Introduction
Breast cancer is the most frequently diagnosed type
of cancer and the second leading cause of cancer-associated
mortality among females. This malignancy accounted for 29%
(232,670) of total novel cancer cases and 15% (40,000) of total
cancer-associated mortalities in 2014 (1). Although estrogen receptor (ER),
progesterone receptor (PR) and human epidermal growth factor
receptor-2 (HER-2) have been used as theranostic references in
clinical practice, triple-negative breast cancers (TNBCs) account
for between 15 and 20% of all breast cancers, which are defined by
the lack of expression of ER, PR and HER-2 (2). No targeted agent is currently available
for TNBC. Cytotoxic chemotherapy is the only option for
postoperative therapy owing to the shortage of specific therapeutic
targets (2). In addition, TNBC is
associated with more aggressive histological characteristics, poor
therapeutic outcome and decreased survival rate compared with other
breast cancer subtypes (3). No
standard therapeutic regimens for TNBC have been established, and
information on TNBC is insufficient. Therefore, identifying novel
prognostic and predictive biomarkers is highly important, and the
development of novel therapeutic options for patients with TNBC is
required.
SWItch/sucrose non-fermentable (SWI/SNF) complex
acts as a conserved chromatin remodeling complex, which performs
key roles in cellular differentiation, proliferation and DNA
repair, in an ATP-dependent manner (4). SWI/SNF includes two major subclasses,
namely BRG1/BRM-associated factor (BAF) and polybromo-associated
BAF (PBAF) complexes (5). AT-rich
interactive domain-containing protein 1B (ARID1B) is a component of
the human SWI/SNF chromatin remodeling complex that is involved in
transcriptional activation and inhibition of selected genes by
chromatin remodeling (6). ARID1B is
important in mammalian development, since it regulates the cell
cycle during differentiation (7).
Previous studies (7,8) have demonstrated that ARID1B performs key
roles in neurodevelopment, and haploinsufficiency of ARID1B is a
frequent cause of intellectual disability. Mutations identified in
ARID1B indicated that this molecule acts as a potential tumorigenic
driver in certain tumors (9). In
breast cancer, ARID1B has been implicated in the development of
breast cancer through the identification of driver mutations, which
confer clonal selective advantage on cancer cells (10). An in vitro study revealed that
ARID1B is a specific determinant of SWI/SNF complexes with an
extensive role in promoting proliferation and an evidently
non-essential role in repressing cell cycle activity, making ARID1B
an attractive target for anticancer therapy (7). Although previous studies have
demonstrated that ARID1B performs an important role in several
types of human malignancy (9,11,12),
ARID1B in patients with TNBC has not been reported.
In the present study, immunohistochemical staining
was performed to analyze ARID1B expression in 142 TNBC tissues from
the Harbin Medical University Cancer Hospital (Harbin, China), and
the data were compared with the clinicopathological features of
patients. To the best of our knowledge, the present study is the
first to associate ARID1B expression with clinicopathological
features and survival rate of patients with TNBC.
Patients and methods
Patients and samples
A total of 142 patients with TNBC were evaluated,
and a complete set of follow-up data was reviewed and analyzed. All
patients were female with a mean age of 48.6 years (range, 32–69
years). Patients with recurrent tumors, distant metastasis sites,
other tumors and bilateral tumors, as well as patients who received
neoadjuvant therapy, were excluded from the present study. A total
of 64 adjacent normal breast tissues were used as controls. All
formalin-fixed paraffin-embedded specimens used in
immunohistochemistry were collected from patients who underwent
surgery in the Harbin Medical University Cancer Hospital. Each
sample was independently examined and analyzed by two pathologists.
All patients were treated postoperatively with adjuvant systemic
therapy according to the National Comprehensive Cancer Network
guidelines (13). Tumors were
confirmed histopathologically and were staged according to
tumor-node-metastasis (TNM) classification. Tumor size was measured
by the pathologists, and normal breast tissues were isolated from
>5 cm outside of the tumor. All patients were routinely tested
for proliferation marker protein Ki67 (Ki67) and p53 using
corresponding antibodies (cat. no. TA500265; dilution, 1:50; cat.
no. TA502780; dilution, 1:100; Origene Technologies Inc.,
Rockville, MD, USA). Samples with at least 14% Ki67+
tumor cells were considered Ki67-positive (14). p53 was considered positive if positive
nuclear staining was ≥10%, regardless of the intensity (15). The present study was approved by the
Ethical Committee of Harbin Medical University (Harbin, China).
Written informed consent was obtained from all study
participants.
Immunohistochemistry
Immunohistochemical staining for ARID1B was
performed on 4-µm thick formalin-fixed and paraffin-embedded
sections. The tissue sections were dried for between 12 and 24 h at
37°C. Subsequently, sections were deparaffinized in xylene and
rehydrated by passing through a graded series of ethanol to
distilled water. The tissue sections were treated with sodium
citrate buffer with a pH of 6.0 at 98°C for 20 min and incubated
with a mouse anti-ARID1B polyclonal antibody (Abcam, Cambridge, UK;
cat. no, ab57461; dilution of 1:300) for 60 min at room
temperature. Subsequent to washing with PBS, sections were
incubated with secondary biotinylated antibody (horseradish
peroxidase-conjugated goat anti-mouse immunoglobulin G; cat. no.
PV6002; Origene Technologies Inc.) for 30 min at 37°C. Subsequent
to washing with PBS, each section was then treated with an
avidin-biotin complex (dilution, 1:1,000) at room temperature for
between 30 and 60 min. The reaction products were visualized with
diaminobenzidine. Finally, the sections were counterstained with
hematoxylin, dehydrated and cleared with xylene, and the sections
were sealed with coverslips. For negative control staining,
sections were treated with 0.01 mol/l PBS in place of primary
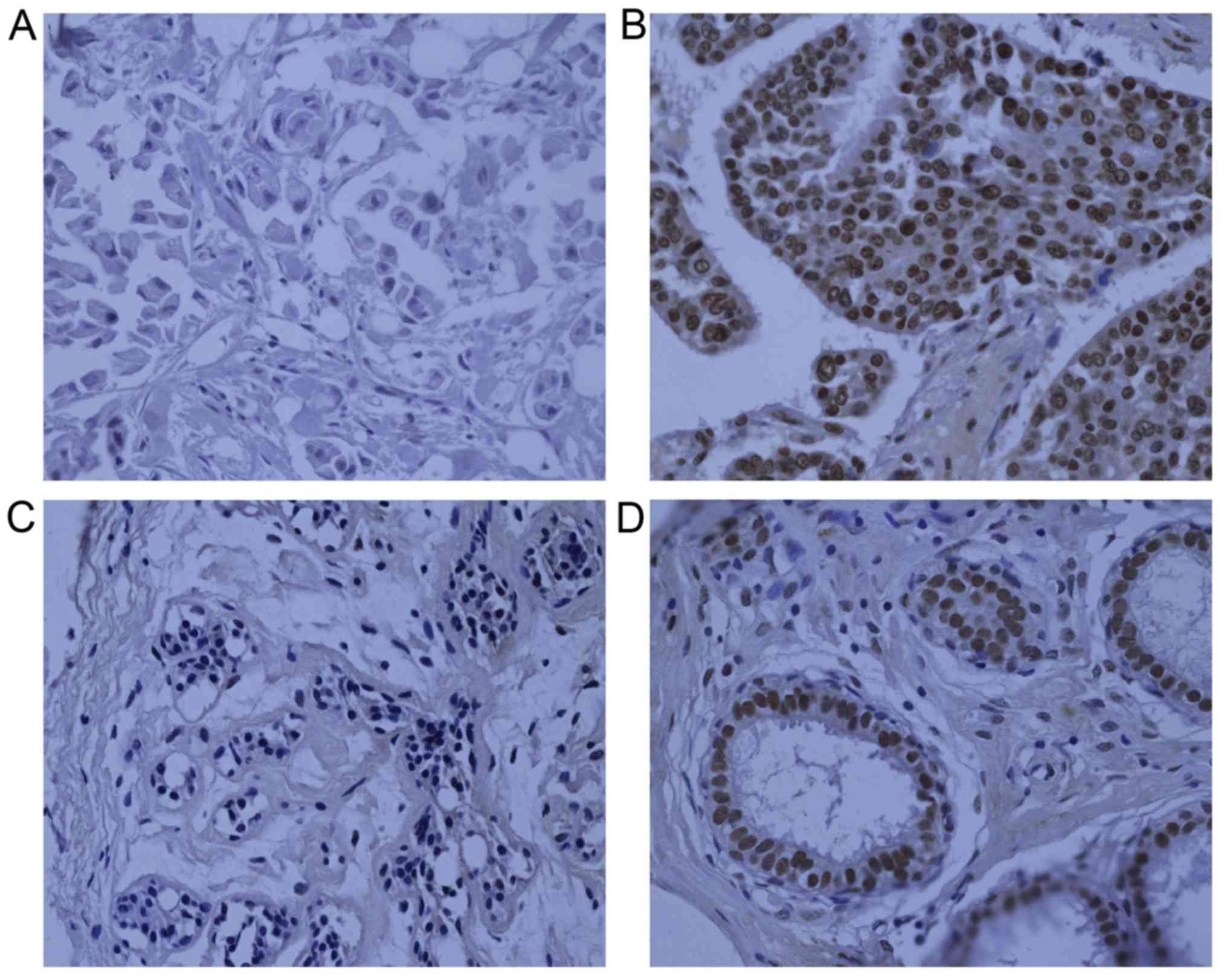
antibodies. Cells with distinct brown granules in the nuclei were
considered positive for ARID1B expression. Sections were evaluated
by two independent investigators who provided a consensus on the
stain patterns using a light microscope at magnification, ×400.
Semi-quantitative expression levels were evaluated on staining
intensity and distribution. Staining intensity was graded as
follows: 0, no staining; 1, light brown; 2, brown; and 3, dark
brown. The extent of reactivity was scored as follows: 0, <10%;
1, between 10 and 25%; 2, between 26 and 50%; 3, between 50 and
75%; and 4, ≥76%. The sum of the intensity and reactivity extension
scores was used as the final staining score for ARID1B. For
statistical analysis, final staining scores of >3 were
classified as high expression, and scores of <3 indicated low
expression.
Statistical analysis
Statistical analyses were performed using SPSS
(version 13.0; SPSS, Inc., Chicago, IL, USA). Potential
associations between ARID1B expression and age, menopausal status,
lymph node metastasis (LNM), histological grade, TNM stage, tumor
size, chemotherapy regimen and status of p53 and Ki67 were analyzed
using a χ2-test. Progression-free survival (PFS) and
overall survival (OS) rates were measured from the date when the
primary surgery started. PFS was measured from the beginning of
therapy until the time of disease progression or at the end of the
observation period in patients without a progressive disease. OS
was measured until mortality from any cause or the end of the
observation period. The Kaplan-Meier estimator method was used to
estimate PFS and OS, and survival differences according to ARID1B
expression were analyzed using a log rank test. Clinicopathological
features known to be associated with prognosis, including age (≥45
vs. <45 years), LNM (positive vs. negative), histological grade
(3 vs. 1+2), TNM stage (III vs. II+I), tumor size (>2 vs. ≤2
cm), p53 (positive vs. negative), Ki67 (positive vs. negative),
menopausal status (postmenopausal vs. premenopausal) and ARID1B
expression (high vs. low) were evaluated by Cox's univariate
analysis. Variables identified to be significant in univariate
analysis were then entered in a multivariate analysis to identify
these variables with independent prognostic value for PFS and OS.
Risk ratios and 95% confidence intervals were recorded for each
marker. P<0.05 was considered to indicate a statistically
significant difference.
Results
Analysis of ARID1B expression in
TNBC
A total of 142 patients with TNBC were enrolled in
the present study and analyzed for ARID1B expression. Expression
scores >3 were classified as positive nuclear ARID1B staining,
and the remainder were classified as negative. ARID1B was highly
expressed in 62.3% (89/142) of the 142 TNBC specimens. ARID1B
protein was significantly upregulated in the nuclei of cancer cells
compared with that in normal tissues (P<0.001). In 64 normal
controls, only 15 (23.4%) samples had positive nuclear ARID1B
staining. The predominant location of ARID1B staining was the
nuclei. ARID1B expression in TNBC and normal tissues is presented
in Fig. 1.
Analysis of association between ARID1B
and clinicopathological characteristics
Associations between ARID1B expression and a series
of clinicopathological factors (age, menstrual status, histological
grade, tumor size, LNM, TNM stage, the status of p53 and Ki67 and
different chemotherapy strategies) are presented in Table I. ARID1B expression was associated
with histological grade, p53 expression and LNM status. In total,
62/88 histological grade 3 patients (70.5%) exhibited significantly
increased expression of ARID1B compared with grade 1 or 2 patients
(50.0%; 27/54 patients; P=0.014). ARID1B overexpression was also
observed in 73.6% of p53-positive patients (53/72) compared with
51.4% of p53-negative patients (36/70 patients; P=0.006). In
addition, ARID1B expression was upregulated in LNM-positive breast
cancer patients (P=0.033). ARID1B expression may be associated with
invasion and metastasis in patients with TNBC.
 | Table I.Association between AT-rich
interactive domain-containing protein 1B and clinicopathological
factors of patients with triple-negative breast cancer. |
Table I.
Association between AT-rich
interactive domain-containing protein 1B and clinicopathological
factors of patients with triple-negative breast cancer.
| Characteristic | Total, n | Negative, n | Positive, n | P-value |
|---|
| Patients | 142 | 53 (37.3) | 89 (62.7) |
|
| Age, years |
|
|
| 0.994 |
| ≤45 | 59 | 22 (37.3) | 37 (62.7) |
|
|
>45 | 83 | 31 (37.3) | 52 (62.7) |
|
| Menopausal
status |
|
|
| 0.708 |
|
Premenopausal | 91 | 35 (38.5) | 56 (61.5) |
|
|
Postmenopausal | 51 | 18 (35.3) | 33 (64.7) |
|
| Lymph node |
|
|
| 0.033 |
|
Negative | 72 | 33 (45.8) | 39 (54.2) |
|
|
Positive | 70 | 20 (28.6) | 50 (71.4) |
|
| Grade |
|
|
| 0.014 |
| 1+2 | 54 | 27 (50.0) | 27 (50.0) |
|
| 3 | 88 | 26 (29.5) | 62 (70.5) |
|
| Tumor size, cm |
|
|
| 0.916 |
| ≤2 | 49 | 18 (36.7) | 31 (63.3) |
|
|
>2 | 93 | 35 (37.6) | 58 (62.4) |
|
| TNM stage |
|
|
| 0.075 |
| I/II | 100 | 42 (42.0) | 58 (58.0) |
|
| III | 42 | 11 (26.2) | 31 (73.8) |
|
| p53 |
|
|
| 0.006 |
|
Negative | 70 | 34 (48.6) | 36 (51.4) |
|
|
Positive | 72 | 19 (26.4) | 53 (73.6) |
|
| Ki67 |
|
|
| 0.240 |
|
Negative | 74 | 31 (41.9) | 43 (58.1) |
|
|
Positive | 68 | 22 (32.4) | 46 (67.6) |
|
| Chemotherapy
regimen |
|
|
| 0.468 |
|
Taxanes | 18 | 9 (50.0) | 9 (50.0) |
|
|
Anthracyclin | 84 | 29 (34.5) | 55 (65.5) |
|
|
Anthracycline + taxanes | 40 | 15 (37.5) | 25 (62.5) |
|
Univariate and multivariate survival
analysis of prognosis
Univariate and multivariate analyses were performed
to evaluate the impact of ARID1B expression and clinicopathological
features on the prognosis of patients with TNBC. Cox's regression
analysis of univariate analysis demonstrated that OS was
significantly associated with LNM (P=0.003), p53 (P=0.025), ARID1B
(P=0.010) and TNM stage (P<0.001). PFS was also significantly
associated with LNM (P=0.001), ARID1B (P=0.003) and TNM stage
(P<0.001). Multivariate analysis was also conducted on the same
set of patients using Cox's regression model. Results from the
multivariate analysis confirmed that ARID1B expression was a
significant independent prognostic factor for OS and PFS of
patients with TNBC (P=0.006 and P=0.002, respectively).
Additionally, TNM stage and p53 were independent prognostic factors
for OS and PFS of patients with TNBC (Table II). These results indicated that
upregulated expression of ARID1B was associated with poor prognosis
in patients with TNBC.
 | Table II.Univariate and multivariate Cox's
regression analysis. |
Table II.
Univariate and multivariate Cox's
regression analysis.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Overall
survival |
|
|
|
|
|
|
| Age
(≥45 vs. <45 years) | 1.274 | 0.534–3.037 | 0.585 |
|
|
|
| Lymph
node (positive vs. negative) | 5.225 | 1.768–15.445 | 0.003 |
|
|
|
| Grade
(3 vs. 1+2) | 2.272 | 0.838–6.160 | 0.107 |
|
|
|
| Tumor
size (>2 vs. ≤2 cm) | 2.503 | 0.847–7.398 | 0.097 |
|
|
|
| TNM
stage (III vs. II+I) | 4.892 | 2.05–11.669 | <0.001 | 4.543 | 1.893–10.902 | 0.001 |
| p53
(positive vs. negative) | 2.920 | 1.142–7.463 | 0.025 | 4.564 | 1.770–11.770 | 0.002 |
| Ki67
(positive vs. negative) | 1.383 | 0.597–3.200 | 0.449 |
|
|
|
|
Menopausal status
(postmenopausal vs. premenopausal) | 1.559 | 0.673–3.609 | 0.300 |
|
|
|
| ARID1B
expression (high vs. low) | 6.742 | 1.575–28.855 | 0.010 | 7.868 | 1.812–34.157 | 0.006 |
| Progression-free
survival |
|
|
|
|
|
|
| Age
(≥45 vs. <45 years) | 0.716 | 0.382–1.343 | 0.298 |
|
|
|
| Lymph
node (positive vs. negative) | 3.155 | 1.570–6.343 | 0.001 |
|
|
|
| Grade
(3 vs. 1+2) | 1.785 | 0.888–3.585 | 0.104 |
|
|
|
| Tumor
size (>2 vs. ≤2 cm) | 1.191 | 0.603–2.351 | 0.615 |
|
|
|
| TNM
stage (III vs. II+I) | 3.133 | 1.670–5.879 | <0.001 | 2.994 | 1.584–5.659 | 0.001 |
| p53
(positive vs. negative) | 1.777 | 0.932–3.389 | 0.081 | 2.663 | 1.375–5.158 | 0.004 |
| Ki67
(positive vs. negative) | 1.230 | 0.656–2.305 | 0.518 |
|
|
|
|
Menopausal status
(postmenopausal vs. pre-menopausal) | 1.005 | 0.522–1.933 | 0.988 |
|
|
|
| ARID1B
expression (high vs. low) | 3.420 | 1.508–7.757 | 0.003 | 3.885 | 1.679–8.988 | 0.002 |
Kaplan-Meier survival analysis
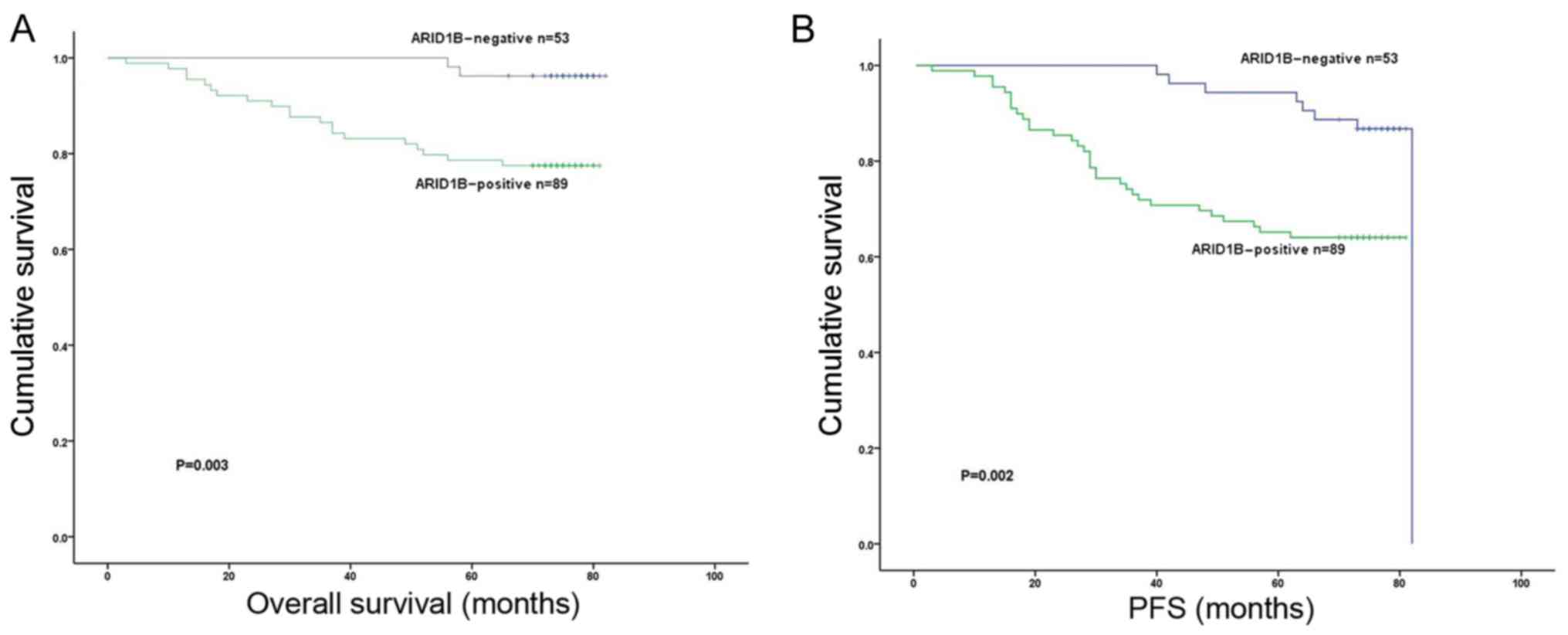
The Kaplan-Meier 5-year survival curves stratified
for ARID1B expression are presented in Fig. 2. Among the 142 patients with TNBC, the
status of nuclear ARID1B expression demonstrated significant
effects on OS (P=0.003; Fig. 2A) and
PFS (P=0.002; Fig. 2B) and. Patients
with TNBC exhibiting high ARID1B expression had significantly
poorer PFS (P=0.002) and OS compared with patients with low ARID1B
expression (P=0.003).
Discussion
TNBC has a poor overall prognosis, and available
hormonal or targeted treatment options for this disease are
insufficient (16,17). No targeted agent is currently
available for TNBC despite the great advances in treating
HER2-positive or ER-positive breast cancer (2). The TNBC phenotype is heterogeneous from
a histopathological and molecular perspective, which indicates that
molecular subsets exist (3).
Therefore, identifying and evaluating predictive molecular
signatures is important. These processes may be advantageous for
the characterization of TNBC and the design of therapeutic
modalities. In the present study, the expression and clinical
significance of ARID1B was first evaluated in 142 cases of TNBC.
Results identified that the status of ARID1B expression may be a
prognostic factor of TNBC.
ARID1A and ARID1B belong to the SWI/SNF chromatin
remodeling complex family, which may enhance or suppress gene
transcription by mobilizing nucleosomes (18). ARID1A and ARID1B subunits are only
present in BAF of SWI/SNF complexes (19). ARID1B is a mutually exclusive subunit
and highly homologous with ARID1A (20). Multiple studies have revealed that
ARID1A is an essential gene that serves a tumor suppressor role,
which is primarily involved in negative regulation of cell cycle
progression and has a tumor suppressor function (21–23).
ARID1A and ARID1B subunits have distinct roles in cell
proliferation; ARID1A exhibits an anti-proliferative function, in
contrast with the pro-proliferative function of ARID1B (7). ARID1B and ARID1A, which reportedly have
opposite functions in cell-cycle arrest, are 60% identical
(24). For instance, mouse embryonic
stem cells with biallelic inactivation of ARID1B revealed decreased
proliferation rate and the abnormality of cell cycle dynamics
(25). In addition,
Arid1b-deficient human fibroblasts exhibited a delayed G1 to
S phase cell cycle progression (26).
ARID1B was demonstrated to be necessary in preosteoblast cell lines
for increased c-Myc oncoprotein expression, which is frequently
observed in various human malignancies and is known to be essential
in preventing cell cycle arrest in response to growth inhibitory
signals (27). In addition, a
previous study demonstrated that ARID1B presents a specific
vulnerability in human cancers with ARID1A mutant alleles,
indicating that ARID1B is required for Arid1a mutations to
promote tumorigenesis (28).
Therefore, in view of its pro-proliferative function, ARID1B may
have an opposing role in the tumorigenesis caused by ARID1A.
In the present study, it was established that ARID1B
is a prognostic biomarker in patients with TNBC, considering that
ARID1B-positive patients presented with a significantly decreased
5-year survival rate compared with ARID1B-negative patients. These
results were consistent with a previous study on ARID1B expression
in breast cancer; high expression of ARID1B was associated with a
decreased 5-year disease-free survival rate (11). In the present study, patients with
TNBC with high histological grade (G3) had increased ARID1B
expression compared with those with low histological grade (G1/G2)
(P=0.014; Table I). ARID1B may serve
a vital role in tumor progression and invasion. However, ARID1B has
been demonstrated to serve as a tumor suppressor in pancreatic
cancer cell lines (12). Therefore,
the roles of ARID1B may differ depending on the cell type.
Additionally, ARID1B expression was associated with LNM (P=0.033),
indicating that ARID1B expression is a potential marker for
predicting LNM in patients with TNBC. These results indicated that
ARID1B serves an important role in the prognosis of TNBC and may be
a novel prognostic factor for patients with TNBC.
To the best of our knowledge, the present study is
the first to investigate the ARID1B expression and its association
with prognosis in TNBC. However, the present study has several
limitations. For instance, a relatively small number of Chinese
patients were evaluated from a single center, and the present study
is retrospective. In addition, the follow-up period was not long,
and the expression status of ARID1B was not analyzed in nodal or
distant metastasis sites. However, the results of the present study
identified that survival and prognosis of patients with TNBC may
depend on ARID1B expression and clinicopathological factors,
including TNM stage. Validation of these results using a larger
sample size with multiple centers is required in future studies to
explore the underlying molecular mechanisms and functional role of
ARID1B.
In conclusion, TNBC may be classified into good and
poor prognostic subtypes according to the ARID1B expression status.
ARID1B may serve as a prognostic biomarker for TNBC. However, more
robust studies are required to investigate the molecular mechanism
underlying the upregulated ARID1B expression in TNBC and determine
whether ARID1B is a potential therapeutic target.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding from the
Heilongjiang Province Applied Technology Research and Development
Project (grant no. GA13C201), the National Key Technology Support
Program (grant no. 2013BAI09B00), the National Natural Science
Foundation of China (grant no. 81172498/H1622), the Heilongjiang
Postdoctoral Fund (grant no. LBH-Z16216), the Foundation of Health
and Family Planning Commission of Heilongjiang Province (grant no.
2016-114) and the Hai Yan Foundation of Harbin Medical University
Cancer Hospital (grant no. JJQN2016-01).
Availability of data and materials
The data used during the current study are available
from the corresponding author on reasonable request.
Authors' contributions
YC contributed to the study design and the writing
of the paper. XZ contributed to analysis and interpretation of
data. XB performed the experiments. YQ contributed to the
pathologic analysis. MN and DP contributed to the study design. All
authors read and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of Harbin Medical University. Written consent was
obtained from all study participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no completing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Q, Liu HY, Liu D and Song YQ:
Ultrasonographic features of triple-negative breast cancer: A
comparison with other breast cancer subtypes. Asian Pac J Cancer
Prev. 16:3229–3232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reisman D, Glaros S and Thompson EA: The
SWI/SNF complex and cancer. Oncogene. 28:1653–1668. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Phelan ML, Sif S, Narlikar GJ and Kingston
RE: Reconstitution of a core chromatin remodeling complex from
SWI/SNF subunits. Mol Cell. 3:247–253. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inoue H, Furukawa T, Giannakopoulos S,
Zhou S, King DS and Tanese N: Largest subunits of the human SWI/SNF
chromatin-remodeling complex promote transcriptional activation by
steroid hormone receptors. J Biol Chem. 277:41674–41685. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagl NG Jr, Wang X, Patsialou A, Van Scoy
M and Moran E: Distinct mammalian SWI/SNF chromatin remodeling
complexes with opposing roles in cell-cycle control. EMBO J.
26:752–763. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoyer J, Ekici AB, Endele S, Popp B,
Zweier C, Wiesener A, Wohlleber E, Dufke A, Rossier E, Petsch C, et
al: Haploinsufficiency of ARID1B, a member of the SWI/SNF-a
chromatin-remodeling complex, is a frequent cause of intellectual
disability. Am J Hum Genet. 90:565–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sausen M, Leary RJ, Jones S, Wu J,
Reynolds CP, Liu X, Blackford A, Parmigiani G, Diaz LA Jr,
Papadopoulos N, et al: Integrated genomic analyses identify ARID1A
and ARID1B alterations in the childhood cancer neuroblastoma. Nat
Genet. 45:12–17. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stephens PJ, Tarpey PS, Davies H, Van Loo
P, Greenman C, Wedge DC, Nik-Zainal S, Martin S, Varela I, Bignell
GR, et al: The landscape of cancer genes and mutational processes
in breast cancer. Nature. 486:400–404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shao F, Guo T, Chua PJ, Tang L, Thike AA,
Tan PH, Bay BH and Baeg GH: Clinicopathological significance of
ARID1B in breast invasive ductal carcinoma. Histopathology.
67:709–718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khursheed M, Kolla JN, Kotapalli V, Gupta
N, Gowrishankar S, Uppin SG, Sastry RA, Koganti S, Sundaram C,
Pollack JR and Bashyam MD: ARID1B, a member of the human SWI/SNF
chromatin remodeling complex, exhibits tumour-suppressor activities
in pancreatic cancer cell lines. Br J Cancer. 108:2056–2062. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berger AM, Mooney K, Alvarez-Perez A,
Breitbart WS, Carpenter KM, Cella D, Cleeland C, Dotan E,
Eisenberger MA, Escalante CP, et al: Cancer-Related Fatigue,
Version 2.2015. J Natl Compr Canc Netw. 13:1012–1039. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheang MC, Chia SK, Voduc D, Gao D, Leung
S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67
index, HER2 status, and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kobayashi T, Iwaya K, Moriya T, Yamasaki
T, Tsuda H, Yamamoto J and Matsubara O: A simple
immunohistochemical panel comprising 2 conventional markers, Ki67
and p53, is a powerful tool for predicting patient outcome in
luminal-type breast cancer. BMC Clin Pathol. 13:52013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bauer KR, Brown M, Cress RD, Parise CA and
Caggiano V: Descriptive analysis of estrogen receptor
(ER)-negative, progesterone receptor (PR)-negative, and
HER2-negative invasive breast cancer, the so-called triple-negative
phenotype: A population-based study from the California cancer
Registry. Cancer. 109:1721–1728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Asleh-Aburaya K and Fried G: Clinical and
molecular characteristics of triple-negative breast cancer patients
in Northern Israel: Single center experience. Springerplus.
4:1322015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weissman B and Knudsen KE: Hijacking the
chromatin remodeling machinery: Impact of SWI/SNF perturbations in
cancer. Cancer Res. 69:8223–8230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wilson BG and Roberts CW: SWI/SNF
nucleosome remodellers and cancer. Nat Rev Cancer. 11:481–492.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Nagl NG, Wilsker D, Van Scoy M,
Pacchione S, Yaciuk P, Dallas PB and Moran E: Two related ARID
family proteins are alternative subunits of human SWI/SNF
complexes. Biochem J. 383:319–325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao J, Liu C and Zhao Z: ARID1A: A
potential prognostic factor for breast cancer. Tumour Biol.
35:4813–4819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mamo A, Cavallone L, Tuzmen S, Chabot C,
Ferrario C, Hassan S, Edgren H, Kallioniemi O, Aleynikova O,
Przybytkowski E, et al: An integrated genomic approach identifies
ARID1A as a candidate tumor-suppressor gene in breast cancer.
Oncogene. 31:2090–2100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guan B, Gao M, Wu CH, Wang TL and Shih Ie
M: Functional analysis of in-frame indel ARID1A mutations reveals
new regulatory mechanisms of its tumor suppressor functions.
Neoplasia. 14:986–993. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu JN and Roberts CW: ARID1A mutations in
cancer: Another epigenetic tumor suppressor? Cancer Discov.
3:35–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Flores-Alcantar A, Gonzalez-Sandoval A,
Escalante-Alcalde D and Lomeli H: Dynamics of expression of ARID1A
and ARID1B subunits in mouse embryos and in cells during the cell
cycle. Cell Tissue Res. 345:137–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sim JC, White SM, Fitzpatrick E, Wilson
GR, Gillies G, Pope K, Mountford HS, Torring PM, McKee S, Vulto-van
Silfhout AT, et al: Expanding the phenotypic spectrum of
ARID1B-mediated disorders and identification of altered cell-cycle
dynamics due to ARID1B haploinsufficiency. Orphanet J Rare Dis.
9:432014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Inghirami G, Chiarle R, Simmons WJ, Piva
R, Schlessinger K and Levy DE: New and old functions of STAT3: A
pivotal target for individualized treatment of cancer. Cell Cycle.
4:1131–1133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Helming KC, Wang X, Wilson BG, Vazquez F,
Haswell JR, Manchester HE, Kim Y, Kryukov GV, Ghandi M, Aguirre AJ,
et al: ARID1B is a specific vulnerability in ARID1A-mutant cancers.
Nat Med. 20:251–254. 2014. View
Article : Google Scholar : PubMed/NCBI
|