Introduction
Liver cancer is a major worldwide healthcare
problem, and one of the leading causes of cancer-associated
mortality globally (1–3). For the majority of patients with
advanced cancer, chemotherapy is accompanied by surgery, but the
long-term prognosis of patients with liver cancer is poor (4). Cisplatin is one of the most useful
anticancer agents available for cancer therapy, with the major
pharmacological effect of inducing apoptosis in cancer cells
(5). The success of cisplatin in
anticancer treatment has triggered a search for additional,
platinum (Pt)-based metal complexes with improved anticancer
properties (6). However, severe side
effects, including drug resistance, nephrotoxicity and
gastrointestinal toxicity, have limited the curative effect of
Pt-based complexes (7). Over the last
decade, a new trend in cancer therapy has emerged from
non-inferiority clinical trials testing combinations of
chemotherapy agents with other ancillary medicine. For example, a
combined treatment of cisplatin with gemcitabine has proven to be
the preferred first-line therapy for patients with metastatic
triple-negative breast cancer (8).
Similarly, combinations of cisplatin with other ancillary medicine
may be beneficial in liver cancer therapy. Therefore, the rational
design and synthesis of potent small-molecule drugs, particularly
plant extracts with low toxicity, may enhance the anticancer
efficacy of cisplatin.
Apoptosis, known as programmed cell death, in which
pro-apoptotic B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax),
cleaved caspase-3 and anti-apoptotic apoptosis regulator Bcl-2 are
commonly used as markers, is accepted as the principal cellular
mechanism contributing to the chemotherapeutic effects of
anticancer drugs, including cisplatin, and is thus associated with
the progression of cancer and the therapy of cancer cells (6). Another biological process, autophagy,
which serves an essential role in cell development, tissue
homeostasis and disease, is considered to exhibit positive and
negative effects in the initiation, development and metastasis of
cancer and is therefore of particular importance in this area
(9,10). Autophagy is thought to prevent cancer
development in non-cancerous cells; however, once cancer has
developed, autophagy facilitates tumor cell survival and
proliferation, and protects tumor cells against stress as an
adaptive response (11–13). Beclin-1 (a putative tumor suppressor),
microtubule-associated protein 1 light chain 3 (LC3)I/II and
adaptor sequestosome 1 (SQSTM1; p62) commonly serve as biomarkers
in autophagy (10,11).
Studies have revealed that interactions among
components of apoptosis and autophagy form a complex signaling
network, which is often induced by similar stimuli (14,15). In
mammalian cells, the phosphoinositide 3-kinase (PI3K)/protein
kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling
pathway is key to physiological cellular processes that are
associated with proliferation, differentiation, autophagy,
apoptosis and metabolism (16,17). In
addition, AMP-activated protein kinase (AMPK), a serine/threonine
protein kinase, is a positive regulator of autophagy by
phosphorylating ULK1 at specific sites (18). However, the detailed molecular
mechanisms of various anticancer drugs involved in autophagy and
apoptosis have not yet been fully investigated, and remain poorly
understood.
Ginkgol C17:1, a natural monophenol refined from the
Ginkgo biloba extract (EGb), is one of the most widely
administered agents with spiritual, medicinal and horticultural
importance worldwide (19). Studies
have demonstrated that EGb has many biological benefits, such as
anti-inflammatory, anti-oxidative and anticancer effects (20,21).
Consistent with the study by Chen et al (22), our previous study revealed that EGb
could effectively inhibit cell division and induce apoptosis in
cancer cell line SMMC-7721 (23).
Recently, ginkgol C17:1 was demonstrated to promote
cisplatin-induced apoptosis and suppress cisplatin-induced
autophagy in liver cancer HepG2 cells (24). Since oral administration is the common
route for chemotherapeutical agents and auxiliary chemotherapy
drugs, normal cells are inevitably also exposed to chemotherapy.
However, as a DNA damaging agent, cisplatin does not selectively
target cancer cells, it affects normal cells as well. The
cytotoxicity to normal cells may be the main cause of
cisplatin-induced side effects. To date, little information exists
on normal hepatocytes treated with ginkgol C17:1 monotherapy or
co-treatment with cisplatin.
In the present study, hepatoma cells and normal
hepatocytes were treated with ginkgol C17:1 and cisplatin in order
to compare their effects on apoptosis and autophagy, and to
investigate underlying molecular mechanisms or pathway
networks.
Materials and methods
Reagents and antibodies
MTT, bisBenzimide H 33342 trihydrochloride (Hoechst
33342) and cisplatin were purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). Penicillin and streptomycin were obtained
from Harbin Pharmaceutical Group, Co., Ltd. (Harbin, China).
Dulbecco's modified Eagle's medium (DMEM), RPMI-1640 medium, fetal
bovine serum (FBS) and trypsin-EDTA solution were bought from
Shanghai ExCell Biology, Inc. (Shanghai, China). Ginkgol C17:1
(high-performance liquid chromatography purity >96.5%) was
obtained from the Laboratory of Food and Biological Engineering
School, Jiangsu University (23,25,26).
Skimmed milk was purchased from Bright Dairy & Food Co., Ltd.
(Harbin, China). Adenovirus (Ad)-monomeric red fluorescent protein
(mRFP)-green fluorescent protein (GFP)-light chain 3 (LC3) was
purchased from Hanheng Biotechnology Co., Ltd. (Shanghai, China;
http://hanhbio.biomart.cn/).
Mouse monoclonal antibody (mAb) against β-actin
(cat. no. sc-47778) was obtained from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Rabbit mAbs against Bax (cat. no. 5023),
cleaved caspase-3 (cat. no. 9661), Beclin-1 (cat. no. 3495),
LC3I/II (cat. no. 12741), phosphorylated (p-)mTOR
(Ser2448; cat. no. 5536), p-ULK1 (Ser555;
cat. no. 5869), p-PI3K (Tyr458; cat. no. 4228) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Anti-Bcl-2 (cat. no. IM001-0363) and anti-p-AKT1/2/3
(Tyr315/316/312; cat. no. IM001-0270) were purchased
from Shanghai ExCell Biology, Inc. Rabbit mAb anti-SQSTM1/p62 (cat.
no. ab91526) and rabbit anti-ULK1 (cat. no. ab128859) were
purchased from Abcam (Cambridge, UK). Mouse anti-AMPKα1 (cat. no.
RLM3361) and anti-p-AMPKα1/2 (Thr172; cat. no. RLM0575)
were obtained from Suzhou Ruiying-Runze Trading Co., Ltd. (Suzhou,
China). Horseradish peroxidase (HRP)-conjugated anti-mouse (cat.
no. A0216) and anti-rabbit (cat. no. A0208) secondary antibodies
were purchased from Beyotime Institute of Biotechnology (Haimen,
China).
Cells and cell culture
Human hepatoma HepG2 cells and human normal L02
hepatocytes were obtained from the Institute of Cell Biology of the
Chinese Academy of Sciences (Shanghai, China). HepG2 cells were
cultured in DMEM and L02 cells were cultured in RPMI-1640 medium
supplemented with 10% FBS at 37°C in a humidified atmosphere
containing 5% CO2. Where indicated, cells were treated
for 24 h with serial dilution concentrations of ginkgol C17:1 (0,
10, 20, 40, 80, or 160 µg/ml) and/or cisplatin (0, 1, 2, 4, 8 or 16
µg/ml), on the basis of previously established concentrations
(24,27).
MTT assay
The cells were pooled and diluted to a density of
105 cells/ml, and 100 µl cell suspension was dispensed
into each well of a 96-well plate. Following incubation for 12 h at
37°C in 5% CO2, the cells were treated with serial
dilutions of ginkgol C17:1 (0, 10, 20, 40, 80, or 160 µg/ml) and/or
cisplatin (0, 1, 2, 4, 8 or 16 µg/ml) for 24 h. A total of 10 µl
MTT (5 mg/ml) was added to each well prior to incubation for an
additional 4–6 h at 37°C. Subsequently, the medium was replaced
with 100 µl dimethylsulfoxide. Following thorough mixing, the
absorbance was measured at an optical density of 490 nm using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Western blotting
Protein extraction was performed according to a
standard procedure outlined in previous studies (24,27). Lysis
buffer (pH 7.4) for protein extraction was composed of 50 mM Tris,
150 mM NaCl, 1 mM ethyl- enediaminetetraacetic acid and 1% Triton
X-100. The sample proteins (5 µg/lane) were separated on 10%
SDS-PAGE (except for LC3 and cleaved caspase 3, which were
separated on 8% SDS-PAGE) and transferred onto polyvinylidene
difluoride (PVDF) membranes (Bio-Rad Laboratories, Inc.). The PVDF
membranes were initially blocked with 5% skimmed milk for 1 h at
room temperature, and were then incubated with the primary
antibodies AKT, p-AKT, AMPK, p-AMPK, β-actin, Bax, Bcl-2, Beclin-1,
cleaved caspase-3, LC3I/II, mTOR, p-mTOR, p62, PI3K, p-PI3K,
ULK1and p-ULK1 (all 1:1,000 dilution) at 4°C overnight. Following
subsequent incubation of the membranes with HRP-conjugated
secondary antibodies (1:1,000 dilution) for 1 h at room
temperature, the western chemiluminescent HRP substrates (EMD
Millipore, Billerica, MA, USA) were applied to reveal the positive
bands on the membrane according to the protocol of the
manufacturer. The bands were detected using MiniChemi™ miniature
chemiluminescence imager (type: MiniChemi 500, software: Lan1D)
(Beijing Sage Creation Science Co. Ltd, Beijing, China).
Cell autophagy flux analysis
Cells were seeded in a 24-well plate to a final
density of 5×104 cells/well. Following incubation for 12
h at 37°C in 5% CO2, the cells were infected with
Ad-mRFP-GFP-LC3 for 12 h and treated with ginkgol C17:1 or/and
cisplatin for 24 h at 37°C. LC3 puncta (green) in different
treatment groups were observed under a fluorescence microscope
(Zeiss, Oberkochen, Germany) at a magnification of ×20.
Calculations were performed (28,29) from
five different wells with same treatment using ImageJ v1.48u
software (imagej.nih.gov). The results are from ≥3
individual experiments.
Hoechst 33342 staining
Cells were cultured in a 24-well plate for 12 h at
37°C in 5% CO2. Following treatment with ginkgol C17:1
or/and cisplatin and incubation at 37°C for 24 h, the cells were
fixed with 4% paraformaldehyde for 2 h at room temperature. To
stain the nucleus, Hoechst 33342 was added to cells for an
additional 15 min (1:5,000 dilution). The cell nuclei in different
treatment groups were visualized under a fluorescence microscope at
a magnification of ×20. The numbers of nucleus aberrations from
five different wells with the same treatment were determined
(24) using Image J. The results are
from ≥3 individual experiments.
Statistical analysis
All data are presented as the mean ± standard
deviation. One-way analysis of variance was used to analyze
differences between experimental groups using SPSS version 16.0
software (SPSS, Inc., Chicago, IL, USA). Dunnett of multiple
comparisons was used as post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of ginkgol C17:1 combined with
cisplatin on cell viability in hepatoma HepG2 and normal L02
hepatocytes
Cell viability was determined using the MTT assay
following 24 h of incubation with ginkgol C17:1 alone, cisplatin
alone or ginkgol C17:1 with cisplatin (co-treatment). As the most
commonly administered chemotherapy drug, cisplatin (2–16 µg/ml)
decreased the viability of hepatoma HepG2 cells (Fig. 1A) and that of normal hepatocytes
(Fig. 1B), in a
concentration-dependent manner. Whereas ginkgol C17:1 significantly
decreased the viability of HepG2 cells at concentrations of 40, 80
and 160 µg/ml (Fig. 1C), this effect
was only observed in L02 cells treated with 80 and 160 µg/ml
ginkgol C17:1 (Fig. 1D). The results
of the co-treatment of cisplatin (2 µg/ml) with ginkgol C17:1 (20,
40 and 80 µg/ml) indicated that ginkgol C17:1 significantly
enhanced the cisplatin-induced inhibition of HepG2 cell viability
(Fig. 1E). However, in normal L02
hepatocytes, enhanced inhibition of cell viability was observed
only for the co-treatment with 80 µg/ml ginkgol C17:1. Notably,
ginkgol C17:1 at the lower concentration of 20 µg/ml reversed the
inhibitory effect of cisplatin in L02 cells (Fig. 1F).
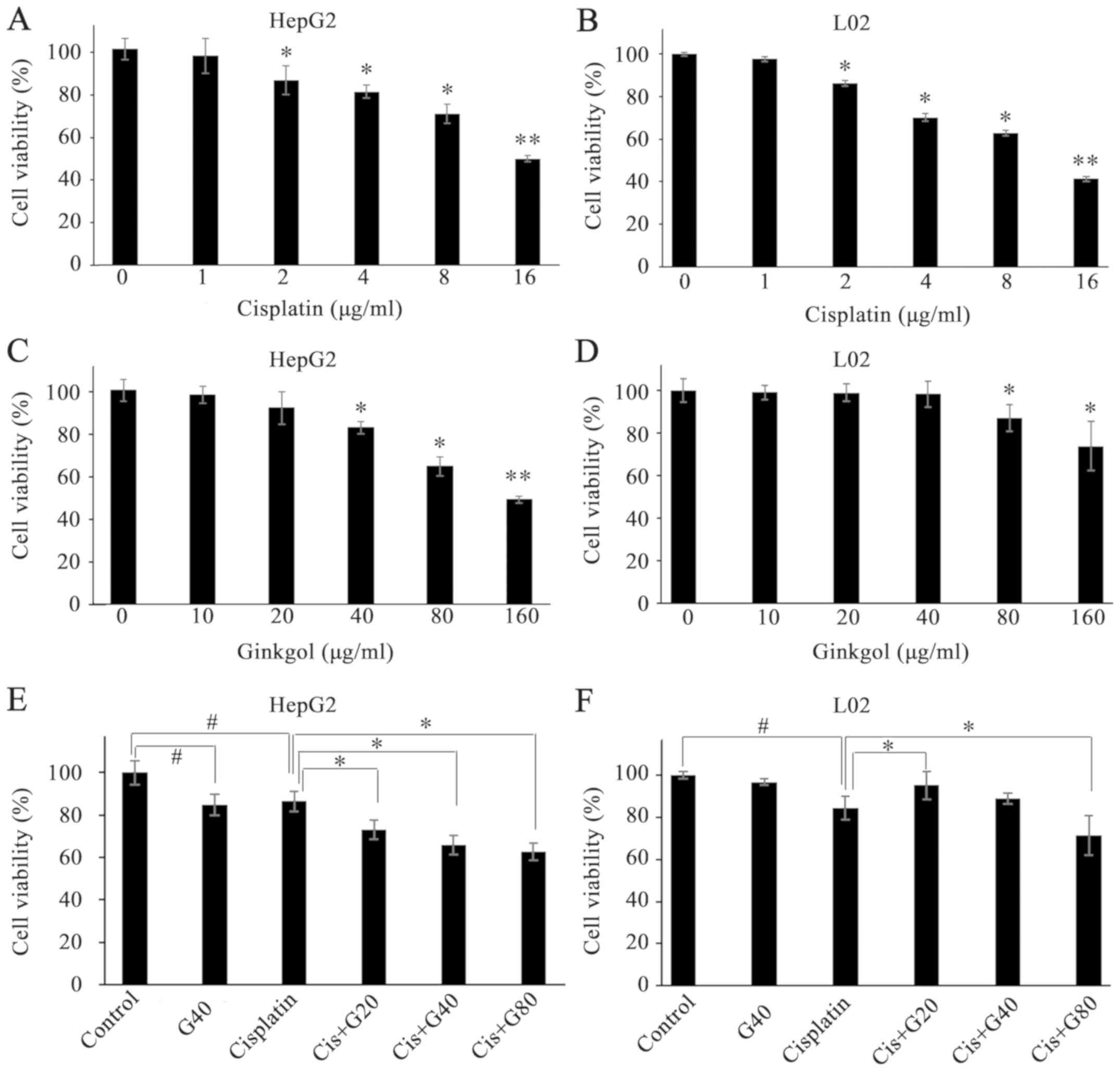 | Figure 1.Effects of ginkgol C17:1 on the
viability of HepG2 and L02 cells. Cell viability was determined
using an MTT assay following treatment with cisplatin alone,
ginkgol C17:1 or co-treatment of ginkgol C17:1 with cisplatin. (A)
HepG2 cell viability following treatment with cisplatin (0, 1, 2,
4, 8 or 16 µg/ml) for 24 h. (B) L02 cell viability following
treatment with cisplatin (0, 1, 2, 4, 8 or 16 µg/ml) for 24 h. (C)
HepG2 cell viability following treatment with ginkgol C17:1 (0, 10,
20, 40, 80 or 160 µg/ml) for 24 h. (D) L02 cell viability following
treatment with ginkgol C17:1 (0, 10, 20, 40, 80 or 160 µg/ml) for
24 h (E) HepG2 cell viability following co-treatment with 2 µg/ml
cisplatin and ginkgol C17:1 (20, 40 or 80 µg/ml). (F) L02 cell
viability following co-treatment with 2 µg/ml cisplatin and ginkgol
C17:1 (20, 40 or 80 µg/ml). *P<0.05, **P<0.01;
#P<0.05 vs. control or as indicated. Cis, cisplatin;
G20, 20 µg/ml ginkgol C17:1; G40, 40 µg/ml ginkgol C17:1; G80, 80
µg/ml ginkgol C17:1. |
Effects of ginkgol C17:1 with
cisplatin on apoptosis in HepG2 and L02 cells
Apoptosis is the primary response of cells to
chemotherapeutic agents, including cisplatin, an effective
anticancer drug with a major clinical effect (6). To determine the occurrence of apoptosis
induced by ginkgol C17:1 alone or by co-treatment with cisplatin,
key proteins involved in apoptosis were analyzed using western
blotting. In hepatoma HepG2 cells, ginkgol C17:1 alone
significantly increased protein levels of pro-apoptotic Bax and
cleaved caspase-3, but decreased the level of the anti-apoptotic
Bcl-2 (Fig. 2A). Along with the trend
of increase, Bax/Bcl2 and cleaved caspase-3 increased significantly
at the doses higher than 20 µg/ml ginkgol C17:1. As demonstrated in
Fig. 2B, no significant effects on
the proteins were observed in normal L02 hepatocytes treated with
ginkgol C17:1, except a significant increase of Bax/Bcl2 at the
higher dose 160 µg/ml (Fig. 2B).
While cisplatin alone induced significant increases of Bax and
cleaved caspase-3, and a decrease in Bcl-2 in the HepG2 and L02
cells, the co-treatment exhibited distinct effects on the two cell
types. In the HepG2 cells, the co-treatment promoted the increased
expression of Bax and cleaved caspase-3 and further decreased the
levels of Bcl-2, showing the significant increases in both Bax/Bcl2
and cleaved caspase-3 except for cleaved caspase-3 at co-treatment
of 20 µg/ml ginkgol C17:1 having no change (Fig. 2C). In the L02 cells, however, the
co-treatment significantly reversed the cisplatin-induced increase
in Bax, and the protein levels of cleaved caspase-3 and Bcl-2 were
comparable with those in the untreated cells except that caspase-3
at co-treatment of 20 µg/ml ginkgol C17:1 was similar to that in
the group of cisplatin alone (Fig.
2D).
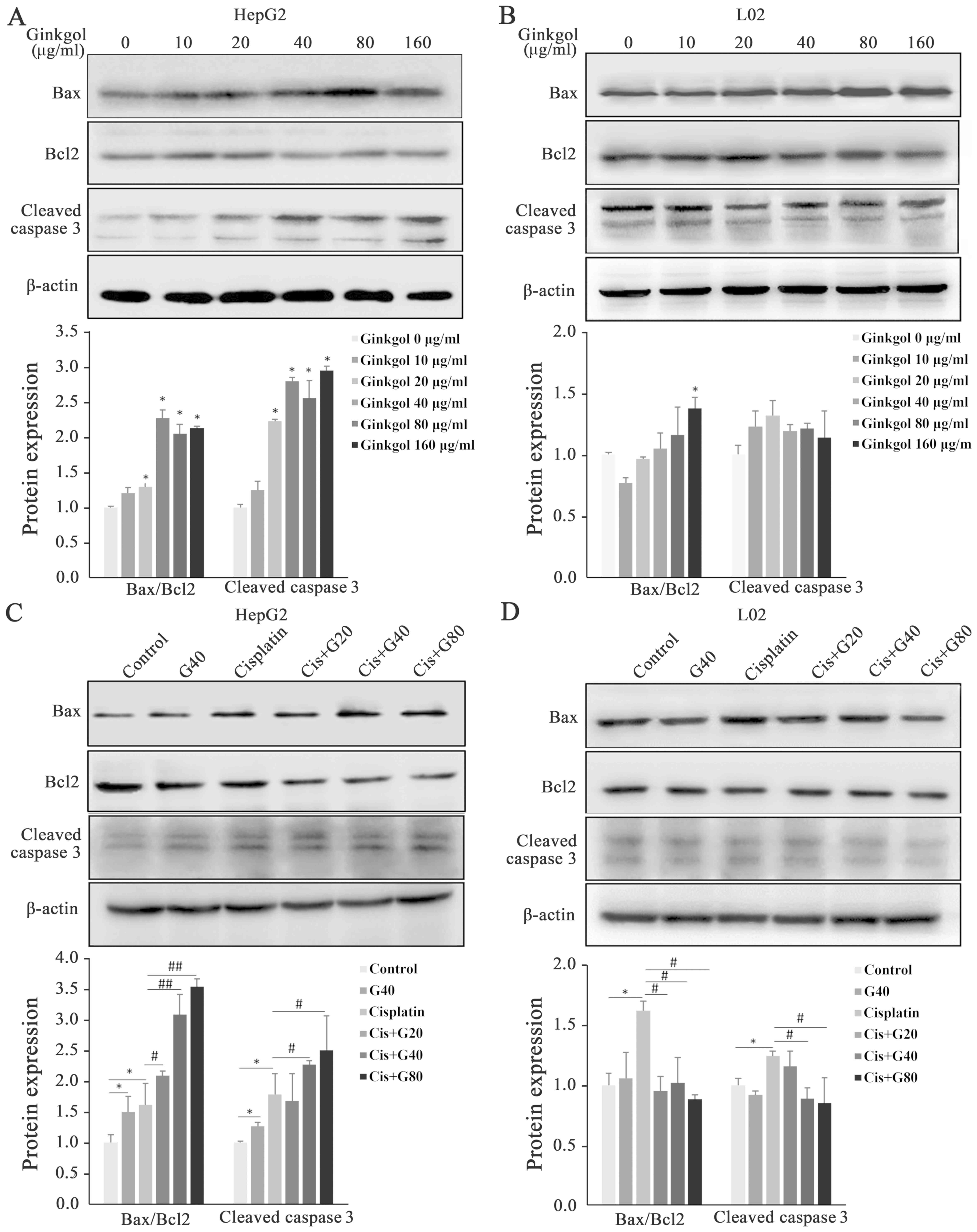 | Figure 2.Expression of Bax, Bcl-2 and cleaved
caspase-3 following treatment with ginkgol C17:1 and/or cisplatin
in HepG2 and L02 cells. Protein expression of Bax, Bcl-2 and
cleaved caspase-3 were analyzed by western blotting. (A) HepG2
cells treated with ginkgol C17:1 (0, 10, 20, 40, 80 or 160 µg/ml)
for 24 h. (B) L02 cells treated with ginkgol C17:1 (0, 10, 20, 40,
80 or 160 µg/ml) for 24 h. (C) HepG2 cells co-treated with 2 µg/ml
cisplatin and ginkgol C17:1 (20, 40 or 80 µg/ml) for 24 h. (D) L02
cells co-treated with 2 µg/ml cisplatin and ginkgol C17:1 (20, 40
or 80 µg/ml) for 24 h. Results are presented as the mean ± standard
deviation for five independent experiments. *P<0.05 vs. control;
#P<0.05 and ##P<0.01 vs. cisplatin
alone. Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein.
Cis, cisplatin; G20, 20 µg/ml ginkgol C17:1; G40, 40 µg/ml ginkgol
C17:1; G80, 80 µg/ml ginkgol C17:1. |
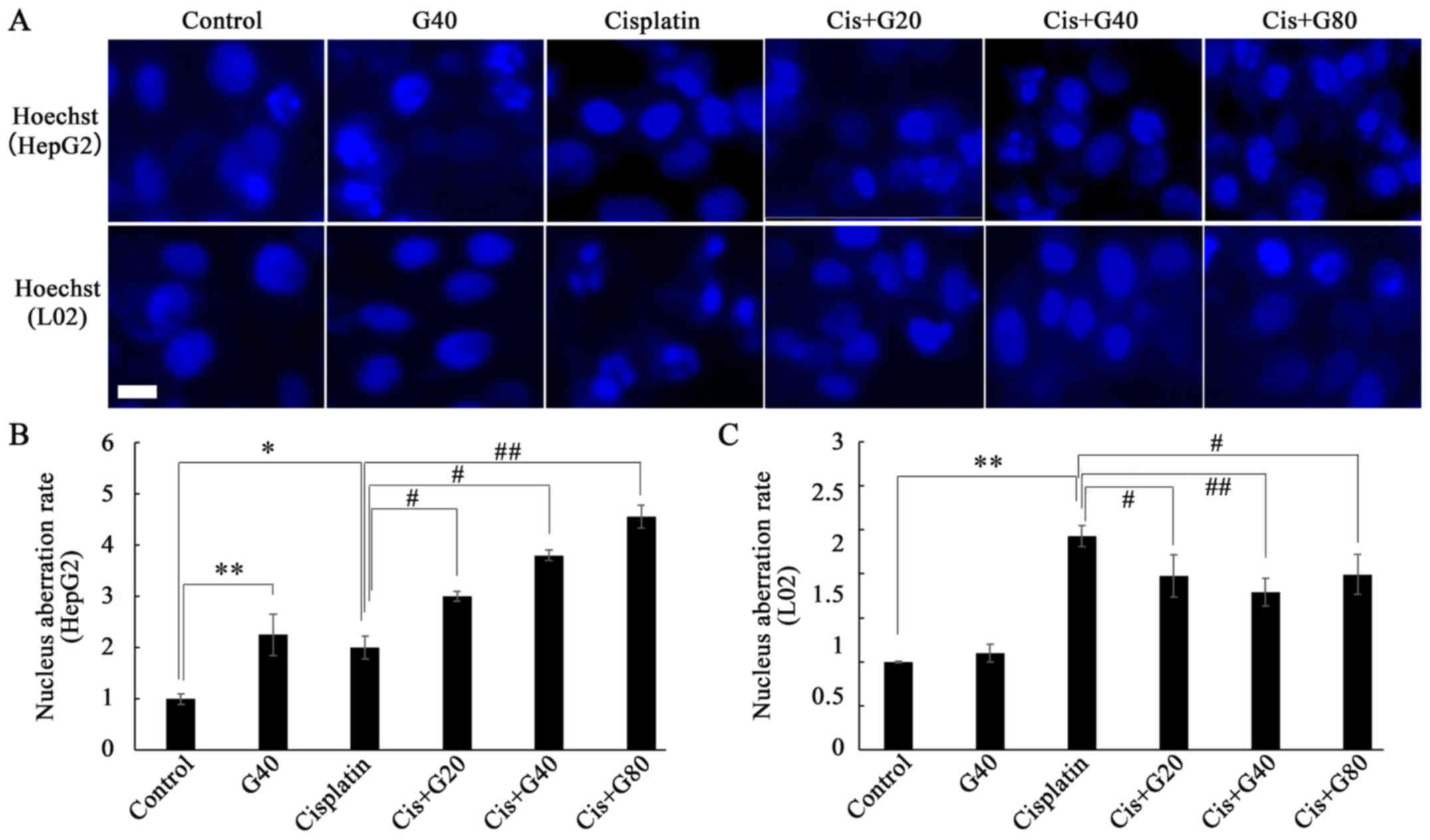
Hoechst 33342 dye was used for visualization of the
apoptotic aberrations in the cell nuclei following various
treatments. As presented in Fig. 3,
cisplatin alone significantly induced nuclear aberrations in HepG2
and L02 cells, whereas ginkgol C17:1 alone caused nuclear
aberrations in HepG2 cells, but did not alter the morphology of
nuclei in L02 cells. Under the co-treatment conditions, ginkgol
C17:1 significantly enhanced the nuclear aberration caused by
cisplatin in the HepG2 cells, in a concentration-dependent manner
(Fig. 3B). In contrast, in L02 cells,
ginkgol C17:1 significantly decreased the effect of cisplatin on
the apoptotic nuclear aberration rate (Fig. 3C).
Effects of ginkgol C17:1 with
cisplatin on autophagy in HepG2 and L02 cells
By detecting the expression of key proteins involved
in the process of autophagy, ginkgol C17:1 alone was revealed to
significantly increase the protein levels of Beclin-1 and LC3I/II,
and decrease p62 levels in hepatoma HepG2 cells (Fig. 4A) and normal L02 hepatocytes (Fig. 4B). As presented in Fig. 4C, cisplatin alone also enhanced
autography in HepG2 cells, indicated by increased protein levels of
Beclin-1 and LC3I/II and decreased levels of p62. However, the
cisplatin-induced autography was significantly attenuated in the
co-treatment (Fig. 4C). In L02 cells,
in comparison with the lack of effect on the Beclin-1, LC3I/II and
p62 protein levels by cisplatin alone, ginkgol C17:1 alone and the
co-treatment induced an increase in Beclin-1 and LC3I/II, and a
decrease in p62 (Fig. 4D).
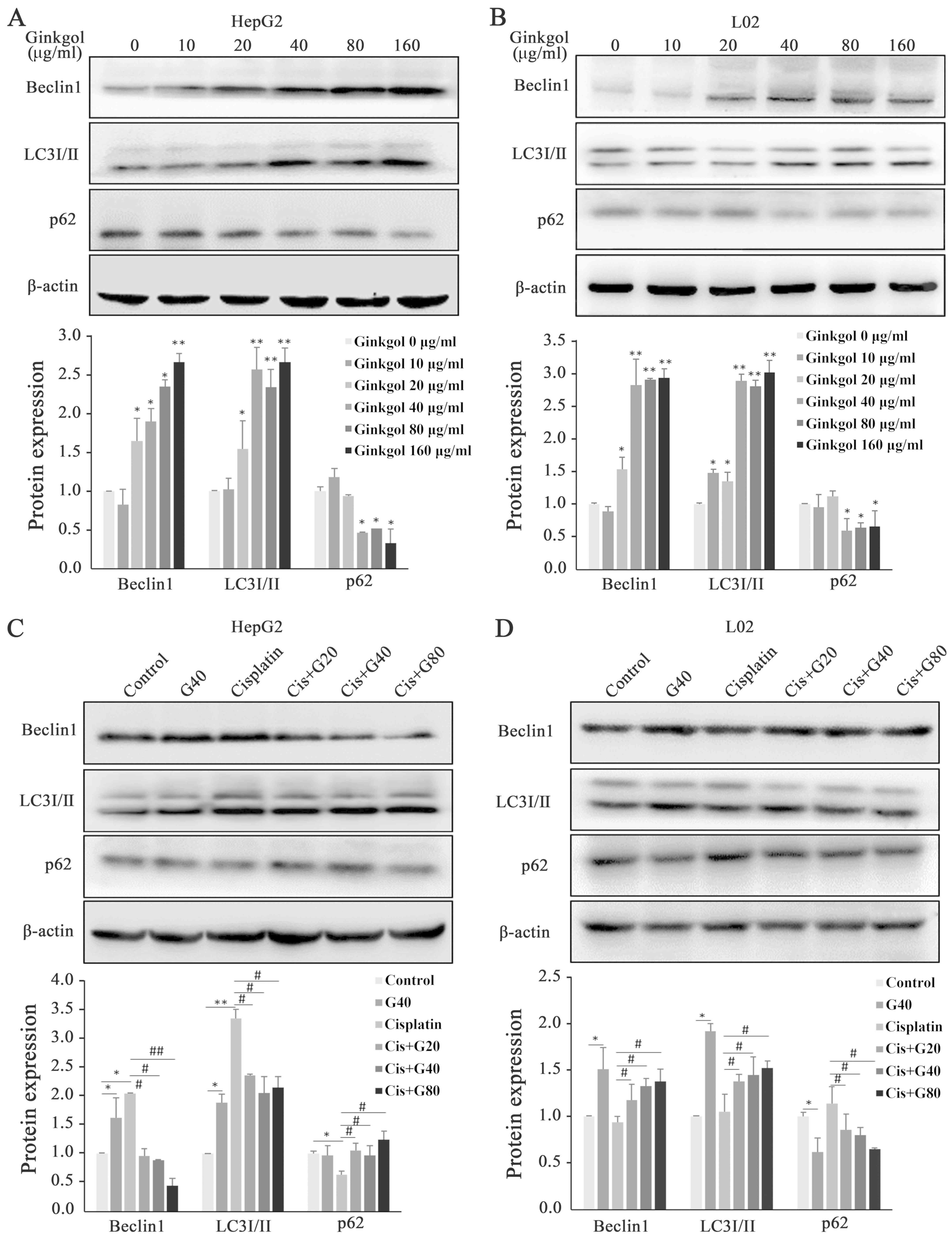 | Figure 4.Expression of Beclin1, LC3I/II and
p62 following treatment with ginkgol C17:1 and/or cisplatin in
HepG2 and L02 cells. The expression of Beclin1, LC3I/II and p62 was
analyzed by western blotting. (A) HepG2 cells treated with ginkgol
C17:1 (0, 10, 20, 40, 80 or 160 µg/ml) for 24 h. (B) L02 cells
treated with ginkgol C17:1 (0, 10, 20, 40, 80 or 160 µg/ml) for 24
h. (C) HepG2 cells co-treated with 2 µg/ml cisplatin and ginkgol
C17:1 (20, 40 or 80 µg/ml) for 24 h. (D) L02 cells treated with 2
µg/ml cisplatin and ginkgol C17:1 (20, 40 or 80 µg/ml) for 24 h.
Results are the mean ± standard deviation for five independent
experiments. *P<0.05 and **P<0.01 vs. control;
#P<0.05 and ##P<0.01 vs. cisplatin
alone. LC3, light chain 3; Cis, cisplatin; G20, 20 µg/ml ginkgol
C17:1; G40, 40 µg/ml ginkgol C17:1; G80, 80 µg/ml ginkgol
C17:1. |
In agreement with the results of the western
blotting, the LC3 punctum assay (Fig.
5) revealed that ginkgol C17:1 or cisplatin alone enhanced
autophagy in HepG2 cells, with the increase in LC3 puncta indicated
by the fluorescence intensity of LC3 scattered throughout the
cytoplasm. However, in the co-treatment, ginkgol C17:1
significantly inhibited cisplatin-induced autophagy (Fig. 5B). The LC3 punctum assay also
confirmed the results of the western blotting in the L02 cells,
revealing that ginkgol C17:1 induced autophagy not only when
administered alone, but also in the co-treatment with cisplatin
(Fig. 5C). Although the difference
between co-treatment with 20 µg/ml ginkgol C17:1 and cisplatin
alone was statistically insignificant, LC3 puncta rate did exhibit
a trend of increase in autophagy.
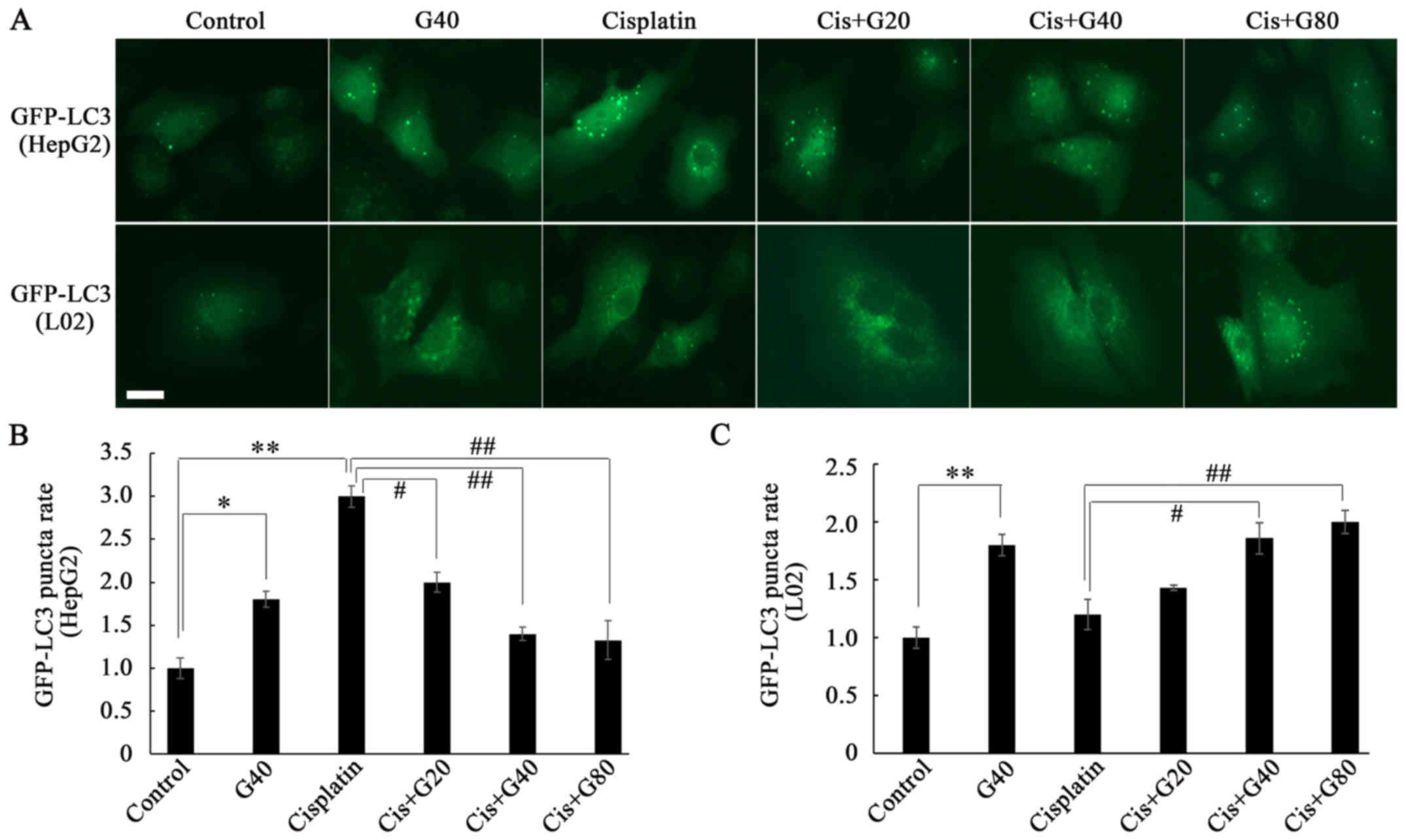 | Figure 5.Formation of GFP-LC3 puncta following
treatment with ginkgol C17:1 and/or cisplatin in HepG2 and L02
cells. Autophagy protein LC3 was determined by immunofluorescence
assay (magnification, ×200) following infection with GFP-LC3
adenovirus and treatment with 2 µg/ml cisplatin and/or ginkgol
C17:1 (20, 40, or 80 µg/ml) for 24 h. (A) GFP-LC3 puncta in HepG2
cells (upper panel) and L02 cells (lower panel). Scale bar, 20 µm.
The relative rates of GFP-LC3 puncta to control group were
determined for (B) HepG2 cells and (C) L02 cells. Results are the
mean ± SD for five independent experiments. *P<0.05,
**P<0.01; #P<0.05, ##P<0.01. GFP,
green fluorescent protein; LC3, light chain 3; Cis, cisplatin; G20,
20 µg/ml ginkgol C17:1; G40, 40 µg/ml ginkgol C17:1; G80, 80 µg/ml
ginkgol C17:1. |
Ginkgol C17:1 affects the AMPK/ULK1
and PI3K/AKT/mTOR signaling pathways in HepG2 and L02 cells
To investigate potential underlying molecular
mechanisms, western blotting was used to determine the levels of
proteins involved in the AMPK/ULK1 and PI3K/AKT/mTOR signaling
pathways, which are associated with autophagy or apoptosis. As
presented in Fig. 6A, increased
amounts of AMPK and ULK1, particularly their phosphorylated forms,
indicating the activation of the AMPK/ULK1 signaling pathway, were
observed following the treatment of HepG2 cells with ginkgol C17:1
or cisplatin alone. However, in comparison with cisplatin alone,
co-treatment with ginkgol C17:1 reversed this activation in
hepatoma HepG2 cells. In normal L02 hepatocytes, ginkgol C17:1
alone activated the AMPK/ULK1 pathway, whereas cisplatin alone did
not significantly alter the protein expression of AMPK and ULK1, or
that of their phosphorylated forms (Fig.
6B). Compared with cisplatin alone, the co-treatment led to an
increase in the levels of AMPK and ULK1, and their phosphorylation
(Fig. 6B).
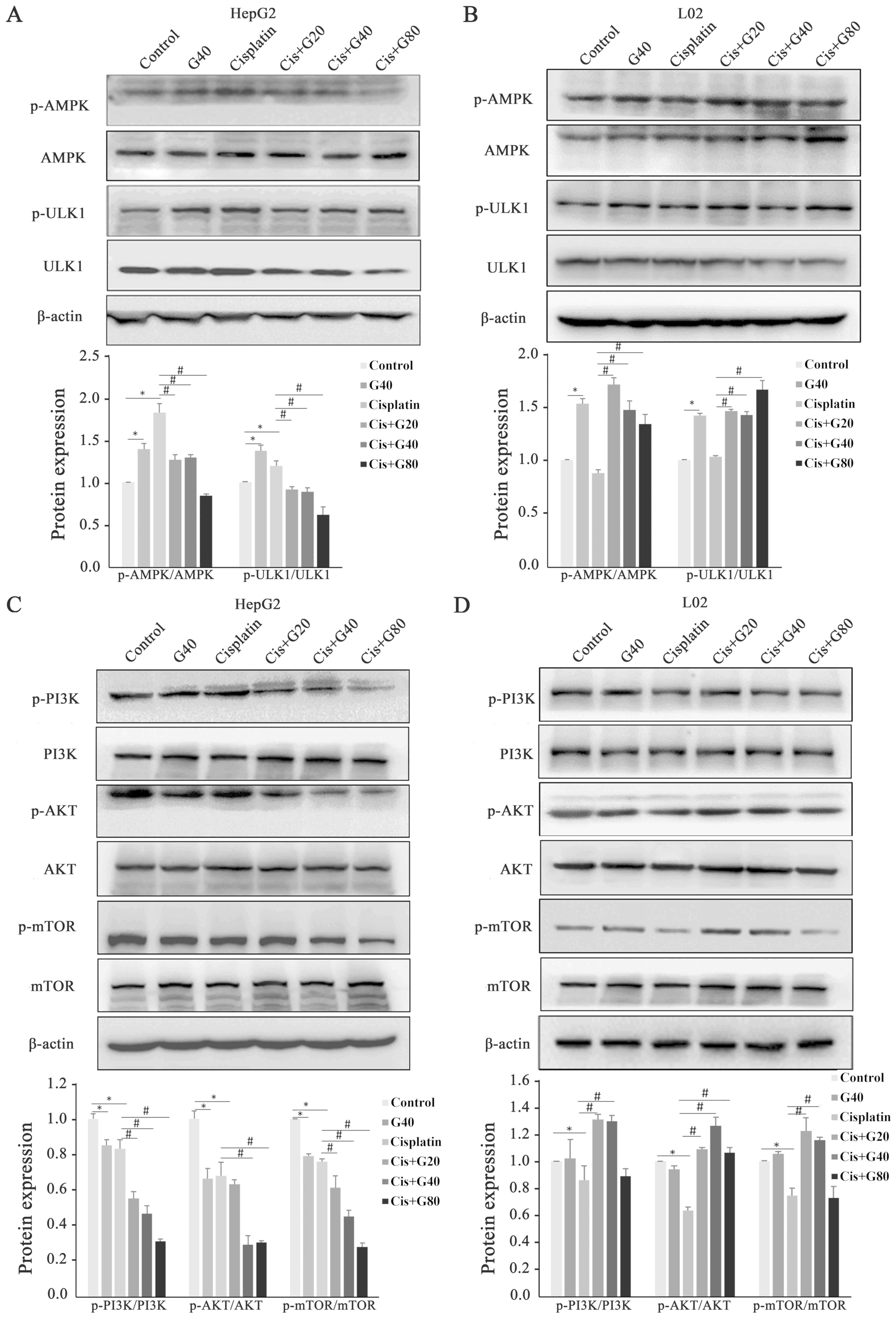 | Figure 6.Effect of ginkgol C17:1 and/or
cisplatin on AMPK/ULK1 and PI3K/AKT/mTOR signaling pathways.
Following treatment with 2 µg/ml cisplatin and/or ginkgol C17:1 (0,
20, 40 or 80 µg/ml) for 24 h, western blotting was performed to
determine the expression of phosphorylated AMPK
(Thr172), phosphorylated ULK1 (Ser555) in (A)
HepG2 cells and (B) L02 cells. The expression of upstream pathway
phosphorylated PI3K (Tyr458), phosphorylated AKT
(Thr308), phosphorylated mTOR (Ser2448) in
(C) HepG2 cells and (D) L02 cells. Results are the mean ± standard
deviation for five independent experiments. *P<0.05 vs. control;
#P<0.05 vs. cisplatin alone. AMPK, AMP-activated
protein kinase; PI3K, phosphoinositide 3-kinase; AKT, protein
kinase B; mTOR, mammalian target of rapamycin; Cis, cisplatin; G20,
20 µg/ml ginkgol C17:1; G40, 40 µg/ml ginkgol C17:1; G80, 80 µg/ml
ginkgol C17:1; p-, phosphor. |
In HepG2 cells (Fig.
6C), ginkgol C17:1 or cisplatin alone inhibited the
phosphorylation of PI3K, AKT and mTOR. Under the conditions of
co-treatment, ginkgol C17:1 further decreased the levels of p-PI3K
and p-mTOR in a concentration-dependent manner, whereas with the
trend of further decline, the levels of p-AKT decreased
significantly at the co-treatment with higher doses than 20 µg/ml.
In L02 cells, however (Fig. 6D),
cisplatin alone inhibited the protein expression of p-PI3K, p-AKT
and p-mTOR, but ginkgol C17:1 did not significantly alter the
protein amounts of p-PI3K, p-AKT and p-mTOR. Co-treatment reversed
the inhibitory effects of cisplatin on PI3K/AKT/mTOR. Co-treatment
doses higher than 20 µg/ml led to further increased amounts of
p-PI3K, p-AKT and p-mTOR compared to untreated cells.
Discussion
Chemotherapy continues to be a common method in the
treatment of cancer. Cisplatin, a metal-based compound which was
identified half a century ago, remains the mainstay of the most
widely used chemotherapeutics for treating cancer (30). However, owing to drug resistance and
multiple side effects of cisplatin, it is imperative for physicians
to identify a novel approach to improve its anticancer effect,
ideally aiming to kill only cancer cells while leaving normal cells
unharmed (31). According to this
concept, auxiliary chemotherapy drugs synergizing the cytotoxicity
at relatively low dose of chemotherapeutical agents would be
beneficial to kill cancer cells with minor side effects on normal
cells.
The cell viability results of the present study
confirmed the comparable cytotoxicity in cancer and normal
hepatocytes. Whereas ginkgol C17:1 induced a cytotoxic effect in
hepatoma cells, it inhibited the viability of normal hepatocytes
only at higher doses (≥80 µg/ml). Importantly, the combination of
ginkgol C17:1 with a low dose of cisplatin (2 µg/ml) exhibited
prominent cytotoxicity in hepatoma cells, but with less (or no)
toxicity in normal hepatocytes.
Previous studies indicated that cisplatin is able to
induce autophagy and apoptosis in cancer cells of different origins
(24,32,33).
Consistently exhibiting the same cisplatin cytotoxicity in liver
cancer HepG2 cells, the results of the present study also confirmed
cisplatin-induced apoptosis in normal hepatocyte L02 cells. Unlike
in HepG2 cells, cisplatin did not cause any significant alteration
in the autophagic activity of L02 cells. These results indicated
that apoptosis may be the primary cytotoxic response to cisplatin
in normal hepatocytes.
Previous studies suggested that ginkgols can inhibit
hepatoma cell viability, migration and invasion, even ultimately
leading to apoptosis by inducing the expression of caspases
(21,34). In the present study, ginkgol C17:1 was
identified to lead to an increase in apoptosis, characterized by
cell shrinkage, nucleus condensation and the increased expression
of apoptotic proteins Bax and cleaved caspase 3 compared with a
decreased expression of Bcl-2 in human liver cancer HepG2 cells. In
the co-treatment, ginkgol C17:1 promoted the cisplatin-induced
apoptosis in HepG2 cells. In contrast, no apoptosis was induced by
ginkgol C17:1 alone in normal hepatocyte L02 cells. When co-treated
with cisplatin, ginkgol C17:1 even inhibited cisplatin-induced
apoptosis in normal hepatocyte L02 cells. These results indicated
the anticancer effect of ginkgol C17:1 in liver cancer cells
without killing normal hepatocytes. More importantly, while
protecting normal cells from cisplatin-induced apoptosis, ginkgol
C17:1 improves the chemotherapeutic effect of cisplatin via
enhancing cisplatin-induced apoptosis in cancer cells.
Autophagy is normally known as a survival mechanism
to clean up the damage signals to protect cells from injury. In
cancer cells, however, induction of autophagy has been implicated
in the resistance to multiple standard chemotherapeutic agents such
as cisplatin (13,35,36). A
number of studies have identified that inhibition of autophagy may
enhance chemosensitization in human cancer cells (37–39). In
the present study, although ginkgol C17:1 alone induced autophagy
in hepatoma HepG2 cells, in conjunction with cisplatin, however,
ginkgol C17:1 inconceivably inhibited the cisplatin-induced
autophagy to a marked extent. The inhibition of cisplatin-induced
autophagy together with the enhancement of cisplatin-induced
apoptosis suggested a promising combination of ginkgol C17:1 with
cisplatin for chemosensitization of cancer cells in cisplatin
chemotherapy. Furthermore, autophagy was increased in L02 cells by
ginkgol C17:1 monotherapy and particularly upon co-treatment with
cisplatin. The induction of autophagy and the inhibition of
cisplatin-induced apoptosis indicated a protective effect of
ginkgol C17:1 in normal hepatocytes, when co-exposed to cisplatin
during chemotherapy. This protective role of ginkgol C17:1 on
cisplatin-induced cytotoxicity in L02 cells further supported the
potential of co-treatment of ginkgol C17:1 with cisplatin with
minor side effects on normal cells in cisplatin chemotherapy.
Previous studies have identified that autophagy
provides a survival advantage against cancer therapies by inducing
AMPK and suppressing apoptosis (40,41). In
liver hepatoma HepG2 cells, although ginkgol C17:1 alone induced
autophagy by activating AMPK signaling pathway, it did inhibit
cisplatin-induced autophagy by decreasing the activity of the AMPK
signaling pathway and enhancing the cisplatin-induced apoptosis.
Suppression of autophagy increased HepG2 cell death through
inhibition of AMPK upon co-treatment of ginkgol C17:1 with
cisplatin. In normal hepatocyte L02 cells, although cisplatin did
not significantly affect the AMPK/ULK1 signaling pathway, ginkgol
C17:1 activated the AMPK/ULK1 signaling pathway to induce autophagy
that can restore the damage caused by cisplatin. Inhibition of the
PI3K/AKT/mTOR signaling pathway is considered to inhibit cell
proliferation and autophagy, thus contributing to anticancer
mechanisms (42,43). In the present study, treatment with
ginkgol C17:1 or cisplatin alone inhibited the PI3K/AKT/mTOR
signaling pathway in liver cancer HepG2 cells and a more marked
inhibition was observed upon co-treatment. In normal hepatocytes,
ginkgol C17:1 alone did not cause a significant change in this
signaling pathway, but it decreased the inhibition caused by
cisplatin. These changes in the PI3K/AKT/mTOR signaling pathway
were consistent with the results of apoptosis and autophagy,
indicating the involvement of the PI3K/AKT/mTOR signaling pathway
in altering apoptosis or autophagy by ginkgol C17:1 or cisplatin.
We hypothesize that ginkgol C17:1 sensitizes liver cancer HepG2
cells to cisplatin chemotherapy via synergistic inhibition of the
PI3K/AKT/mTOR signaling pathway, whereas repairing the
PI3K/AKT/mTOR signaling pathway may contribute to the mechanism of
ginkgol C17:1 in protecting normal hepatocyte L02 cells against
cisplatin-induced cytotoxicity. However, the exact molecular
mechanisms such as which upstream targets are involved require
further clarification.
In conclusion, to the best of our knowledge, the
results of the present study provided the first evidence that,
ginkgol C17:1 can protect normal hepatocytes against
cisplatin-caused cytotoxicity while potentiating the anticancer
effect of cisplatin chemotherapy. The differential effects upon
co-treatment of ginkgol C17:1 with cisplatin in hepatoma and normal
hepatocytes suggest that ginkgol C17:1 may be a promising candidate
for auxiliary chemotherapy, meeting the modern concept of
developing therapeutics that would selectively target cancer cells
and leave healthy cells unharmed or less harmed. Further
investigations such as in vivo studies on animal models are
currently underway.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 81372404) and the College
Students' Scientific Research Project of Jiangsu University (grant
nos. 16A511 and 16A513).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YL designed the study, measured the signaling
pathways and participated in manuscript preparation. XZ performed
experiments concerning apoptosis and autophagy. XY and JL isolated
ginkgol C17:1, participated in data analysis and prepared the
figures, LL performed experiments on immunofluorescence. WM
performed the MTT assay and statistical analysis. MC analyzed data
and was a major contributor in writing the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pascual S, Herrera I and Irurzun J: New
advances in hepatocellular carcinoma. World J Hepatol. 8:421–438.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marquardt JU, Andersen JB and Thorgeirsson
SS: Functional and genetic deconstruction of the cellular origin in
liver cancer. Nat Rev Cancer. 15:653–667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun BS, Dong QZ, Ye QH, Sun HJ, Jia HL,
Zhu XQ, Liu DY, Chen J, Xue Q, Zhou HJ, et al: Lentiviral-mediated
miRNA against osteopontin suppresses tumor growth and metastasis of
human hepatocellular carcinoma. Hepatology. 48:1834–1842. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Y, Lu Y, Wang C, Bai W, Qu J, Chen Y,
Chang X, An L, Zhou L, Zeng Z, et al: Cryotherapy is associated
with improved clinical outcomes of Sorafenib therapy for advanced
hepatocellular carcinoma. Cell Biochem Biophys. 63:159–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boulikas T and Vougiouka M: Cisplatin and
platinum drugs at the molecular level (Review). Oncol Rep.
10:1663–1682. 2003.PubMed/NCBI
|
|
6
|
Kelland L: The resurgence of
platinum-based cancer chemotherapy. Nat Rev Cancer. 7:573–584.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Markman M: Toxicities of the platinum
antineoplastic agents. Expert Opin Drug Saf. 2:597–607. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu XC, Zhang J, Xu BH, Cai L, Ragaz J,
Wang ZH, Wang BY, Teng YE, Tong ZS, Pan YY, et al: Cisplatin plus
gemcitabine versus paclitaxel plus gemcitabine as first-line
therapy for metastatic triple-negative breast cancer (CBCSG006): A
randomised, open-label, multicentre, phase 3 trial. Lancet Oncol.
16:436–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu SZ and Harrison-Findik DD: Autophagy
and cancer. World J Biol Chem. 4:64–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
White E: The role for autophagy in cancer.
J Clin Invest. 125:42–46. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Amaravadi R, Kimmelman AC and White E:
Recent insights into the function of autophagy in cancer. Genes
Dev. 30:1913–1930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Wang Q, Yin FQ, Zhang W, Yan LH
and L Li: MTRR silencing inhibits growth and cisplatin resistance
of ovarian carcinoma via inducing apoptosis and reducing autophagy.
Am J Transl Res. 7:1510–1527. 2015.PubMed/NCBI
|
|
14
|
Nikoletopoulou V, Markaki M, Palikaras K
and Tavernarakis N: Crosstalk between apoptosis, necrosis and
autophagy. Biochim Biophys Acta 1833. 3448–3359. 2013.
|
|
15
|
Li F, Zeng J, Gao Y, Guan Z, Ma Z, Shi Q,
Du C, Jia J, Xu S, Wang X, et al: G9a inhibition induces autophagic
cell death via AMPK/mTOR pathway in bladder transitional cell
carcinoma. PLoS One. 10:e01383902015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vanhaesebroeck B, Stephens L and Hawkins
P: PI3K signaling: The path to discovery and understanding. Nat Rev
Mol Cell Biol. 13:195–203. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu K, Liu P and Wie W: mTOR signaling in
tumorigenesis. Biochim Biophys Acta 1846. 638–654. 2014.
|
|
18
|
Cheong H, Lindsten T, Wu J, Lu C and
Thompson CB: Ammonia-induced autophagy is independent of ULK1/ULK2
kinases. Proc Natl Acad Sci USA. 108:11121–11126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nash KM and Shah ZA: Current perspectives
on the beneficial role of ginkgo biloba in neurological and
cerebrovascular disorders. Integr Med Insights. 10:1–9. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ni XW and Wu MC: Study on antitumor
activities of ginkgolic acids from Ginkgo sarcotestas. J Huazhong
Agricul Univ. 4:49–51. 2006.
|
|
21
|
Wang YF, Yang XM, Li YY, Li J, Huang BZ,
Guo CY, Yi N and Xing CH: Inhibitory effect of ginkgols on
SMMC-7721 liver cancer cells in vitro and liver cancer H22-bearing
mice in vivo. J Jiangsu Univ. 23:2332013.
|
|
22
|
Chen Q, Yang GW and An LG: Apoptosis of
hepatoma cells SMMC-7721 induced by Ginkgo biloba seed
polysaccharide. World J Gastroenterol. 8:832–836. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang XM, Wang YF, Li YY and Ma HL: Thermal
stability of ginkgolic acids from Ginkgo biloba and the effects of
ginkgol C17:1 on the apoptosis and migration of SMMMC7721 cells.
Fitoterapia. 98:66–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu J, Li Y, Yang X, Dong Y, Wu J and Chen
M: Effects of ginkgol C17:1 on cisplatin-induced autophagy and
apoptosis in HepG2 cells. Oncol Lett. 15:1021–1029. 2018.PubMed/NCBI
|
|
25
|
Yang XM, Zhang XL, Chen YC and Liu F: LC
method for determination of ginkgolic acids in mice plasma and its
application to pharmacokinetic study. Chromatographia. 69:593–596.
2009. View Article : Google Scholar
|
|
26
|
Fang YY, Yang XM, Li YY and Feng CL:
Spectroscopic studies on the interaction of bovine serum albumin
with Ginkgol C15:1 from Ginkgo biloba L. J Luminesceence.
162:203–211. 2015. View Article : Google Scholar
|
|
27
|
Li Y, Liu J, Yang X, Dong Y, Liu Y and
Chen M: Ginkgol C17:1 inhibits tumor growth by blunting the EGF-
PI3K/Akt signaling pathway. J Biomed Res. 31:232–239.
2017.PubMed/NCBI
|
|
28
|
Ni HM, Bockus A, Wozniak AL, Jones K,
Weinman S, Yin XM and Ding WX: Dissecting the dynamic turnover of
GFP-LC3 in the autolysosome. Autophagy. 7:188–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu D, Wu J, Xu L, Zhang R and Chen L: A
method for the establishment of a cell line with stable expression
of the GFP-LC3 reporter protein. Mol Med Rep. 6:783–786. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ndagi U, Mhlongo N and Soliman ME: Metal
complexes in cancer therapy-an update from drug design perspective.
Drug Des Devel Ther. 11:599–616. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ziko L, Riad S, Amer M, Zdero R,
Bougherara H and Amleh A: Mechanical stress promotes
cisplatin-induced hepatocellular carcinoma cell death. Biomed Res
Int 2015. 4305692015.
|
|
32
|
Cavallo F, Feldman DR and Barchi M:
Revisiting DNA damage repair, p53-mediated apoptosis and cisplatin
sensitivity in germ cell tumors. Int J Dev Biol. 57:273–280. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang HQ, He B, Fang N, Lu S, Liao YQ and
Wan YY: Autophagy inhibition sensitizes cisplatin cytotoxicity in
human gastric cancer cell line SGC7901. Asian Pac J Cancer Prev.
14:4685–4688. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Liu J, Liu Y, Yang X, Huang B and
Chen M: Inhibitory effect of Ginkgol C17:1 on the biological
behavior of tumor cells. Oncol Lett. 13:1873–1879. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang J and Wu GS: Role of autophagy in
cisplatin resistance in ovarian cancer cells. J Biol Chem.
289:17163–17173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu L, Gu C, Zhong D, Shi L, Kong Y, Zhou Z
and Liu S: Induction of autophagy counteracts the anticancer effect
of cisplatin in human esophageal cancer cells with acquired drug
resistance. Cancer Lett. 355:34–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu Y, Yu H, Qin H, Kang J, Yu C, Zhong J,
Su J, Li H and Sun L: Inhibition of autophagy enhances cisplatin
cytotoxicity through endoplasmic reticulum stress in human cervical
cancer cells. Cancer Lett. 314:232–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yan MM, Ni JD, Song D, Ding M and Huang J:
Interplay between unfolded protein response and autophagy promotes
tumor drug resistance. Oncol Lett. 10:1959–1969. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao J, Nie Y, Wang H and Lin Y: MiR-181a
suppresses autophagy and sensitizes gastric cancer cells to
cisplatin. Gene. 576:828–833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie BS, Zhao HC, Yao SK, Zhou DX, Jin B,
Lv DC, Wu CL, Ma DL, Gao C, Shu XM and Ai ZL: Autophagy inhibition
enhances etoposide-induced cell death in human hepatoma G2 cells.
Int J Mol Med. 27:599–606. 2011.PubMed/NCBI
|
|
42
|
Shi SQ and Cao H: Shikonin promotes
autophagy in BXPC-3 human pancreatic cancer cells through the
PI3K/Akt signaling pathway. Oncol Lett. 8:1087–1089. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sharma N, Nanta R, Sharma J, Gunewardena
S, Singh KP, Shankar S and Srivastava RK: PI3K/AKT/mTOR and sonic
hedgehog pathways cooperate together to inhibit human pancreatic
cancer stem cell characteristics and tumor growth. Oncotarget.
6:32039–32060. 2015. View Article : Google Scholar : PubMed/NCBI
|