Introduction
Pancreatic cancer is a malignant tumor of the
digestive system identified by an insidious clinical manifestation,
rapid progression and a poor prognosis. Furthermore, there are no
acceptable screening methods to detect early-stage disease, so the
majority of patients present with advanced disease and diagnosis at
early stages of the disease is difficult (1–3).
Pancreatic cancer has become the fourth leading cause of
cancer-associated mortality in the Western world (4,5).
Pancreatic cancer is often resistant to known anticancer drugs
(6–8).
Globally, there has been an increased focus on discovering
alternative and more efficient anti-pancreatic cancer agents and
gemcitabine sensitizers (8–10). There is a clear requirement to
investigate more potential therapeutic agents for the treatment of
pancreatic cancer.
MicroRNAs (miRs/miRNAs) are a group of small
non-coding RNAs composed of 18–25 nucleotides that regulate
protein-coding genes by binding to the 3′-untranslated regions
(UTRs) of their target mRNAs (11–15). Since
initial discovery by Lee et al (16) in 1993, increasing evidence has
revealed that miRNAs serve important functions in the generation or
development of various tumors, particularly with roles in
processes, including cell cycle, apoptosis, migration and invasion
(17–21).
Similar studies have also been performed in
pancreatic cancer cells (22,23). In a recent study by the present
authors (24), miR-221 was found to
be differentially expressed between highly and weakly invasive PC
cells (PC-1.0 and PC-1) when using miRNA microarray. The PC-1 cell
line was established from pancreatic ductal adenocarcinomas induced
by N-Nitrosobis (2-oxopropyl) amine in a Syrian golden hamster
(25). The PC-1.0 cell line was
established from a subcutaneous tumor produced following
inoculation of PC-1 cells. PC-1 cells exhibited a low aggressive
potential, whilst PC-1.0 cells exhibited a high aggressive
potential (26). These two cell lines
also exhibited different growth rates and morphology in
vitro. PC-1 cells formed island-like cell colonies, whereas
PC-1.0 cells primarily grew as single cells (27). The results verified using two hamster
pancreatic cancer cell lines (PC-1 and PC-1.0) and two human
pancreatic cancer cell lines (Capan-2 and AsPC-1) by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Capan-2 cells exhibited a low aggressive potential, similar to PC-1
cells, whereas the AsPC-1 cell line exhibited a high aggressive
potential, similar to PC-1.0 cells. The results revealed that six
upregulated microRNAs (miR-34a, miR-193a, miR-221, miR-222, miR-484
and miR-502) exhibited differential expression between PC-1.0/PC-1
and AsPC-1/Capan-2 pancreatic cancer cells. Additionally, Gene
Ontology and Kyoto Encyclopedia of Genes and Genomes enrichment
analysis revealed that miR-221 were involved in multiple biological
functions in tumorigenesis of pancreatic cancer. A number of
studies have documented that miR-221 serves an important function
in regulating the tumorigenesis and metastasis of glioblastoma,
breast and gastric cancer, by affecting processes, including cell
cycle, apoptosis, migration and autophagy (13,18). These
results suggest that miR-221 may participate in regulating the
progression of pancreatic cancer.
Histone deacetylases (HDACs), which are often
deregulated in pancreatic cancer and other solid tumor types
(28,29), are implicated in the regulation of
molecules in growth regulatory and/or apoptotic pathways (28,29).
Unique among the HDAC family members, HDAC6 has intrinsic
ubiquitin-binding activity and associates with microtubules and the
F-actin cytoskeleton (30–33). It has been demonstrated that HDAC6 was
able to associate with and deacetylate α-tubulin in vitro
and in vivo. Overexpression of HDAC6 induced the expression
of deacetylated α-tubulin and promoted cell motility. Consistent
with its effect on cell motility, HDAC6 is predominantly localized
in the cytoplasm (34). Notably,
malignant mammary epithelial cells exhibit increased HDAC6
cytosolic localization compared with normal cells (35). It has also been reported that HDAC6
also serves a function in the clearance of aggresomes, thereby
implying a functional association between HDAC6 and autophagy
(36). Giaginis et al
(36) revealed that HDAC6 may be
implicated in pancreatic malignant disease progression, and is
considered to have clinical utility with potential use as a
therapeutic target. Bae et al (37) revealed that c-Jun N-terminal kinase
stimulated by hepatocyte growth factor led to the activation of Jun
proto-oncogene, AP-1 transcription factor subunit and the
transcriptional activation of miR-221, which increased miR-221
expression, thereby serving a critical function in suppressing
HDAC6 expression in hepatocellular carcinoma cells. However, there
are no studies on miR-221 regulating autophagy in pancreatic cancer
via the regulation of HDAC6 expression.
In the present study, the aim was to elucidate the
potential function of miR-221 on the apoptosis and autophagy of
pancreatic cancer cells. In addition, it was confirmed that miR-221
regulates apoptosis and autophagy in pancreatic cancer cells by
regulating HDAC6 expression. This will provide novel treatment
methods and strategies for pancreatic cancer.
Materials and methods
Cell culture and cell
transfection
PC-1.0 cell line (hamster; sourced from the Kumamoto
University Medical School, Kumamoto, Japan) and the AsPC-1 cell
line (human; purchased from Sigma-Aldrich, Merck KGaA, Darmstadt,
Germany) were cultured in RPMI-1640, supplemented with 10% fetal
bovine serum (both Gibco; Thermo Fisher Scientific, Inc.), 1%
penicillin-streptomycin (Thermo Fisher Scientific, Inc.) at 37°C in
a humidified atmosphere of 5% CO2. The growth of cells
was observed under an inverted microscope. When the cells had
reached 70–80% confluence, they were detached using 0.25% trypsin
(Gibco; Thermo Fisher Scientific, Inc.). The medium was changed
every other day, and the cells were passaged every 3–4 days. The
cells in the exponential phase were selected for further
experiments.
The cultured PC-1.0 and AsPC-1 cells were uniformly
seeded in 6-well cultured plates at a density of
3×105/ml (2 ml in each well). Subsequent to the cells
reaching complete adherence, transiently transfections with miR-221
mimic and mimic negative control (NC; both designed and synthesized
by Invitrogen; Thermo Fisher Scientific, Inc.) at a final
concentration of 30 nM using 9 µl Lipofectamine® RNAiMAX
reagent and 150 µl OPTI-MEM I reduced serum medium (both
Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 24 h
according to the manufacturer's protocol. Untransfected cells were
not used as a control. Downregulation of HDAC6 was achieved using
190 nM Apicidin (190 nM; Abcam, Cambridge, UK) at 37°C for 2 h. The
cells that were treated alone with DMSO were used as the NC. The
cells were serum-starved overnight prior to transfection. The
sequences are as follows: miR-221 mimic,
5′-AGCUACAUUGUCUGCUGGGUUUC-3′; mimic negative control,
5′-UUCUCCGAACGUGUCACGUTT-3′.
RNA isolation and RT-qPCR
miRNA were extracted using the mirVana™
microRNA isolation kit (Ambion; Thermo Fisher Scientific, Inc.) and
the levels of miRNA were determined using the TaqMan®
MicroRNA Assay kit (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocols. cDNA was amplified using mature
microRNA-specific RT primers and the TaqMan® MicroRNA
Reverse Transcription kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocols. RT-qPCR was performed on
an ABI 7500 Real-Time PCR system (Thermo Fisher Scientific, Inc.).
The amplification reactions were performed in triplicate in a
96-well plate using the following thermocycling conditions: 10 min
at 95°C, followed by 40 cycles of 15 sec at 95°C and 1 min at 60°C.
The Cq values were calculated using ABI Sequence
Detection System software (version 2.1; Thermo Fisher Scientific,
Inc.). The relative fold-change of each miRNA was calculated using
the comparative Cq (2−ΔΔCq) method (38). The noncoding small nuclear RNA U6
(Thermo Fisher Scientific, Inc.) was used as the endogenous
control. The primer sequences were as follows: hsa-miR-221 forward,
5′-GAAACCCAGCAGACAATGTAGCT-3′ and reverse,
5′-CTTTGGTGTTTGAGATGTTTGG-3′; and U6 forward,
5′-GCGAATTCTTAAACAGCTCGAATTAAGAATATGTTTCC-3′ and reverse,
5′-GCGGATCCGCTATGGAAGTTTTCTTTATTGATTACTTAATGTG-3′.
Flow cytometric analysis of
apoptosis
Apoptosis was detected using flow cytometric
analysis. Following transfection, the cells were washed and
re-suspended in 0.5 ml phosphate-buffered saline (PBS; pH 8.0) at a
density of 1×106 cells/ml. Then, Annexin V-FITC and
propidium iodide dye (Nanjing KeyGEN Biotech Co., Ltd., Nanjing,
China) were added to the cells and incubated at room temperature in
the dark for 15 min. Apoptosis was detected using FACS LSR II (BD
Immunocytometry Systems; BD Biosciences, San Jose, CA, USA) and
analyzed using CXP software 2.2 (Beckman Coulter, Inc., Brea, CA,
USA).
Western blot analysis
The expression of HDAC6 and LC3 proteins in PC-1.0
and AsPC-1 cells was analyzed. PBS was used as the wash water. The
cells were washed three times prior to lysing. The cells were lysed
in radioimmunoprecipitation assay buffer containing protease
inhibitor cocktail (both Beyotime Institute of Biotechnology,
Haimen, China). Total protein was quantified using a Bradford
protein assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Briefly, samples containing equivalent amounts of total protein (20
µg) were run on 7.5–15% SDS-PAGE gels and then transferred onto
PVDF membranes (Merck KGaA, Darmstadt, Germany). Blocking solution
[PBS + 5% BSA (Sigma-Aldrich; Merck KGaA) + 10% goat serum] was
then added at room temperature for 30 min to prevent non-specific
binding. Membranes were incubated overnight at 4°C using primary
antibodies. The blots were then incubated with an anti-mouse (cat
no. sc-2005) or anti-rabbit (cat no. sc-2004; both Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) horseradish
peroxidase-conjugated secondary antibody (diluted 1:5,000 in 0.1%
Tween-20/PBS) at room temperature for 4 h. The membranes were
subsequently visualized using an enhanced chemiluminescence
detection system (GE Healthcare, Chicago, IL, USA). Protein bands
were quantified using densitometric analysis with ImageJ software
(39). The primary antibodies used
were as follows: HDAC6 (1:1,000; cat no. ab82667; rabbit
polyclonal; Abcam), microtubule associated protein 1 light chain 3
(LC3; 1:500; cat no. sc-398822; mouse polyclonal; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). GAPDH (1:1,000; cat no.
sc-25778; rabbit polyclonal; Santa Cruz Biotechnology, Inc.) was
used as a protein-loading control.
Immunofluorescence microscopy
Individual sterile coverslips were placed in the
wells of a 4-well plate. PC-1.0 and AsPC-1 cells were added and
incubated at 37°C for 24 h. Following treatment with Apicidin at
37°C for 2 h, the cells were washed using PBS, and then fixed in
3.7% paraformaldehyde for 20 min at room temperature. Subsequently,
paraformaldehyde was removed and the cells were washed using PBS.
Blocking solution (PBS + 5% BSA + 10% goat serum) was then added at
room temperature for 30 min to prevent non-specific binding, and
then the cells were incubated with the primary antibodies against
HDAC6 (1:200; cat no. ab82667; rabbit polyclonal; Abcam) and LC3
(1:100; cat no. sc-398822; mouse polyclonal; Santa Cruz
Biotechnology, Inc.), overnight at 4°C, on a shaker. The washed
slides were incubated for 1 h at room temperature with 1:100
dilutions of Alexa-488 anti-rabbit immunoglobulin IgG (H+L; cat no.
ab150077) and Alexa-568 goat anti-mouse IgG (H+L; cat no. ab175473;
both Abcam) secondary antibodies. The slides were washed again with
PBS, mounted with Vectashield mounting medium (Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA), and were observed under
a confocal laser scanning microscope (Leica Microsystems GmbH,
Wetzlar, Germany).
Statistical analysis
All experiments were performed at least three times
in triplicate for each group. The data are presented as the mean ±
standard deviation. The differences between groups were calculated
with Student's paired t-test using GraphPad Prism software (version
6.0; GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression levels of miR-221 following
miR-221 mimic transfection
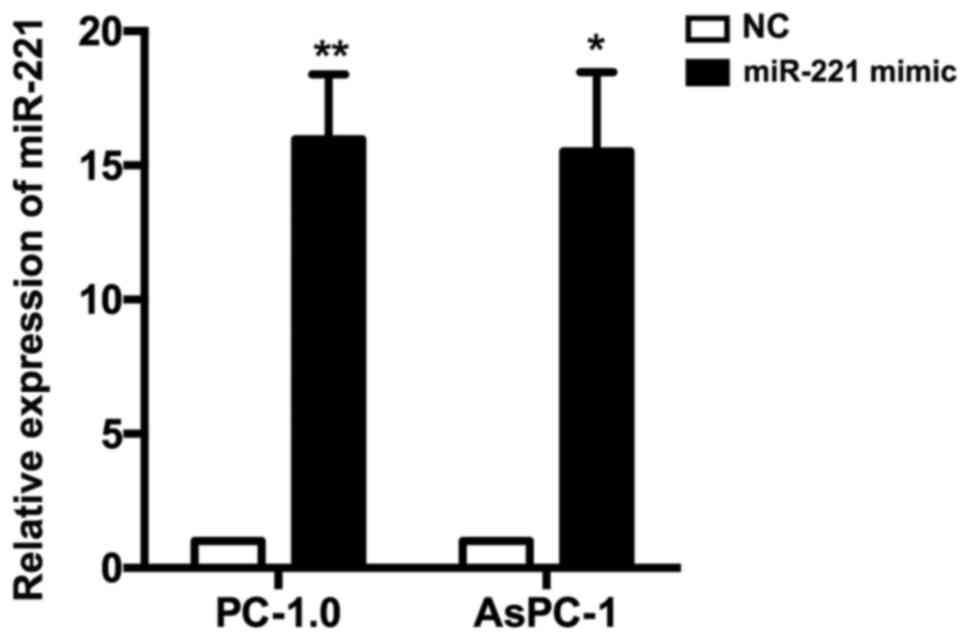
As indicated in in Fig.
1, the levels of miR-221 expression in PC-1.0 and AsPC-1 cells
that were transfected with the miR-221 mimic were significantly
increased compared with the NC (15.973±2.416 and 15.533±2.936-fold,
respectively).
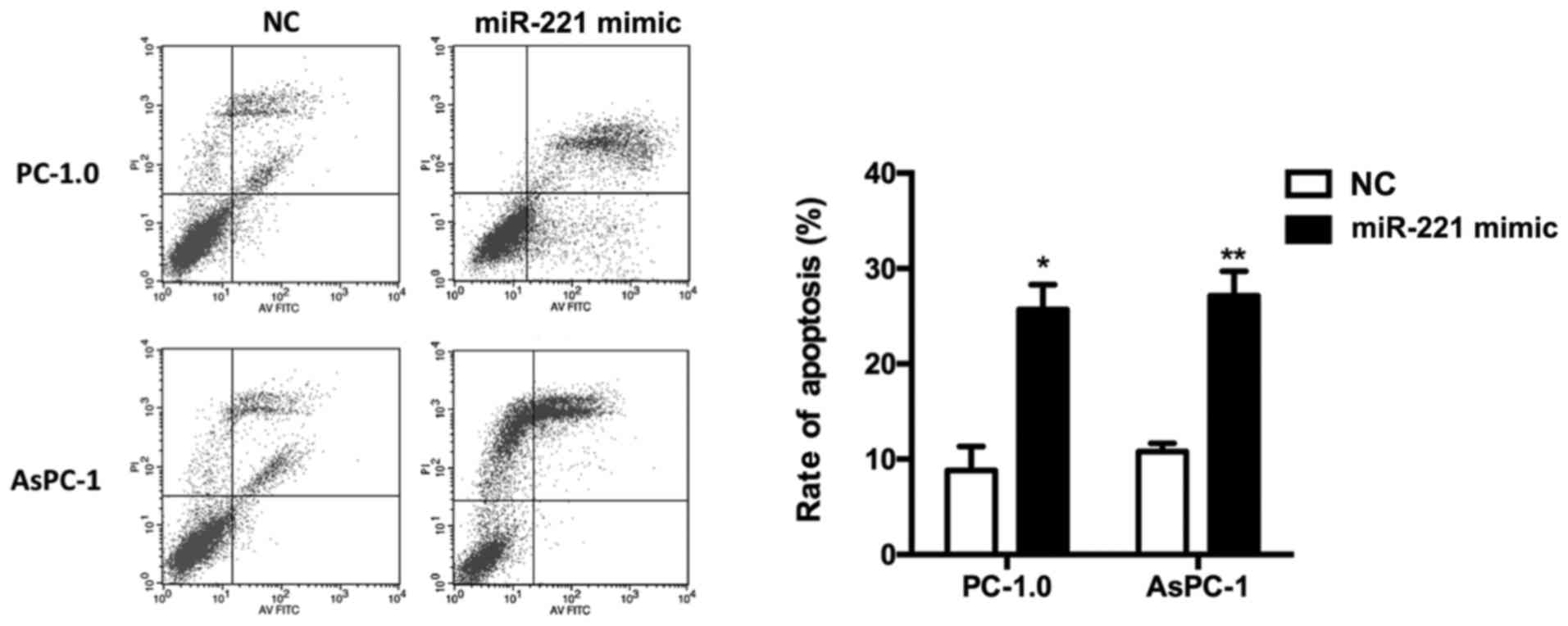
Cell apoptosis is induced by
miR-221
Flow cytometric analysis revealed that the apoptotic
rate of PC-1.0 and AsPC-1 cells in the miR-221 mimic group were
significantly increased compared with the miR-221 NC group
following transfection (PC-1.0, P<0.05; AsPC-1, P<0.01;
Fig. 2).
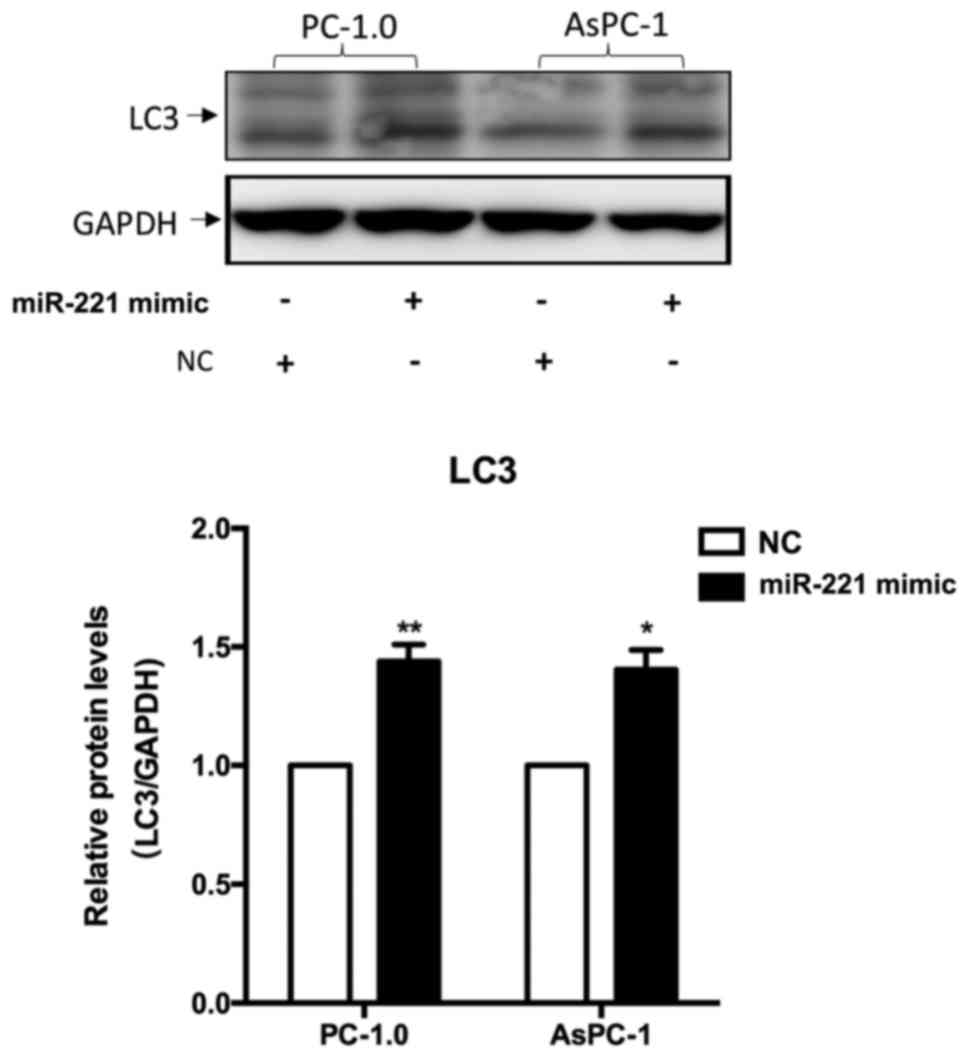
Cell autophagy is induced by
miR-221
Induction of autophagy was confirmed by western blot
analysis of modifications to the endogenous LC3. Data revealed that
the protein levels of LC3-II and LC3-I were significantly increased
following transfection with the miR-221 mimic group compared with
NC group (PC-1.0, P<0.01; AsPC-1, P<0.05; Fig. 3). These results suggest that miR-221
was able to induce autophagy in pancreatic cancer cells.
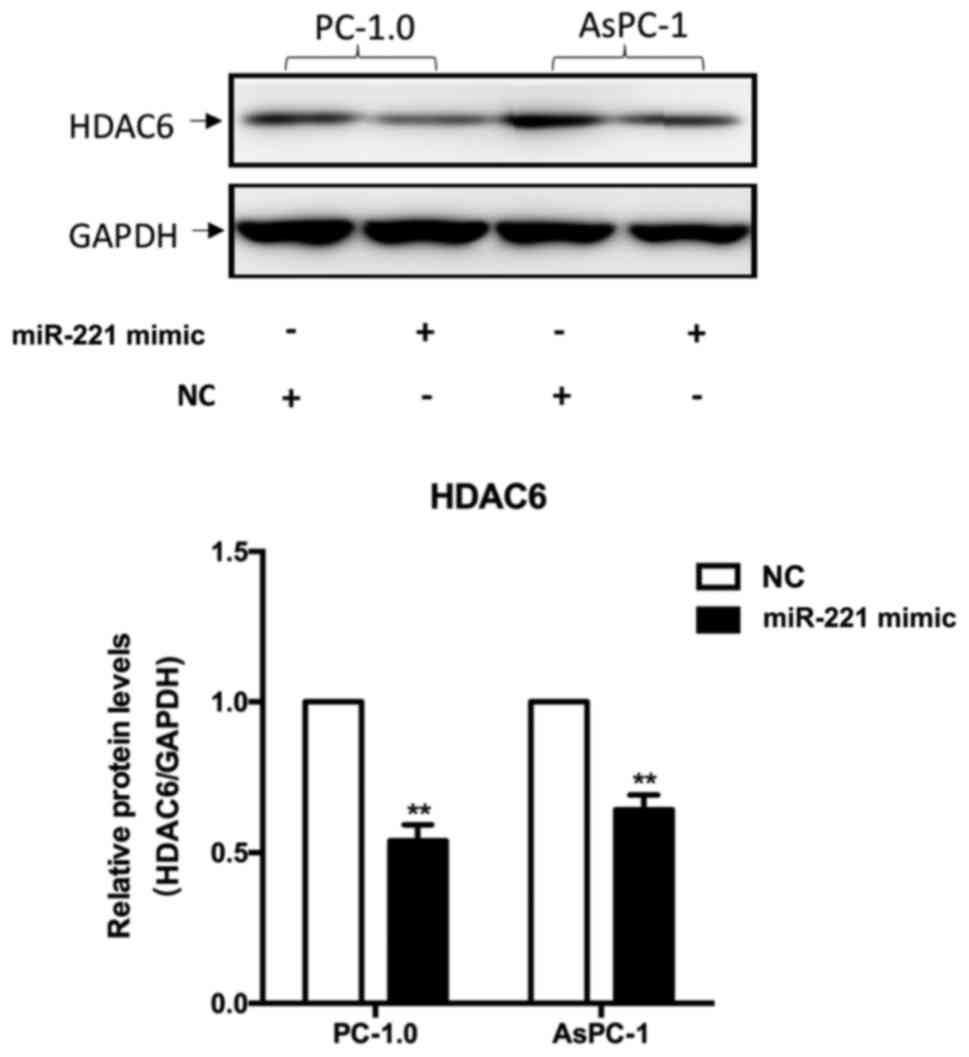
HDAC6 expression is inhibited by
miR-221
The levels of HDAC6 expression were detected using
western blot analysis following transfection. Data revealed that
the expression levels of HDAC6 were significantly decreased
following transfection compared with the miR-221 NC group
(P<0.01; Fig. 4).
Autophagy is induced by the
downregulation of HDAC6
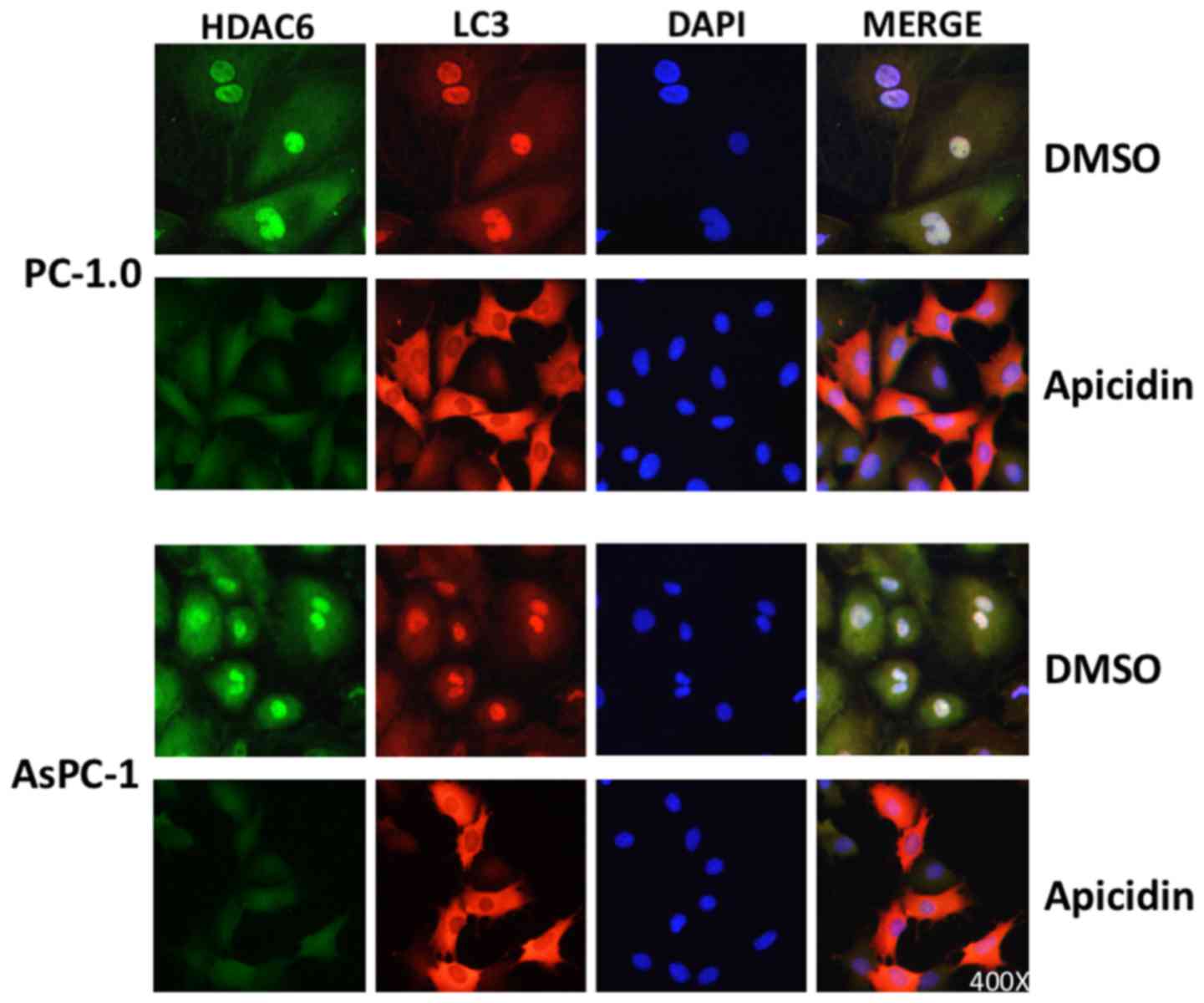
To confirm the association between HDAC6 and
autophagic activity in pancreatic cancer cells, immunofluorescence
was performed on PC-1.0 and AsPC-1 cells using LC3 and HDAC6
antibodies. The immunofluorescence data revealed that LC3 was
initially located in the nucleus and cytoplasm (Fig. 5). However, after a 2 h treatment with
apicidin, the expression of LC3 was markedly increased in the
cytoplasm. Additionally, the results revealed that a decrease in
HDAC6 expression was associated with autophagic activity and
suggests that HDAC6 may serve a key function in modulating
autophagy activity in pancreatic cancer cells.
Discussion
Pancreatic cancer is known to be a major cause of
cancer mortality globally, with the overall survival time of the
majority of patients being <1 year following diagnosis (40,41), and
the 5-year survival rate being <5% of all patients (4,5). At time
of diagnosis, extensive local invasion and metastasis are observed
in patients, thereby precluding curative surgical resection
(42). Therefore, understanding the
molecular mechanisms underlying high propensity of pancreatic
cancer for invasion and metastasis and developing novel therapeutic
targets for pancreatic cancer is important.
miRNAs have been revealed to serve important
functions in the generation or development of various types of
cancer (16–18). The expression and functions of
cancer-related miRNAs in pancreatic cancer have been extensively
investigated (43,44). A recent study by the present authors
indicated that miR-221 was found to be differentially expressed
between highly and weakly invasive PC cells and may serve an
important role in the regulation of pancreatic cancer (24). This previous data suggests that
miR-221 may function as a tumor suppressor in pancreatic cancer.
miR-221 is highly expressed in various cancer-derived cells,
including pancreatic carcinoma, prostate carcinoma and thyroid
carcinoma cells (45–47).
However, the function of miR-221 in pancreatic
cancer remains to be determined. In the present study, the
carcinogenic function of miR-221 in pancreatic cancer was examined.
Flow cytometric analysis revealed that transfection with miR-221
mimic significantly increased the apoptotic rate of PC-1.0 and
AsPC-1 cells. Furthermore, western blot analysis also revealed that
miR-221 may promote autophagy in pancreatic cancer cells.
HDAC6, a distinct cytoplasmic deacetylase, targets
tubulin, heat shock protein 90 and cortactin and therefore may
regulate cell adhesion, motility and chaperone function (48). HDAC6 has been demonstrated to be
involved in carcinogenic transformation and may modulate the
epithelial mesenchymal transition in several types of cancer by
regulating a number of critical cellular components. Accumulating
evidence indicates that the expression of HDAC6 is associated with
oncogenic transformation, anchorage-independent proliferation and
tumor aggressiveness (49,50). In the present study, a western blot
analysis was performed to reveal that the expression of HDAC6 was
suppressed by miR-221 in PC-1.0 and AsPC-1 cells. The
immunofluorescence data on PC-1.0 and AsPC-1 cells revealed that
LC3 was markedly increased in the cytoplasm following treatment
with apicidin, compared with its location in the nucleus and
cytoplasm in untreated cells. The results additionally revealed
that a decrease in HDAC6 expression may increase autophagic
activity and suggested that HDAC6 may serve a key function in
modulating autophagic activity in pancreatic cancer cells. The
results of the present study indicated that HDAC6 serves as a
target of miR-221 and is function-related in pancreatic cancer
cells. Taken together with previous studies, it appears that
miR-221 may promote autophagy by downregulating HDAC6 expression in
pancreatic cancer cells.
In conclusion, miR-221 may significantly induce
autophagy by negatively regulating HDAC6 expression and inducing
the apoptosis of pancreatic cancer cells. Increasing the levels of
miR-221 expression may be a potentially beneficial treatment
strategy for patients with pancreatic cancer.
Acknowledgements
The authors wish to acknowledge Professor Hideo Baba
(Department of Gastroenterological Surgery, Graduate School of
Medical Sciences, Kumamoto University, Kumamoto, Japan) for the
gift of the PC-1.0 cell line.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 30973501).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY, YS and XT conceived and designed the study. YY,
YS, HW, XM, YW, PL, XL and JZ performed the study. HL, YY, MZ and
LZ analyzed the data. YY wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Zhang S, Zhao P, Zeng H,
Zou X and He J: Annual report on status of cancer in China, 2010.
Chin J Cancer Res. 26:48–58. 2014.PubMed/NCBI
|
|
2
|
Rossi RE, Massironi S, Conte D and
Peracchi M: Therapy for metastatic pancreatic neuroendocrine
tumors. Ann Transl Med. 2:82014.PubMed/NCBI
|
|
3
|
Arslan C and Yalcin Y: Current and future
systemic treatment options in metastatic pancreatic cancer. J
Gastrointest Oncol. 5:280–295. 2014.PubMed/NCBI
|
|
4
|
Rozengurt E: Mechanistic target of
rapamycin (mTOR): A point of convergence in the action of
insulin/IGF-1 and G protein-coupled receptor agonists in pancreatic
cancer cells. Front Physiol. 5:3572014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Song R and Cui M: Numerical
simulation on hydromechanical coupling in porous media adopting
three-dimensional pore-scale model. ScientificWorldJournal.
2014:1412062014.
|
|
6
|
Costello E and Neoptolemos JP: Pancreatic
cancer in 2010: New insights for early intervention and detection.
Nat Rev Gastroenterol Hepatol. 8:71–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hidalgo M: Pancreatic Cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ducreux M, Boige V and Malka D: Treatment
of advanced pancreatic cancer. Semin Oncol. 34 2 Suppl 1:S25–S30.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blaszkowsky L: Treatment of advanced and
metastatic pancreatic cancer. Front Biosci. 3:E214–E225. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song B, Zhang C, Li G, Jin G and Liu C:
MiR-940 inhibited pancreatic ductal adenocarcinoma growth by
targeting MyD88. Cell Physiol Biochem. 35:1167–1177. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ambros V: MicroRNAs: Tiny regulators with
great potential. Cell. 107:823–826. 2011. View Article : Google Scholar
|
|
14
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
Downing JR, et al: MicroRNA expression profiles classify human
cancers. Nature. 435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song W, Li Q and Wang L and Wang L:
Modulation of FoxO1 expression by miR-21 to promote growth of
pancreatic ductal adenocarcinoma. Cell Physiol Biochem. 35:184–190.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee RC, Feinbaum RL and Ambros V: The
C.elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shenouda SK and Alahari SK: MicroRNA
function in cancer: Oncogene or a tumor suppressor? Cancer
Metastasis Rev. 28:369–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iorio MV and Croce CM: MicroRNAs in
cancer: Small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Palumbo S, Miracco C, Pirtoli L and
Comincini S: Emerging roles of microRNA in modulating cell-death
processes in malignant glioma. J Cell Physiol. 229:277–286. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ghosh A and Berger A: Opioids, adjuvants,
and interventional options for pain management of symptomatic
metastases. Ann Palliat Med. 3:172–191. 2014.PubMed/NCBI
|
|
21
|
Francis T, Graf A, Hodges K, Kennedy L,
Hargrove L, Price M, Kearney K and Francis H: Histamine regulation
of pancreatitis and pancreatic cancer: A review of recent findings.
Hepatobiliary Surg Nutr. 2:216–226. 2013.PubMed/NCBI
|
|
22
|
Wang J, Paris PL, Chen J, Ngo V, Yao H,
Frazier ML, Killary AM, Liu CG, Liang H, Mathy C, et al: Next
generation sequencing of pancreatic cyst fluid microRNAs from low
grade-benign and high grade-invasive lesions. Cancer Lett.
356:404–409. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Raimondo M, Guha S, Chen J, Diao
L, Dong X, Wallace MB, Killary AM, Frazier ML, Woodward TA, et al:
Circulating microRNAs in pancreatic juice as candidate biomarkers
of pancreatic cancer. J Cancer. 5:696–705. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan X, Zhou L, Wang H, Yang Y, Sun Y, Wang
Z, Zhang X, Gao F and Li H: Differential expression profiles of
microRNAs in highly and weakly invasive and metastatic pancreatic
cancer cells. Oncol Lett. (In Press).
|
|
25
|
Egami H, Takiyama Y, Cano M, Houser WH and
Pour PM: Establishment of hamster pancreatic ductal carcinoma cell
line (PC-1) producing blood group-related antigens. Carcinogenesis.
10:861–869. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Egami H, Tomioka T, Tempero M, Kay D and
Pour PM: Development of intrapancreatic transplantable model of
pancreatic duct adenocarcinoma in Syrian golden hamsters. Am J
Pathol. 138:557–561. 1991.PubMed/NCBI
|
|
27
|
Giaginis C, Damaskos C, Koutsounas I,
Zizi-Serbetzoglou A, Tsoukalas N, Patsouris E, Kouraklis G and
Theocharis S: Histone deacetylase (HDAC)-1, −2, −4 and −6
expression in human pancreatic adenocarcinoma: Associations with
clinicopathological parameters, tumor proliferative capacity and
patients' survival. BMC Gastroenterol. 15:1482015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Ruijter AJ, van Gennip AH, Caron HN,
Kemp S and van Kuilenburg AB: Histone deacetylases (HDACs):
Characterization of the classical HDAC family. Biochem J.
370:737–749. 2013. View Article : Google Scholar
|
|
29
|
Marks P, Rifkind RA, Richon VM, Breslow R,
Miller T and Kelly WK: Histone deacetylases and cancer: Causes and
therapies. Nat Rev Cancer. 1:194–202. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Seigneurin-Berny D, Verdel A, Curtet S,
Lemercier C, Garin J, Rousseaux S and Khochbin S: Identification of
components of the murine histone deacetylase 6 complex: Link
between acetylation and ubiquitination signaling pathways. Mol Cell
Biol. 21:8035–8044. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hubbert C, Guardiola A, Shao R, Kawaguchi
Y, Ito A, Nixon A, Yoshida M, Wang XF and Yao TP: HDAC6 is a
microtubule-associated deacetylase. Nature. 417:455–458. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao YS, Hubbert CC, Lu J, Lee YS, Lee JY
and Yao TP: Histone deacetylase 6 regulates growth factor-induced
actin remodeling and endocytosis. Mol Cell Biol. 27:8637–8647.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang X, Yuan Z, Zhang Y, Yong S,
Salas-Burgos A, Koomen J, Olashaw N, Parsons JT, Yang XJ, Dent SR,
et al: HDAC6 modulates cell motility by altering the acetylation
level of cortactin. Mol Cell. 27:197–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lafarga V, Aymerich I, Tapia O, Mayor F Jr
and Penelab P: A novel GRK2/HDAC6 interaction modulates cell
spreading and motility. EMBO J. 31:856–869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshida N, Omoto Y, Inoue A, Eguchi H,
Kobayashi Y, Kurosumi M, Saji S, Suemasu K, Okazaki T, Nakachi K,
et al: Prediction of prognosis of estrogen receptor-positive breast
cancer with combination of selected estrogen-regulated genes.
Cancer Sci. 95:496–502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iwata A, Riley BE, Johnston JA and Kopito
RR: HDAC6 and microtubules are required for autophagic degradation
of aggregated huntingtin. J Biol Chem. 280:40282–40292. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bae HJ, Jung KH, Eun JW, Shen Q, Kim HS,
Park SJ, Shin WC, Yang HD, Park WS, Lee JY and Nam SW: MicroRNA-221
governs tumor suppressor HDAC6 to potentiate malignant progression
of liver cancer. J Hepatol. 63:408–419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livaka KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
39
|
Rasband WS and Image J US: National
Institutes of Health. Bethesda, Maryland, USA: https://imagej.nih.gov/ij/1997–2016
|
|
40
|
Ma J, Siegel R and Jemal A: Pancreatic
cancer death rates by race among US men and women, 1970–2009. J
Natl Cancer Inst. 105:1694–1700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu
L and He J: Report of incidence and mortality in China cancer
registries, 2009. Chin J Cancer Res. 25:10–21. 2013.PubMed/NCBI
|
|
42
|
Yamamoto H, Itoh F, Iku S, Adachi Y,
Fukushima H, Sasaki S, Mukaiya M, Hirata K and Imai K: Expression
of matrix metalloproteinases and tissue inhibitors of
metalloproteinases in human pancreatic adenocarcinomas:
Clinicopathologic and prognostic significance of matrilysin
expression. J Clin Oncol. 19:1118–1127. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Saito Y, Suzuki H and Hibi T: The role of
microRNAs in gastrointestinal cancers. J Gastroenterol. 44:18–22.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ryu JK, Hong SM, Karikari CA, Hruban RH,
Goggins MG and Maitra A: Aberrant MicroRNA-155 expression is an
early event in the multistep progression of pancreatic
adenocarcinoma. Pancreatology. 10:66–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Davis BN, Hilyard AC, Nguyen PH, Lagna G
and Hata A: Induction of MicroRNA-221 by platelet-derived growth
factor signaling is critical for modulation of vascular smooth
muscle phenotype. J Biol Chem. 284:3728–3738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kawaguchi T, Komatsu S, Ichikawa D,
Morimura R, Tsujiura M, Konishi H, Takeshita H, Nagata H, Arita T,
Hirajima S, et al: Clinical impact of circulating miR-221 in plasma
of patients with pancreatic cancer. Br J Cancer. 108:361–369. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
le Sage C, Nagel R, Egan DA, Schrier M,
Mesman E, Mangiola A, Anile C, Maira G, Mercatelli N, Ciafrè SA, et
al: Regulation of the p27(Kip1) tumor suppressor by miR-221 and
miR-222 promotes cancer cell proliferation. EMBO J. 26:3699–3708.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li J, Lau G, Chen L, Yuan YF, Huang J, Luk
JM, Xie D and Guan XY: Interleukin 23 promotes hepatocellular
carcinoma metastasis via NF-Kappa B induced matrix
metalloproteinase 9 expression. PLoS One. 7:e462642012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lee YS, Lim KH, Guo X, Kawaguchi Y, Gao Y,
Barrientos T, Ordentlich P, Wang XF, Counter CM and Yao TP: The
cytoplasmic deacetylase HDAC6 is required for efficient oncogenic
tumorigenesis. Cancer Res. 68:7561–7569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shan B, Yao T, Nguyen HT, Zhuo Y, Levy DR,
Klingsberg RC, Tao H, Palmer ML, Holder KN and Lasky JA:
Requirement of HDAC6 for transforming growth factor-1-induced
epithelial-mesenchymal transition. J Biol Chem. 283:21065–21073.
2008. View Article : Google Scholar : PubMed/NCBI
|