Introduction
Osteosarcoma, also known as osteogenic sarcoma, is
the most common type of bone malignancy. Despite its low incidence
in the general population (<1/100,000), osteosarcoma is one of
the primary causes of cancer-associated mortality among young
adults and children (1,2). Osteosarcoma mainly affects long bones,
and commonly develops in the distal femur (43%), proximal tibia
(23%) and humerus (10%) (3). Patients
with osteosarcoma usually suffer from swelling combined with severe
pain in the affected bone (4). Due to
great efforts being made regarding the prevention and treatment of
this disease, the long-term survival rate of patients has increased
to 70%; however, ~20% of patients exhibit distant tumor metastasis
at the time of diagnosis, which markedly impairs their survival
(4). The early diagnosis of
osteosarcoma and the development of efficient treatment are
therefore critical to improve the survival rate of patients with
osteosarcoma.
Osteosarcoma progression is a complex and multi-step
process involving numerous external and internal factors (5). It has been revealed that progression of
osteosarcoma is accompanied by alterations in the expression
pattern of a large set of long non-coding RNAs (lncRNAs) (6). lncRNAs consist of >200 nucleotides
and have critical functions in normal and pathological processes
(7–9).
E2F-mediated cell proliferation enhancing lncRNA (EPEL) is a novel
lncRNA involved in the regulation of lung cancer cell proliferation
(10); however, its role in other
types of cancer remains unknown. In the present study, the role of
EPEL in osteosarcoma was investigated. The results demonstrated
that EPEL may promote the migration and invasion of osteosarcoma
cells by upregulating Rho-associated coiled-coil containing protein
kinase 1 (ROCK1).
Materials and methods
Patients
The present study recruited 39 patients with
osteosarcoma who were diagnosed and treated at the Jingzhou Central
Hospital (Hubei, China) between March 2009 and January 2013.
Following diagnosis, patients with other types of malignancies or
severe diseases were not included. The 39 patients included 22 men
and 17 women, aged between 11 and 54 years (mean age, 32±7.7
years). Distant tumor metastases were found in 22 cases. In
addition, 42 healthy volunteers were included as the control group.
This group included 22 men and 20 women, aged between 15 and 52
years (mean age, 34±7.1 years). Following osteosarcoma tumor
resection, all patients were followed-up for 5 years to record
survival conditions. The study was approved by the Ethics Committee
of the Jingzhou Central Hospital, and all patients and their
guardians provided written informed consent.
Specimen collection
Patients with osteosarcoma underwent surgical tumor
resection. Tumor tissues and adjacent healthy tissues (5 cm around
the tumor) were collected during surgical resection. Whole blood
(10 ml) was extracted from the elbow vein of patients and healthy
controls. Blood was maintained at room temperature for 2 h, and
centrifuged at 1,175 × g for 20 min to collect serum. All specimens
were stored in liquid nitrogen for long-term use.
Cell lines and cell culture
The normal bone cell line hFOB, and osteosarcoma
cell lines U2OS, MG-63 and SAOS-2, were purchased from the American
Type Culture Collection (ATCC, Manassas, VA, USA). The SAOS-2 and
MG-63 cell lines were cultured in Eagle's minimum essential medium
(cat. no. 30-2003; ATCC) supplemented with 10% heat-inactivated
fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). The U2OS and hFOB cell lines were cultured in
ATCC-formulated McCoy's 5a medium (cat. no. 30-2007; ATCC)
supplememted with 10% FBS. All cells were cultured at 37°C in a
humidified incubator containing 5% CO2. No serum was
added to the culture media during treatment with Stemolecule™ ROCK
I Inhibitor (10 nM; cat. no. 203911-26-6; Stemgent, Inc.,
Cambridge, MA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Tumor and adjacent healthy tissues were ground in
liquid nitrogen, and incubated with TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), in
order to extract total RNA. TRIzol® reagent was directly
mixed with serum samples and in vitro cultivated cells to
extract total RNA. The NanoDrop™ 2000 Spectrophotometer (NanoDrop;
Thermo Fisher Scientific, Inc., Wilmington, DE, USA) was used to
determine the quantity and quality of extracted RNA. The RNA
samples of satisfactory quality (A260/A280 between 1.8 and 2.0)
were subjected to reverse transcription using SuperScript III
Reverse Transcriptase (Thermo Fisher Scientific, Inc.) to
synthesize cDNA according to the manufacturers protocol. The PCR
reaction system was prepared using SYBR®−Green Real-Time
PCR Master Mixes (Thermo Fisher Scientific, Inc.) with the
following primers: EPEL forward, 5′-GAGGCAGACCACGTGAGAG-3′ and
reverse, 5′-CAGATTTAAACCCCGCACTG-3′; β-actin forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′.
PCR reactions were conducted using a CFX96 Touch™ Real-Time PCR
Detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
with the following reaction conditions: 95°C for 50 sec, followed
by 40 cycles at 95°C for 10 sec and 60°C for 40 sec. Data analysis
was performed using the 2−ΔΔCq method (11) and EPEL expression was normalized to
the endogenous control β-actin.
Construction of EPEL expression vector
and transfection
Full length EPEL cDNA was provided by Sangon Biotech
Co., Ltd., (Shanghai, China) and inserted into a pIRSE2-EGFP vector
(Clontech Laboratories, Inc., Mountainview, CA, USA) to construct
an EPEL expression vector. The EPEL small interfering (si)RNA,
5′-UACAAAACUCUGGAACCUC(dTdT)-3′ and negative control siRNA,
5′-CCUACGCCACCAAUUUCGU(dTdT)-3′ were synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China). U2OS, MG-63 and SAOS-2
cells were cultured overnight to reach 80–90% confluence prior to
transfection. Lipofectamine® 2000 reagent (cat. no.
11668-019; Invitrogen; Thermo Fisher Scientific, Inc.) was used to
transfect cells (5×105/sample) with 10 nM vector or 50
nM siRNA. Transfection with an empty vector or negative control
siRNA was used as a negative control. Overexpression rate >200%
and knockdown rate <50% were confirmed by RT-qPCR compared with
control cells.
Cell migration and invasion
assays
Cells were collected during the logarithmic growth
phase 24 h post-transfection, and single cell suspensions of
5×104 cells/ml were prepared. Cell migration and
invasion were measured by Transwell migration and invasion assays.
For the migration assay, 5×104 cells in 0.1 ml
serum-free culture medium were added into the upper chamber, and
the lower chamber was filled with RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 20% fetal calf serum
(Sigma-Aldrich; Merck KGaA). After 24 h, membranes were collected
and stained with 0.5% crystal violet (Sigma-Aldrich; Merck KGaA) at
room temperature for 20 min. The same procedure was followed for
the invasion assay, with the exception that the upper chamber was
pre-coated with Matrigel (cat. no. 356234; EMD Millipore,
Billerica, MA, USA). Cells were observed using the CX33 optical
microscope (Olympus Corporation, Tokyo, Japan). In cases of
Stemolecule™ ROCK I Inhibitor (10 nM; cat. no. 203911-26-6;
Stemgent, Inc.) treatment, cells were pretreated with Stemolecule™
ROCK I Inhibitor for 12 h at 37°C in a humidified incubator
containing 5% CO2 before use.
Western blotting
Cells were collected 3 days post-transfection. Cells
were mixed with radioimmunoprecipitation assay lysis and extraction
Buffer (Thermo Fisher Scientific, Inc.) on ice to extract the total
protein. The bicinchoninic acid method was used to quantify protein
concentration. SDS-PAGE was performed with a 10% gel (20 µg protein
loaded per lane), followed by protein transfer onto polyvinylidene
fluoride membranes. Membranes were blocked with 5% skimmed milk for
1 h at room temperature, followed by incubation with rabbit
anti-ROCK1 (cat. no. ab45171; 1:2,000; Abcam, Cambridge, UK) and
anti-β-actin (cat. no. ab8227; 1:1,000; Abcam) primary antibodies
overnight at 4°C. Membranes were subsequently incubated with the
anti-rabbit immunoglobulin G-horseradish peroxidase secondary
antibody (cat. no. MBS435036; 1:1,000; MyBioSource, San Diego, CA,
USA) or goat anti-mouse IgG (H+L, cat. no. A-11001, Thermo Fisher
Scientific, Inc.) for 2 h at room temperature. Enhanced
chemiluminescence detection reagent (Sigma-Aldrich; Merck KGaA) was
used to measure signal development. Relative expression levels of
ROCK1 were normalized to the endogenous control β-actin using
ImageJ v1.46 (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used for
all statistical analyses. All data are presented as the means ±
standard deviation, and comparisons among multiple groups were
performed using one-way analysis of variance and the least
significant difference test. The receiver operating characteristic
(ROC) curve analysis was performed to evaluate the diagnostic value
of serum EPEL for osteosarcoma. Patients were then divided into the
high expression group (n=20) and low expression group (n=19)
according to the median serum levels of EPEL. Survival curves of
these two groups were plotted using the Kaplan-Meier method, and
compared by log rank test. Categorical data were processed by
χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of EPEL in tumor and
adjacent healthy tissues of 39 patients with osteosarcoma
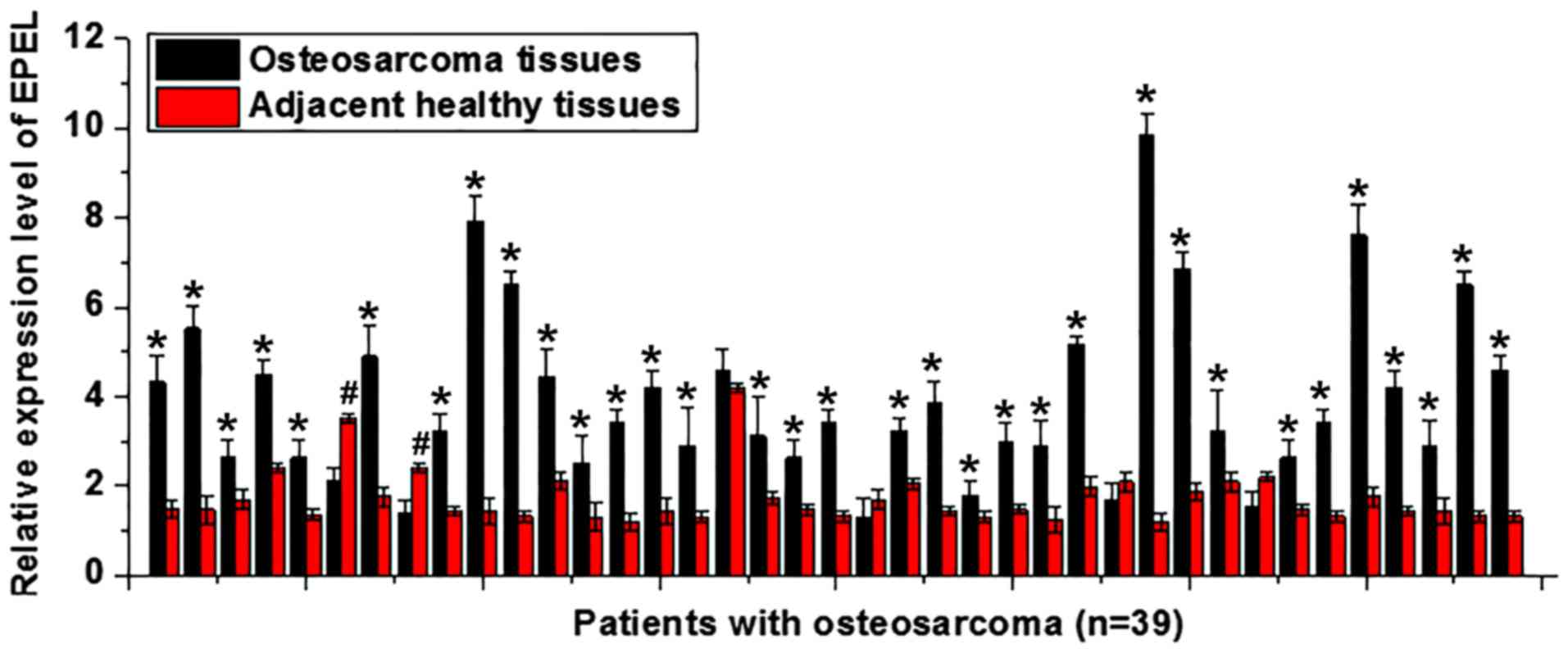
EPEL expression levels in osteosarcoma and adjacent
healthy tissues were detected by RT-qPCR. As illustrated in
Fig. 1, in samples from ~85% (33/39)
of patients, the expression levels of EPEL were upregulated in
tumor tissues compared with adjacent healthy tissues (P<0.05). A
total of 2 patients had significantly higher expression levels of
EPEL in adjacent healthy tissues compared with those in tumor
tissues (P<0.05). No significant differences were observed in
the remaining three patients (P>0.05). The upregulation of EPEL
may therefore be involved in the pathogenesis of osteosarcoma.
Expression levels of EPEL in the serum
of healthy controls and patients with osteosarcoma, and diagnostic
and prognostic values
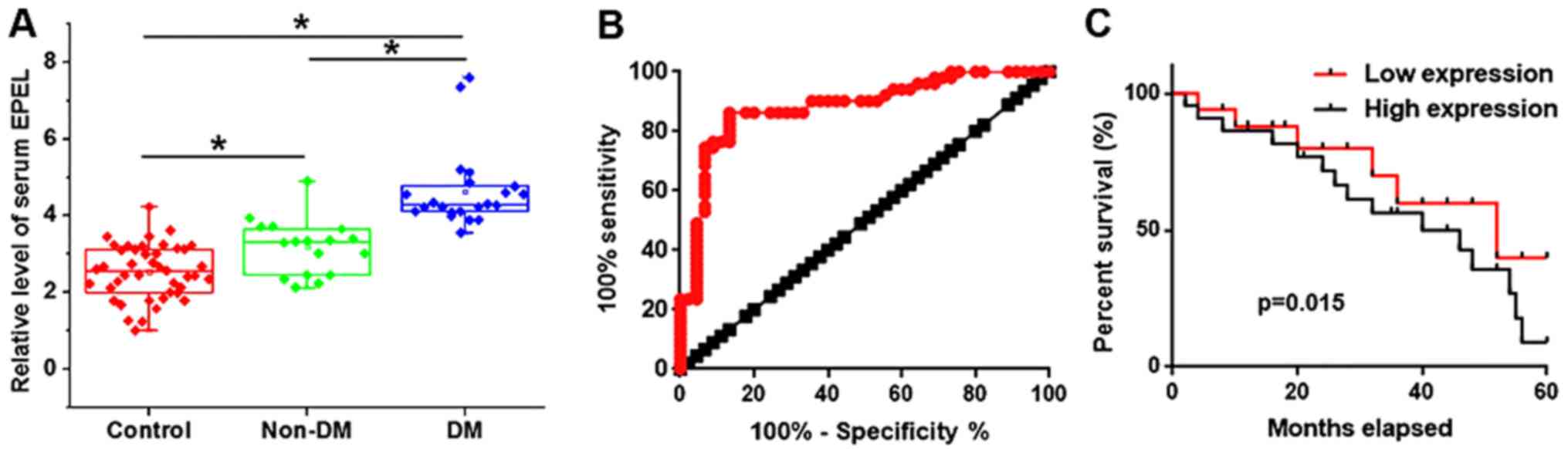
Distant tumor metastasis was observed in 22
patients, who formed the distant metastasis (DM) group. The
remaining 17 patients formed the non-distant metastasis (non-DM)
group. The expression levels of EPEL in the two groups were
detected by RT-qPCR. As illustrated in Fig. 2A, serum levels of EPEL were higher in
the DM and non-DM groups compared with the control group
(P<0.05). In addition, serum levels of EPEL were higher in the
DM group compared with the non-DM group (P<0.05). The receiver
operating characteristic (ROC) curve analysis was performed to
evaluate the diagnostic value of serum EPEL for osteosarcoma. As
shown in Fig. 2B, the area under the
curve was 0.8817, with a 95% confidence interval of 0.8111–0.9523
(P<0.0001), suggesting that serum EPEL may serve as a potential
biomarker for osteosarcoma. Patients were then divided into the
high expression (n=20) and low expression (n=19) groups according
to the median serum levels of EPEL. Survival curves of these two
groups were plotted using the Kaplan-Meier method and compared by
log rank test. As illustrated in Fig.
2C, the overall survival rate of patients in the high
expression group was significantly lower than that in the low
expression group (P=0.015), suggesting that high serum levels of
EPEL may be associated with poor survival of patients with
osteosarcoma.
Association between serum levels of
EPEL and clinical data of patients with osteosarcoma
Associations between serum levels of EPEL (high and
low) and the clinical data of patients with osteosarcoma were
analyzed by χ2 test. As displayed in Table I, serum levels of EPEL were not
associated with sex, age, tumor size or lifestyle habits, including
smoking and drinking. However, EPEL serum levels were significantly
associated with distant tumor metastasis.
 | Table I.Association between serum levels of
EPEL and clinical data of patients with osteosarcoma. |
Table I.
Association between serum levels of
EPEL and clinical data of patients with osteosarcoma.
| Variable | Cases | High-expression | Low-expression | χ2 | P-value |
|---|
| Sex |
|
|
| 0.69 | 0.41 |
| Male | 22 | 10 | 12 |
|
|
|
Female | 17 | 10 | 7 |
|
|
| Age (years) |
|
|
| 0.65 | 0.42 |
|
>35 | 19 | 11 | 8 |
|
|
|
<35 | 20 | 9 | 11 |
|
|
| Drinking |
|
|
| 0.64 | 0.42 |
| Yes | 27 | 15 | 12 |
|
|
| No | 12 | 5 | 7 |
|
|
| Smoking |
|
|
| 0.63 | 0.43 |
| Yes | 21 | 12 | 9 |
|
|
| No | 18 | 8 | 10 |
|
|
| Tumor diameter |
|
|
| 1.34 | 0.24 |
| ≥5
cm | 16 | 10 | 6 |
|
|
| <5
cm | 23 | 10 | 13 |
|
|
| Distant
metastasis |
|
|
| 7.69 | 0.006 |
| Yes | 22 | 15 | 5 |
|
|
| No | 17 | 5 | 12 |
|
|
EPEL overexpression upregulates the
expression of ROCK1 in osteosarcoma cell lines, but not in a normal
bone cell line
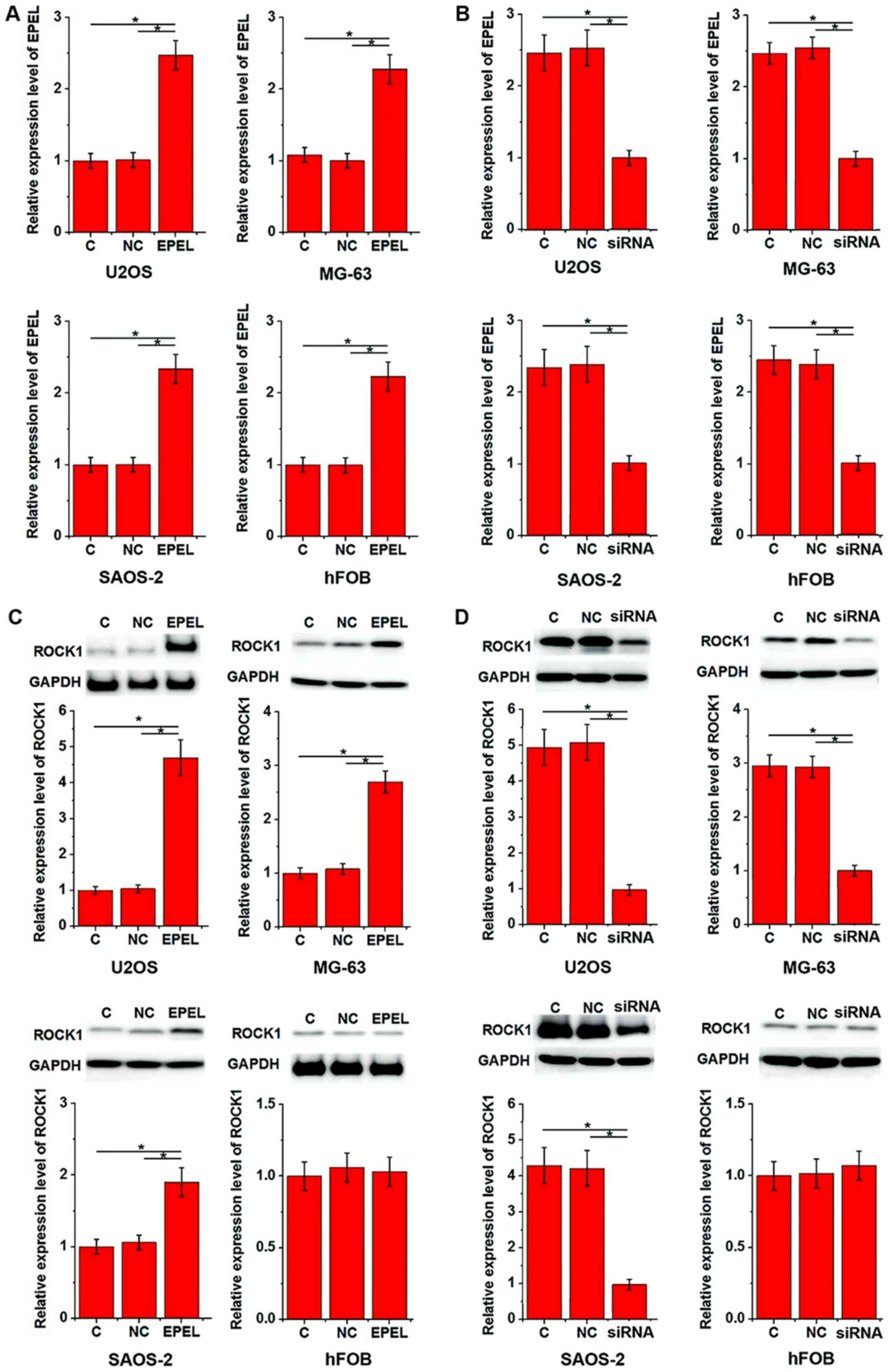
Based on the aforementioned data, it was
hypothesized that EPEL may be involved in osteosarcoma metastasis.
ROCK1 is known to be involved in the migration and invasion of
osteosarcoma cells (10). In the
present study, the EPEL expression vector and siRNA were
transfected into all cell lines prior to assessing ROCK1 expression
levels. As displayed in Fig. 3, EPEL
overexpression (Fig. 3A) and
silencing (Fig. 3B) were reached
following transfection in all cell lines. EPEL overexpression
significantly upregulated the expression of ROCK1 in U2OS, MG-63
and SAOS-2 cell lines (P<0.05), but not in the hFOB cell line
(Fig. 3C). In addition, EPEL
siRNA-induced silencing significantly downregulated the expression
of ROCK1 in U2OS, MG-63 and SAOS-2 cell lines (P<0.05), but not
in hFOB cells (Fig. 3D).
EPEL overexpression promotes migration
and invasion of osteosarcoma cells possibly by upregulating
ROCK1
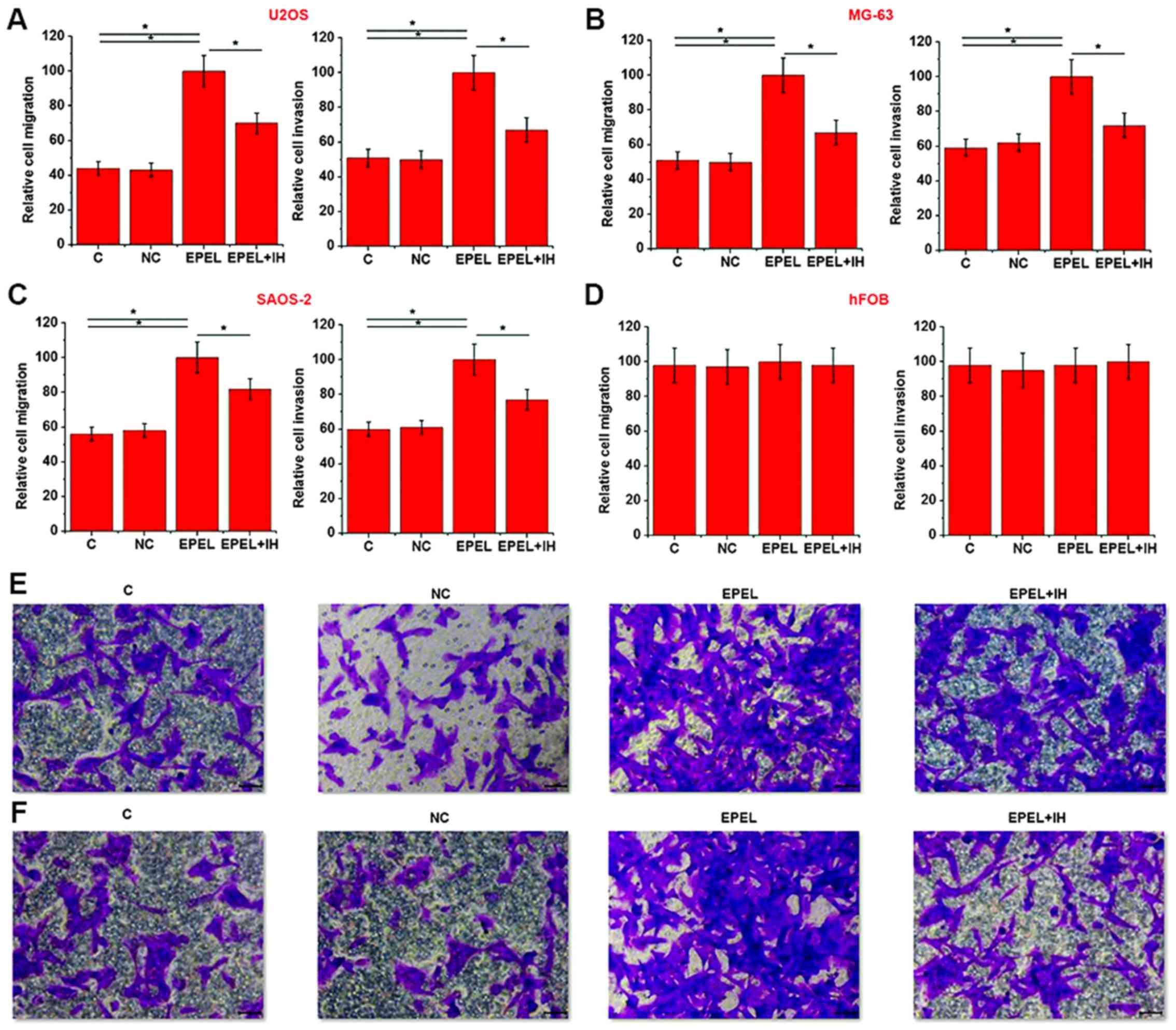
Transwell migration and invasion assays were
performed to investigate the effects of EPEL overexpression on cell
migration and invasion of the osteosarcoma cell lines U2OS, MG-63
and SAOS-2, and the normal bone cell line hFOB. As illustrated in
Fig. 4, EPEL expression vector
transfection significantly promoted cell migration and invasion of
the osteosarcoma cell lines U2OS (Fig.
4A), MG-63 (Fig. 4B) and SAOS-2
(Fig. 4C) (P<0.05), but not the
normal bone cell line hFOB (Fig. 4D)
(P>0.05). However, cell treatment with Stemolecule™ ROCK I
Inhibitor (10 nM; cat no. 203911-26-6; Stemgent, Inc.)
significantly reduced the enhancing effects of EPEL overexpression
on cell migration and invasion of osteosarcoma cell lines. Fig. 4E and F represent cell migration and
invasion results corresponding to Fig.
4A, respectively. In addition, EPEL siRNA transfection
significantly inhibited cell migration and invasion of osteosarcoma
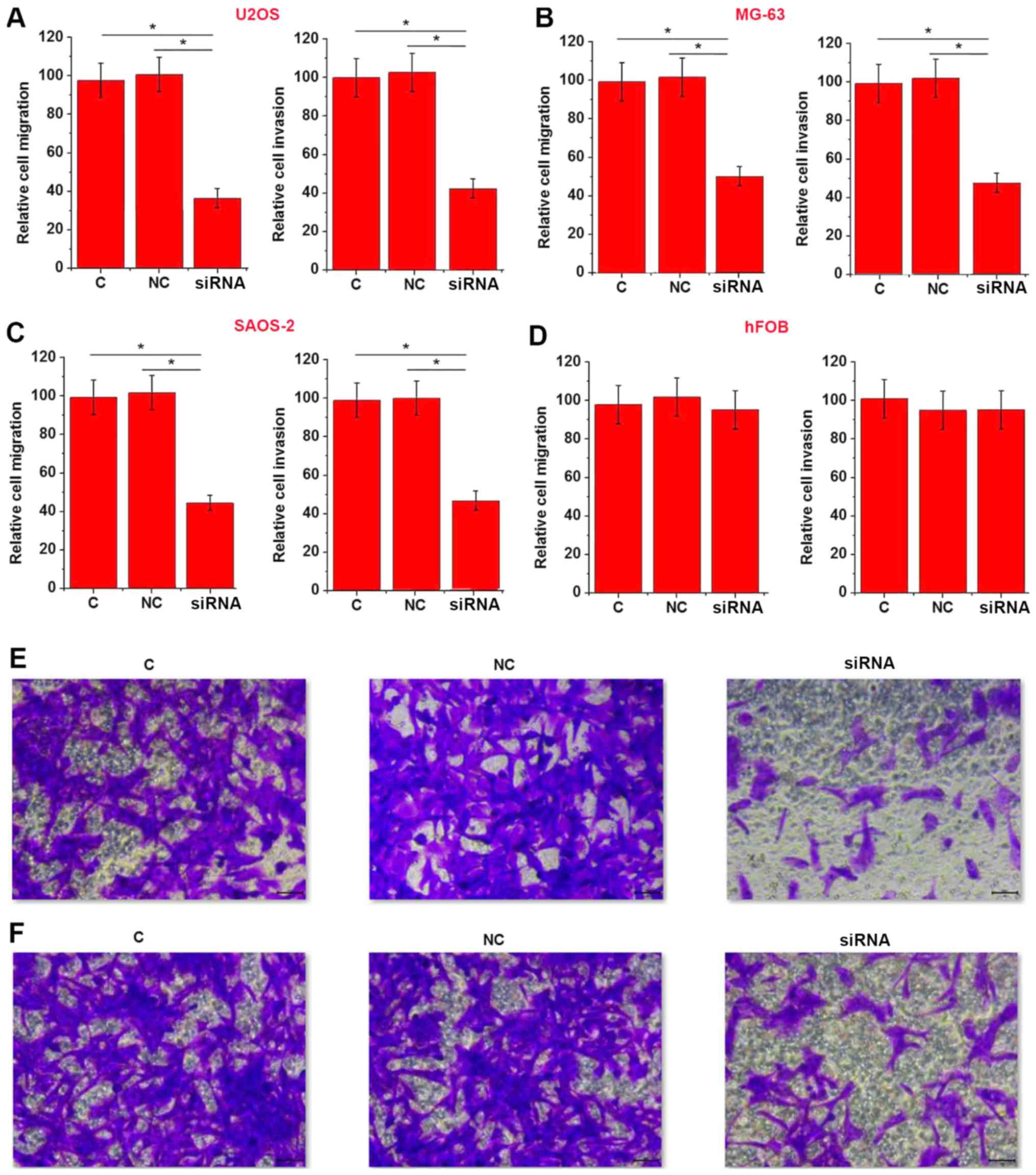
cell lines U2OS (Fig. 5A), MG-63
(Fig. 5B) and SAOS-2 (Fig. 5C) (P<0.05), but not normal bone
cell line hFOB (Fig. 5D) (P>0.05).
Fig. 5E and F represent cell
migration and invasion results corresponding to Fig. 5A, respectively. These data suggested
that EPEL may promote cell migration and invasion of osteosarcoma
cells by upregulating ROCK1.
Discussion
A previous study reported that EPEL is upregulated
in lung cancer (10). The present
study demonstrated that the expression levels of EPEL were
significantly upregulated in the tissue samples of most patients
with osteosarcoma. In addition, serum circulating levels of EPEL
were significnatly higher in patients with osteosarcoma than in
healthy controls, suggesting that EPEL may serve as an oncogene in
the development of osteosarcoma. In addition, a previous study has
demonstrated that EPEL promotes lung cancer cell proliferation
(10), indicating the stimulating
effects of EPEL on tumor growth. In the present study, serum levels
of EPEL were not significantly associated with tumor size,
suggesting that EPEL may not be associated with osteosarcoma
growth. Conversely, EPEL serum levels were significantly associated
with distant tumor metastasis. In addition, transfection with an
EPEL expression vector significantly promoted cell migration and
invasion of osteosarcoma cell lines. These data indicated that EPEL
may be involved in the regulation of osteosarcoma metastasis but
not tumor growth. This suggested that the pathogenesis of
osteosarcoma may be different from that of lung cancer.
According to previous studies, ~20% of patients with
osteosarcoma present distant tumor metastasis at the time of
diagnosis; these patients have a poor prognosis (4,12). Early
diagnosis and treatment are therefore crucial for the survival of
these patients. Development of human diseases is usually
accompanied by blood marker modifications, and monitoring the
changes in these markers aids in the diagnosis of human disease
(13). In the present study, ROC
curve analysis revealed that serum EPEL may be used to distinguish
patients with osteosarcoma from healthy conrols. In addition,
survial analysis indicated that high expression levels of EPEL were
associated with poor patient survival. These data suggested that
serum EPEL may serve as a potential diagnostic and prognostic
biomarker for osteosarcoma. The expression of some lncRNAs however
are affected by internal and external factors, including aging
(14), alcohol consumption (15) and smoking (16), which can affect the accuracy of
certain lncRNAs in the diagnosis of human diseases. In the present
study, serum levels of EPEL were not associated with sex, age,
tumor size or lifestyle habits, including smoking and drinking.
This indicated that EPEL may be highly accurate in the diagnosis
and prognosis of osteosarcoma. In addition, since EPEL is a novel
lncRNA unkown in other diseases except lung cancer, the combined
use of multiple biomarkers, such as alkaline phosphatase, may
imporve the diagnosis and prognosis of osteosarcoma.
ROCK1 is a protein serine/threonine kinase with
prominent functions in cancer cell motility, metastasis and
angiogenesis (17). It is well known
that ROCK1 is involved in the migration and invasion of
osteosarcoma cells; inhibition of ROCK1 may therefore serve as a
potential therapeutic target in the treatment of osteosarcoma
(10,18–20). In
the present study, EPEL transfection significantly promoted the
expression of ROCK1 in three osteosarcoma cell lines. In addition,
cell treatment with a ROCK1 inhibitor significantly reduced the
enhancing effects of EPEL overexpression on cell migration and
invasion. These data suggested that EPEL may promote cell migration
and invasion of osteosarcoma cells by upregulating ROCK1. Notably,
EPEL overexpression had no effects on hFOB cells, suggesting that
EPEL may serve a potential therapeutic target in the treatment of
osteosarcoma. However, the study only elucidated EPEL-ROCK1
sequential signaling in osteosarcoma; whether this interaction is
direct or indirect is still unknown and requires further
investigation.
In conclusion, the present study revealed that EPEL
was upregulated in osteosarcoma, and that serum levels of EPEL may
serve as a promising diagnostic and prognostic marker for
osteosarcoma. In addition, EPEL overexpression promoted the
migration and invasion of osteosarcoma cells and ROCK1 expression,
whereas siRNA silencing inhibited these phenomena. Conversely, cell
treatment with a ROCK1 inhibitor reduced the enhancing effects of
EPEL overexpression on cancer cell migration and invasion. These
results suggested that EPEL may promote the migration and invasion
of osteosarcoma cells by upregulating ROCK1. Due to the low
incidence of this disease, only 39 patients were included in this
study. Future studies with a larger sample size are required to
confirm the present findings.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SC designed the experiments. SC and ZL performed the
experiments. SL and BH analyzed data. SC drafted the manuscript and
all authors reviewed and approved the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Jingzhou Central Hospital. All patients and their guardians
provided written informed consent.
Patient consent for publication
Patients signed informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Botter SM, Neri D and Fuchs B: Recent
advances in osteosarcoma. Curr Opin Pharmacol. 16:15–23. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gattia M, Solari A, Pattarozzi A,
Campanella C, Thellung S, Maniscalco L, De Maria R, Würth R,
Corsaro A, Bajetto A, et al: In vitro and in vivo characterization
of stem-like cells from canine osteosarcoma and assessment of drug
sensitivity. Exp Cell Res. 363:48–64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kansara M and Thomas DM: Molecular
pathogenesis of osteosarcoma. DNA Cell Biol. 26:1–18. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li JP, Liu LH, Li J, Chen Y, Jiang XW,
Ouyang YR, Liu YQ, Zhong H, Li H and Xiao T: Microarray expression
profile of long noncoding RNAs in human osteosarcoma. Biochem
Biophys Res Commun. 433:200–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao JZ, Li J, DU JL and Li XL: Long
non-coding RNA HOTAIR is a marker for hepatocellular carcinoma
progression and tumor recurrence. Oncol Lett. 11:1791–1798. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ning S, Zhang J, Wang P, Zhi H, Wang J,
Liu Y, Gao Y, Guo M, Yue M, Wang L and Li X: Lnc2Cancer: A manually
curated database of experimentally supported lncRNAs associated
with various human cancers. Nucleic Acids Res. 44:D980–D985. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Augoff K, McCue B, Plow EF and
Sossey-Alaoui K: miR-31 and its host gene lncRNA LOC554202 are
regulated by promoter hypermethylation in triple-negative breast
cancer. Mol Cancer. 11:52012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Zhao W and Fu Q: miR-335
suppresses migration and invasion by targeting ROCK1 in
osteosarcoma cells. Mol Cell Biochem. 384:105–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harting MT, Blakely ML, Jaffe N, Cox CS
Jr, Hayes-Jordan A, Benjamin RS, Raymond AK, Andrassy RJ and Lally
KP: Long-term survival after aggressive resection of pulmonary
metastases among children and adolescents with osteosarcoma. J
Pediatr Surg. 41:194–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hori SS and Gambhir SS: Mathematical model
identifies blood biomarker-based early cancer detection strategies
and limitations. Sci Transl Med. 3:109ra–116ra. 2011. View Article : Google Scholar
|
|
14
|
Grammatikakis I, Panda AC, Abdelmohsen K
and Gorospe M: Long noncoding RNAs (lncRNAs) and the molecular
hallmarks of aging. Aging (Albany NY). 6:992–1009. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mayfield RD: Emerging roles for ncRNAs in
alcohol use disorders. Alcohol. 60:31–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Qiu M, Xu Y, Li M, Dong G, Mao Q,
Yin R and Xu L: Long noncoding RNA CCAT2 correlates with smoking in
esophageal squamous cell carcinoma. Tumour Biol. 36:5523–5528.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rath N and Olson MF: Rho-associated
kinases in tumorigenesis: Re-considering ROCK inhibition for cancer
therapy. EMBO Rep. 13:900–908. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou X, Wei M and Wang W: MicroRNA-340
suppresses osteosarcoma tumor growth and metastasis by directly
targeting ROCK1. Biochem Biophys Res Commun. 437:653–658. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Choy E, Hornicek FJ, Yang S, Yang
C, Harmon D, Mankin H and Duan Z: ROCK1 as a potential therapeutic
target in osteosarcoma. J Orthop Res. 29:1259–1266. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li E, Zhang J, Yuan T and Ma B: MiR-145
inhibits osteosarcoma cells proliferation and invasion by targeting
ROCK1. Tumour Biol. 35:7645–7650. 2014. View Article : Google Scholar : PubMed/NCBI
|