Turin. Clinical, epidemiological and
histological data were collected from the medical history of
patients, all diagnosed, treated and followed-up according to
previously reported protocols, after written informed consent
(6–8).
Disease specific survival (DSS) was calculated from the date of
primary lesion diagnosis to the date of patient death or last
follow-up. Disease free interval (DFI) was calculated from the date
of primary lesion diagnosis to the date of tumour
progression/recurrence or last follow-up. Ethical approval for the
present study was obtained from the Ethical Committee of our
Institution.
Mutational status assessment
Metastatic tumor sections were submitted to DNA
extraction as previously described (9). Mutational detection was performed using
the Sequenom MassARRAY® system (Sequenom, San Diego, CA,
USA) in conjunction with The Myriapod Colon Status kit that
identifies 58, 54, 23 and 66 nucleotide substitutions in the
KRAS, NRAS, BRAF and PIK3CA genes, respectively.
Mutant and wild type alleles were discriminated using the Sequenom
MassARRAY® Analyser 4 platform.
ALK immunostaining
Melanoma metastases used for the study were
previously fixed in 4% buffered formaldehyde, routinely processed
and paraffin embedded. For each case, three micrometer-thick
paraffin sections were collected on superfrost plus slides and
tested by immunohistochemistry using anti-ALK rabbit monoclonal
antibody (clone D5F3; Ventana Medical Systems, Inc., Tucson, AZ,
USA). ALK detection was performed on the fully automated Ventana
BenchMark XT System using the recommended protocol. ALK
immunostaining was evaluated by two independent pathologists
applying a 4-tier (0–3) scoring scheme (negative: 0; mild
cytoplasmic: 1; moderate smooth cytoplasmic: 2; intense granular
cytoplasmic staining: 3) with either diffuse or focal pattern.
ALK transcript detection
Total RNA from formalin-fixed paraffin embedded
(FFPE) samples were extracted using the miRNeasy FFPE Kit (QIAGEN),
according to the manufacturer's protocols. cDNA was obtained from
0.2 µg of total RNA treated previously with RNase-free DNase
(Promega Corporation, Madison, WI, USA) using reverse transcriptase
SuperScript III (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and gene-specific reverse primers (Table I). RT-qPCR was performed with Thermal
iCycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using iQ
SYBR Green Supermix (Bio-Rad Laboratories, Inc.), according to the
manufacturer's instructions. The PCR cycling conditions were as
follows: 95°C for 5 min, followed by 40 cycles at 94°C for 10 sec
and 60°C for 30 sec. Oligonucleotide primer pairs used for RT-qPCR
were designed with PrimerBLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) to
obtain amplicons of 70–110 bp (Table
I). To confirm amplification specificity, PCR products were
subjected to the analysis of melting curve, linearity, and slope of
standard curve. PCR assays were performed in triplicate.
Gene-expression results were normalized to GAPDH
(glyceraldehyde-3-phosphate dehydrogenase) expressions and
quantified using the ΔCt method, as previously described (10). Samples with GAPDH Ct value >30 were
excluded from the analysis. Expression levels of ALK exons were
compared to commercially available RNA from SH-SY5Y neuroblastoma
cell line (Applied Biological Materials Inc., Richmond, BC,
Canada), which expresses full length ALK transcripts (11,12). ALK
primers used to verify the human origin of SH-SY5Y neuroblastoma
cell line and detect full length ALK expression were designed
according to the following ALK mRNA reference sequences:
ENST00000389048.7 (human); ENSMUST00000086639.4 (murine) (13–15).
Specifically, ALK1798R oligonucletiode and ALK1718F and ALK1790R
primers were used for gene-specific retrotranscription (exon 3) and
qPCR (exons 2–3), respectively (Table
I).
 | Table I.ALK gene-specific reverse primers. |
Table I.
ALK gene-specific reverse primers.
| Gene | Forward primer
5′-3′ | Reverse primer
5′-3′ | Use |
|---|
| ALK Ex 2/3 | ALK1718F
CTGTCTCATCGCAGCCGATA | ALK1790R
GTGGAGGGGAATACTCCAGC | RT |
|
|
| ALK1798R
GTCATGCAGTGGAGGGGAAT | RT-qPCR |
| ALK Ex 20/21 | ALK4284F
TGCCGCGGAAAAACATCACCCTG | ALK4371R
TTGGGCATTCCGGACAC | RT |
|
|
| ALK4380R
CTTGGGTCGTTGGGCATTC | RT-qPCR |
| ALK Ex 28/29 | ALK5050F
GCAACATCAGCCTGAAGACACCG | ALK5140R
AGCGGTGTTGATTACAT | RT |
|
|
| ALK5144R
GCAAAGCGGTGTTGATTACA | RT-qPCR |
| HK | GAPDHF
TCTTTTGCGTCGCCAGCCGAGCGAC | GAPDH150R
TGACCAGGCGCCCAATA | RT-qPCR |
Statistical analysis
Statistical analyses were performed using
Stata/SE12.0 statistical software (STATA, College Station, TX,
USA). P<0.05 was considered to indicate a statistically
significant difference. Differences in ALK expression were analyzed
using the χ2 test and the Fisher's exact test for small
numbers. Survival curves between different groups, according to ALK
expression, were plotted using the Kaplan-Meier method and the
statistical comparisons were performed with Log-rank test.
Results
The clinico-pathological features of the included
cases are shown in Table II.
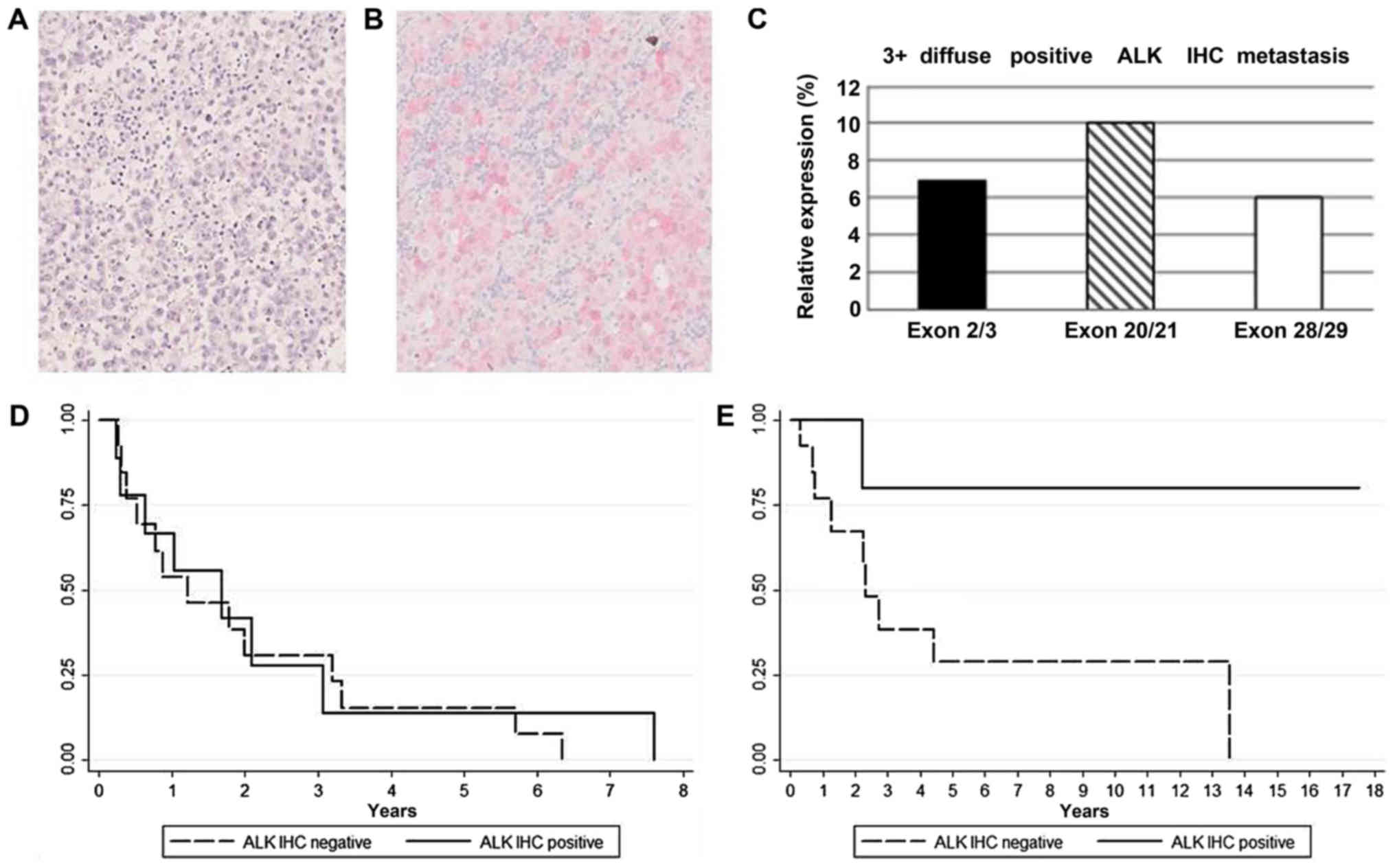
Immunohistochemical analysis identified 10/71 (14%) ALK positive
metastases (4 regional skin, 4 lymph node, 1 lung, 1 spleen
metastasis sites), showing a predominantly cytoplasmic staining
(Fig. 1A and B). Among these, 4 cases
were scored 1+, 3 cases 2+, and 3 cases 3+. Transcriptional
analysis by RT-qPCR using 5′ and 3′ exon specific primers detected
the expression of full-length ALK mRNA in 8/10 ALK positive samples
(Fig. 1C) and in 2/61 ALK negative
samples, thus supporting the immunohistochemical observations
(P<0,001). No significant imbalances of ALK exons expression
suggestive of ALK fusions or ALKATI isoform were
detected.
 | Table II.Clinical characteristics of patients
across mutational status. |
Table II.
Clinical characteristics of patients
across mutational status.
| Characteristics | Total | WT (n=19) | BRAF-mutated
(n=30) | NRAS-mutated
(n=22) | P-value |
|---|
| Sex |
|
|
|
|
|
|
Female | 29 | 8 | 14 | 7 | 0.556 |
|
Male | 42 | 11 | 16 | 15 |
|
| Age, years, median
(range) | 62 (25–86) | 66 (39–83) | 53 (25–83) | 67 (32–79) | 0.002 |
| Breslow, mean ±
SD | 3.67±2.61 | 4.26±2.57 | 3.44±2.95 | 3.33±1.96 | 0.500 |
| Histotype |
|
|
|
|
|
|
SSM | 35 | 7 | 16 | 12 | 0.749 |
| NM | 12 | 4 | 4 | 4 |
|
|
Othera | 24 | 8 | 10 | 6 |
|
| Ulceration |
|
|
|
|
|
|
Absent | 53 | 14 | 20 | 19 | 0.271 |
|
Present | 18 | 5 | 10 | 3 |
|
| Mitosis |
|
|
|
|
|
|
<1 | 36 | 8 | 14 | 14 | 0.328 |
| ≥1 | 35 | 11 | 16 | 8 |
|
| Stage at
diagnosis |
|
|
|
|
|
|
I/II | 37 | 11 | 18 | 8 | 0.206 |
|
III | 27 | 8 | 10 | 9 |
|
| IV | 7 | 0 | 2 | 5 |
|
| ALK IHC |
|
|
|
|
|
|
Negative | 61 | 19 | 29 | 13 | <0.001 |
|
Positive | 10 | 0 | 1 | 9 |
|
ALK expression was not associated with gender, age,
or classical melanoma prognostic factors such as Breslow thickness,
ulceration, or mitoses. BRAF, KRAS, NRAS and PIK3CA
mutational analysis recognized 9/10 ALK positive samples as
NRAS mutated, one BRAF mutated (score 3+ diffuse),
and none KRAS or PIK3CA mutated, nor wild-type.
Therefore, ALK expression was significantly (p=<0.001) enriched
in NRAS mutated metastases (9 out of 22; Table II). No statistically significant
difference in DFI or DSS was observed between ALK positive and
negative patients overall. However, among NRAS mutated
patients (11 NRAS Q61R, 11 NRAS Q61K), ALK positive
samples (5 NRAS Q61R, 4 NRAS Q61K; Tables III and IV) displayed a significantly better DSS
compared to ALK negatives (P=0.050; Fig.
1D and E). In NRAS mutated patients ALK expression was
not correlated with other clinico-pathological variables.
 | Table III.Clinical characteristics of NRAS
mutated patients. |
Table III.
Clinical characteristics of NRAS
mutated patients.
|
Characteristics | Total | ALK IHC
negative | ALK IHC
positive | P-value |
|---|
| Sex |
|
|
| 0.899 |
|
Female | 7 | 4 | 3 |
|
|
Male | 15 | 9 | 6 |
|
| Age, years, median
(interval) | 67 (32–79) | 68 (32–78) | 66 (46–79) | 1.000 |
| Breslow, mean ±
SD | 3.33±1.96 | 3.17±1.32 | 3.58±2.81 | 0.707 |
| Histotype |
|
|
| 0.868 |
|
SSM | 12 | 7 | 5 |
|
| NM | 4 | 2 | 2 |
|
|
Othera | 6 | 4 | 2 |
|
| Ulceration |
|
|
| 0.271 |
|
Absent | 19 | 12 | 7 |
|
|
Present | 3 | 1 | 2 |
|
| Mitosis |
|
|
| 0.378 |
|
<1 | 14 | 9 | 5 |
|
| ≥1 | 8 | 4 | 4 |
|
| Stage at
diagnosis |
|
|
| 0.542 |
| II | 8 | 4 | 4 |
|
|
III | 9 | 5 | 4 |
|
| IV | 5 | 4 | 1 |
|
| First site of
progression |
|
|
| 0.683 |
|
Regional | 18 | 11 | 7 |
|
|
Distant | 4 | 2 | 2 |
|
 | Table IV.Clinical and pathologic features of
ALK-positive metastatic melanoma in NRAS mutated patients. |
Table IV.
Clinical and pathologic features of
ALK-positive metastatic melanoma in NRAS mutated patients.
| Case | Age | Sex | Site of
metastasis | Site of
primary | Breslow
thickness | ALK IHC
metastasis | RT-PCR | NRAS mutation | Therapy at first
progression | Therapy at 2nd
progression |
|---|
| 1 | 47 | M | Skin | Trunk | 3,20 | 2+ focal | Positive | Q61K | Surgery alone | Ipilimumab |
| 2 | 74 | M | Lung | Arm | 9,00 | 2+ focal | Positive | Q61K | Ipilimumab | – |
| 3 | 74 | F | Skin | Arm | 1,60 | 1+ focal | Positive | Q61R | Surgery alone | – |
| 4 | 66 | M | LN | Leg | 2,5 | 3+ diffuse | Positive | Q61K | Surgery alone | Anti-PD1 |
| 5 | 62 | M | Skin | Back | 3 | 2+ focal | Positive | Q61K | Surgery alone | Anti-PD1 |
| 6 | 77 | F | LN | Leg | 1,2 | 1+ focal | Negative | Q61R | Surgery alone |
Electro-chemotherapy |
| 7 | 46 | M | Spleen | Trunk | 3 | 1+ focal | Negative | Q61R | Surgery alone | – |
| 8 | 61 | M | LN | Head | 1.3 | 1+ focal | Positive | Q61R | Surgery alone | – |
| 9 | 79 | F | LN | Back | 3,50 | 3+ diffuse | Positive | Q61R | Surgery alone | Ipilimumab |
Discussion
Melanoma represents the fifth most common tumour in
humans and is considered one of the most invasive,
therapy-resistant and metastatic malignancy, with only 10% of
metastatic patients surviving 5 years post-diagnosis. In addition,
over the past decades, its incidence has been increasing by 3–8%
per year in Western countries (16,17).
Therefore, a deeper understanding of the molecular events
regulating melanoma aggressiveness and metastatic dissemination is
essential to develop new relevant biomarkers and therapeutic
strategies.
In the present study, according to previous reports
(5), we have documented that ALK
protein expression can be detected by immunohistochemistry in a
significant subset of metastatic melanomas (10 out 71, 14%), with
variable immunoreactivity scores ranging from focal/weak to
diffuse/strong. Interestingly, in our series 9 out of 10 ALK
positive patients were NRAS mutated. Busam and colleagues
detected ALK immunoreactivity in metastatic tumors independently on
the BRAF or NRAS mutational status (5).
In melanomas, NRAS activating mutations
(present in 15–20% of cases) have been associated with aggressive
clinical behaviour, and lack of effective treatment options, as
well as with poor outcome and lower median overall survival
(18,19). In particular, the presence of
NRAS mutations correlates to shorter survival in stage IV
melanomas and it is associated with a higher risk of central
nervous system involvement (19). In
our study we observed that, among NRAS-mutated patients,
those ALK positive showed a more favourable outcome in term of DSS,
when compared to ALK negative ones. Even though the statistical
significance of this observation needs to be confirmed in a larger
cohort of patients, its interpretation could open new scenarios.
The observation that ALK expression in metastatic melanomas plays a
physiological or pathological function still remains an open issue.
In our series no ALK translocations or alternative isoforms were
detected. This observation suggests that ALK expression is most
likely related to the neuroectodermal origin of melanoma cells
(20). Indeed, it has been shown that
ALK protein is variably expressed in the cytoplasm and/or nucleus
of developing central and peripheral nervous system during
embryogenesis, and its expression is maintained in the adult at
lower level in several tissues, including keratinocytes and
melanocytes (http://www.proteinatlas.org/ENSG00000171094-ALK/tissue/skin)
(2,13,21,22). The
correlation between ALK expression and NRAS mutation could
be ascribed to the fact that NRAS mutations occur at the
stage of neural crest and are an early somatic event in the
development of the majority of melanomas (22,23). The
central nervous system (CNS) is a frequent site of disease
progression in melanoma patients, with palliative radiotherapy
usually being administered to the CNS metastasis. Recently, a
combination of RT and systemic immunotherapy has been proposed for
the treatment of stage IV melanoma (24). ALK positive patients could represent a
novel subgroup to treat with multimodal therapy comprehensive of
TKI, as described in NSCLC brain metastases (25). However, additional clinical studies
are needed to determine the efficacy of targeted therapies in
melanomas expressing ALK (26).
A larger study is desirable to better clarify,
confirm, or possibly rule out the effective ALK reliability as a
possible indicator of less aggressive pattern in
NRAS-mutated metastatic melanoma patients. If confirmed, ALK
positive/NRAS mutated metastatic melanomas could represent a
novel clinical entity and a new therapeutic challenge in metastatic
melanoma patients.
Acknowledgements
The authors would like to acknowledge technical
support in immunohistochemical procedures to Dr Maria Stella
Scalzo, Dr Francesca Veneziano and Dr Chiara Musuraca (Department
of Medical Sciences, Pathology Unit, University of Torino).
Funding
This research received funding specifically
appointed to the Department of Medical Sciences from the Italian
Ministry for Education, University and Research under the programme
‘Dipartimenti di Eccellenza 2018–2022’ and has been supported by
grants from Ministero dell'Istruzione, dell'Università e della
Ricerca (grant no. Ex60% 2015; received by SOA),
Lanzavecchia-Lastretti Foundation for ‘Progetto Melanoma’,
Associazione Italiana per la Ricerca sul Cancro (grant no.
IG-13358), Compagnia di San Paolo (grant no. TO_Call2_2012_0061)
and Fondazione CRT (grant no. 2014_1105; received by RP).
Availability of data and materials
The data that support the findings of the present
study are available from Città della Salute e della Scienza
Hospital of Torino, Department of Medical Sciences University of
Torino, but restrictions apply to the availability of these data,
which were used under license for the current study, and so are not
publicly available. Data are however available from the authors
upon reasonable request and with permission of Città della Salute e
della Scienza Hospital of Torino and Department of Medical Sciences
University of Torino.
Authors' contributions
RP, EM and SOA designed and supervised the study,
obtained funding and wrote the manuscript approved by all authors.
EP and EB performed the RT-PCR experiments. FL contributed
substantially to the design and execution of immunohistochemistry
experiments, and preparation of the manuscript. LB and MP provided
samples, and designed and supervised the pathological evaluation of
samples. SR and MTF supervised the clinical data management and
contributed to the statistical analysis.
Ethics approval and consent to
participate
The present study was performed in accordance with
The Code of Ethics of the World Medical Association (Declaration of
Helsinki) for experiments involving humans and within guidelines
and regulations by the Research Ethics Committee of the University
of Turin. The present study was approved by the Research Ethics
Committee of the University of Turin.
Patient consent for publication
Written informed consent was obtained from all
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chiarle R, Voena C, Ambrogio C, Piva R and
Inghirami G: The anaplastic lymphoma kinase in the pathogenesis of
cancer. Nat Rev Cancer. 8:11–23. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dirks WG, Fähnrich S, Lis Y, Becker E,
MacLeod RA and Drexler HG: Expression and functional analysis of
the anaplastic lymphoma kinase (ALK) gene in tumor cell lines. Int
J Cancer. 100:49–56. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niu HT, Zhou QM, Wang F, Shao Q, Guan YX,
Wen XZ, Chen LZ, Feng QS, Li W, Zeng YX and Zhang XS:
Identification of anaplastic lymphoma kinase break points and
oncogenic mutation profiles in acral/mucosal melanomas. Pigment
Cell Melanoma Res. 26:646–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wiesner T, Lee W, Obenauf AC, Ran L,
Murali R, Zhang QF, Wong EW, Hu W, Scott SN, Shah RH, et al:
Alternative transcription initiation leads to expression of a novel
ALK isoform in cancer. Nature. 526:453–457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Busam KJ, Vilain RE, Lum T, Busam JA,
Hollmann TJ, Saw RP, Coit DC, Scolyer RA and Wiesner T: Primary and
metastatic cutaneous melanomas express ALK through alternative
transcriptional initiation. Am J Surg Pathol. 40:786–795. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sanlorenzo M, Ribero S, Osella-Abate S,
Zugna D, Marenco F, Macripò G, Fierro MT, Bernengo MG and Quaglino
P: Prognostic differences across sexes in melanoma patients: What
has changed from the past? Melanoma Res. 24:568–576. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ribero S, Osella-Abate S, Sanlorenzo M,
Balagna E, Senetta R, Fierro MT, Macripò G, Macrì L, Sapino A and
Quaglino P: Sentinel lymph node biopsy in thick-melanoma patients
(N=350): What is its prognostic role? Ann Surg Oncol. 22:1967–1973.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Osella-Abate S, Ribero S, Sanlorenzo M,
Maule MM, Richiardi L, Merletti F, Tomasini C, Marra E, Macripò G,
Fierro MT and Quaglino P: Risk factors related to late metastases
in 1,372 melanoma patients disease free more than 10 years. Int J
Cancer. 136:2453–2457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mariani S, Di Bello C, Bonello L, Tondat
F, Pacchioni D, Molinaro L, Barreca A, Macrì L, Chiusa L, di Celle
PF, et al: Flexible lab-tailored cut-offs for suitability of
formalin-fixed tumor samples for diagnostic mutational analyses.
PLoS One. 10:e01218152015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Agnelli L, Mereu E, Pellegrino E, Limongi
T, Kwee I, Bergaggio E, Ponzoni M, Zamò A, Iqbal J, Piccaluga PP,
et al: Identification of a 3-gene model as a powerful diagnostic
tool for the recognition of ALK-negative anaplastic large-cell
lymphoma. Blood. 120:1274–1284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scarfò I, Pellegrino E, Mereu E, Kwee I,
Agnelli L, Bergaggio E, Garaffo G, Vitale N, Caputo M, Machiorlatti
R, et al: Identification of a new subclass of ALK-negative ALCL
expressing aberrant levels of ERBB4 transcripts. Blood.
127:221–232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
George RE, Sanda T, Hanna M, Fröhling S,
Luther W II, Zhang J, Ahn Y, Zhou W, London WB, McGrady P, et al:
Activating mutations in ALK provide a therapeutic target in
neuroblastoma. Nature. 455:975–978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iwahara T, Fujimoto J, Wen D, Cupples R,
Bucay N, Arakawa T, Mori S, Ratzkin B and Yamamoto T: Molecular
characterization of ALK, a receptor tyrosine kinase expressed
specifically in the nervous system. Oncogene. 14:439–449. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
https://www.ncbi.nlm.nih.gov/nuccore/NM_004304?report=GenBankHomo
sapiens ALK receptor tyrosine kinase (ALK), transcript variant 1.
mRNA.
|
|
15
|
https://www.ncbi.nlm.nih.gov/nuccore/NM_007439.2?report=GenBankMus
musculus anaplastic lymphoma kinase (Alk). mRNA.
|
|
16
|
Forsea AM, Del Marmol V, de Vries E,
Bailey EE and Geller AC: Melanoma incidence and mortality in
Europe: New estimates, persistent disparities. Br J Dermatol.
167:1124–1130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Svedman FC, Pillas D, Taylor A, Kaur M,
Linder R and Hansson J: Stage-specific survival and recurrence in
patients with cutaneous malignant melanoma in Europe-a systematic
review of the literature. Clin Epidemiol. 8:109–122. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnson DB, Lovly CM, Flavin M, Panageas
KS, Ayers GD, Zhao Z, Iams WT, Colgan M, DeNoble S, Terry CR, et
al: Impact of NRAS mutations for patients with advanced melanoma
treated with immune therapies. Cancer Immunol Res. 3:288–295. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jakob JA, Bassett RL Jr, Ng CS, Curry JL,
Joseph RW, Alvarado GC, Rohlfs ML, Richard J, Gershenwald JE, Kim
KB, et al: NRAS mutation status is an independent prognostic factor
in metastatic melanoma. Cancer. 118:4014–4023. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arozarena I and Wellbrock C: Targeting
invasive properties of melanoma cells. FEBS J. 284:2148–2162. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thul PJ, Åkesson L, Wiking M, Mahdessian
D, Geladaki A, Ait Blal H, Alm T, Asplund A, Björk L, Breckels LM,
et al: A subcellular map of the human proteome. Science. 356(pii):
eaal33212017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morris SW, Kirstein MN, Valentine MB,
Dittmer K, Shapiro DN, Look AT and Saltman DL: Fusion of a kinase
gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's
lymphoma. Science. 267:316–317. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Platz A, Egyhazi S, Ringborg U and Hansson
J: Human cutaneous melanoma; a review of NRAS and BRAF mutation
frequencies in relation to histogenetic subclass and body site. Mol
Oncol. 1:395–405. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hiniker SM, Reddy SA, Maecker HT,
Subrahmanyam PB, Rosenberg-Hasson Y, Swetter SM, Saha S, Shura L
and Knox SJ: A prospective clinical trial combining radiation
therapy with systemic immunotherapy in metastatic melanoma. Int J
Radiat Oncol Biol Phys. 96:578–588. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Churilla TM and Weiss SE: Emerging trends
in the management of brain metastases from non-small cell lung
cancer. Curr Oncol Rep. 20:542018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Couts KL, Bemis J, Turner JA, Bagby SM,
Murphy D, Christiansen J, Hintzsche JD, Le A, Pitts TM, Wells K, et
al: ALK inhibitor response in melanomas expressing EML4-ALK fusions
and alternate ALK isoforms. Mol Cancer Ther. 17:222–231. 2018.
View Article : Google Scholar : PubMed/NCBI
|