Introduction
Osteosarcoma is a common type of primary malignant
tumor of bone that predominantly occurs in children and
adolescents, with a high propensity for local invasion and early
systemic metastasis (1). It occurs
most frequently in the long bones while they are growing, including
the humerus, femur and tibia (2). At
present, treatment typically includes neoadjuvant chemotherapy,
surgical resection and a successive course of chemotherapy
following surgery (2). Although the
5-year survival rate for patients with localized osteosarcoma is
60% (3–5), the rate has remained relatively
unchanged during the previous three decades. The prognosis is
particularly poor for patients with metastasis or relapse, with
survival rates of 20–30% (6–8).
The gold standard for osteosarcoma chemotherapy is
based on 5 drugs: High-dose methotrexate with leucovorin rescue,
doxorubicin, cisplatin, ifosfamide and etoposide (9). However, high-dose chemotherapeutic
drugs are not always effective and may cause severe side effects;
in addition, multidrug resistant cases are common, particularly to
cisplatin, doxorubicin and the majority of neoadjuvant chemotherapy
drugs (4,5). Therefore, it is critical to develop
novel therapies for the management of osteosarcoma.
Celastrol is a triterpenoid isolated from the
‘Thunder of God Vine’, which is used in traditional Chinese
medicine. Previous studies have demonstrated that it may inhibit
growth and encourage apoptosis in a variety of human cancer cell
lines, including hepatoma, myeloma, and breast, pancreatic and
gastric cancer cell lines (10–14).
Celastrol may also induce the apoptosis of human osteosarcoma
cells, via the mitochondrial pathway of apoptosis (15). Therefore, as tumor cells exhibit
increasing resistance to traditional chemotherapy drugs including
cisplatin, combinatorial treatments, including a combination of
cisplatin with celastrol, may assist in improving the efficacy of
chemotherapy.
In the present study, the effects of cisplatin,
celastrol and combined cisplatin/celastrol on the human
osteosarcoma U-2OS cell line was investigated, and the molecular
mechanism by which celastrol/cisplatin induce the apoptosis of
osteosarcoma cells was examined, with the aim of providing a
theoretical basis for their clinical combination.
Materials and methods
Materials and reagents
Dulbecco's modified Eagle's medium (DMEM), PBS,
dimethyl sulfoxide (DMSO) and MTT were purchased from Beijing
Solarbio Science & Technology Co., Ltd. (Beijing, China). Fetal
bovine serum (FBS) was purchased from Beijing Transgen Biotech Co.,
Ltd. (Beijing, China) and a Hoechst 33258 staining kit was provided
by Nanjing Keygen Biotech Co., Ltd. (Nanjing, China). Antibodies
against Bcl-2 (cat no. ab32124), Bcl-2-associated X protein (Bax;
cat no. ab32503), caspase-3 (cat no. ab13847), caspase-8 (cat no.
ab108333), caspase-9 (cat no. ab32539), poly(ADP-ribose) polymerase
(PARP; cat no. ab219953), cytochrome c (cat no. ab133504),
78 kDa glucose-regulated protein (GRP78; cat no. ab21685) and
C/EBP-homologous protein (CHOP; cat no. ab11419) were purchased
from Abcam (Cambridge, MA, USA). β-actin (cat no. 8H10D10) was
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Horseradish peroxidase (HRP)-conjugated secondary antibodies
(anti-mouse antibody; cat no. 14709S; and anti-rabbit antibody; cat
no. ZB-2306) were purchased from Cell Signaling Technology, Inc.
and Beijing Transgen Biotech Co., Ltd., respectively. An Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis
detection kit was provided by Nanjing Keygen Biotech Co., Ltd.
Celastrol and cisplatin were obtained from Nanjing
Zelang Medical Technology Co., Ltd. (Nanjing, China). Stock
solutions of celastrol were prepared by dissolving the celastrol
powder in DMSO to a concentration of 20 M, and stock solutions of
cisplatin were prepared by dissolving the cisplatin powder in
saline to 1 mg/l; these were stored at −20°C. Working solutions of
celastrol and cisplatin were prepared by diluting the stock
solution with culture medium. The final concentration of DMSO in
the medium was <0.1%.
Cell culture
Cells of the human osteosarcoma U-2OS cell line were
obtained from the American Type Culture Collection (Manassas, VA,
USA). Cells were cultured in DMEM supplemented with 10% (v/v) FBS,
100 µ/ml penicillin, and 100 µg/ml streptomycin. Cells were kept in
a humidified atmosphere containing 5% CO2 at 37°C. Cells
used in the present study had been subjected to <20 cell
passages and were in the logarithmic growth phase.
Quantification of cell viability by
MTT assay
Cells were cultured in 96-well plates at a
concentration of 1×104 cells/well and cell viability was
determined using an MTT colorimetric assay. Cells were treated with
various concentrations of celastrol (1, 2, 3, 4 and 5 µM),
cisplatin (2, 4, 6, 8 and 10 µg/ml), or a combination of
celastrol/cisplatin at each final concentration, for 24, 36 or 48
h; control cells were treated with 0.02% DMSO. Following the
incubation period, 20 µl of MTT (5 mg/ml in PBS) was added and the
plates were incubated at 37°C for an additional 4 h. The formazan
precipitate was then dissolved in 150 µl DMSO and agitated for 10
min. Absorbance was measured at 490 nm using a universal microplate
reader (ELISA Reader Model EXl800; BioTek Instruments, Inc.,
Winooski, VT, USA). Cell growth was expressed as the relative
percentage of viability by comparing the absorbance of treated vs.
control cells. Each experiment was repeated 3 times at each time
point/dose.
Quantification of apoptosis by Annexin
V-FITC/PI staining assay
To assess the induction of apoptosis by celastrol
and cisplatin, U-2OS cells were stained with the Annexin V-FITC/PI
kit. U-2OS cells were seeded in 6-well culture plates
(1.5×105 cells/well) and incubated for 24 h; the cells
were incubated with celastrol (2.6 µM) and/or cisplatin (6.1 mg/l)
for 48 h and collected by trypsinization, without EDTA. Following
two rounds of washing with PBS at 4°C, the cell pellets were
re-suspended in 400 µl ice-cold 1X binding buffer at a density of
~1×106 cells/ml and incubated in Annexin V-FITC and PI
(10 µg/ml) at room temperature for 10 min in the dark. Samples were
analyzed using a flow cytometer within 1 h of staining. BD Accuri
C6 Software 1.0.264.21 (BD Biosciences, Franklin Lakes, NJ, USA)
was used for analysis, and the experiment was repeated 3 times.
Hoechst 33258 staining of U-2OS
cells
Cells were incubated for 48 h with the
IC50 of celastrol, cisplatin or celastrol combined with
cisplatin, harvested, fixed with 4% paraformaldehyde for 30 min at
25°C, washed 3 times with ice-cold PBS and stained with 10 mg/l
Hoechst 33258 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for
10 min in the dark at room temperature. The stained nuclei were
observed under a fluorescence microscope (Olympus Corporation,
Tokyo, Japan; magnification, ×100) with excitation at 350 nm and
emission at 460 nm. This experiment was repeated 3 times.
Western blot analysis
U-2OS cells were cultured in 6-well plates
(2×105 cells/well). Following treatment with the
indicated concentration of celastrol and/or cisplatin [group 1,
celastrol (2.6 µM); group 2, cisplatin (6.1 mg/l); group 3,
celastrol (2.6 µM) combined with cisplatin (6.1 mg/l)] for 48 h
and, subsequent to washing with ice-cold PBS, the cells were
collected and lysed in radioimmunoprecipitation assay buffer
containing a protease inhibitor cocktail (Sigma-Aldrich; Merck
KGaA). The homogenates were centrifuged at 24,148.8 × g for 10 min
at 4°C and the supernatant fraction was collected for
immunoblotting. Protein concentrations were determined with a BCA
assay using bovine serum albumin as the standard. Equal quantities
(45 µg) of protein were loaded and separated by electrophoresis on
12% SDS-PAGE gels under reducing conditions at 110 V for 2 h.
Following electrophoresis, the proteins were transferred to
polyvinylidene fluoride (PVDF) membranes in a Tris-glycine transfer
buffer (3.05 g Tris, 14.4 g glycine and 200 ml methanol solute in
1,000 ml water) using a semi-dry blotting system, and incubated
with antibodies against Bcl-2, Bax, cytochrome c, caspase-3,
−8 and −9, GRP78, CHOP, PARP and β-actin (dilution, 1:1,000)
overnight at 4°C. The PVDF membranes were washed in Tris-buffered
saline with Tween-20 (TBST) 3 times and secondary HRP-conjugated
antibodies (dilution, 1:2,000) were added for 2 h at room
temperature. The PVDF membranes were then re-washed in TBST 3
times. Bound antibodies were detected using the ECL Plus Western
Blotting Detection system (GE Healthcare Bio-Sciences, Pittsburgh,
PA, USA) and a LAS-1000 Plus Image Analyzer (Fujifilm Holdings
Corporation, Tokyo, Japan). The western blots were quantified by
densitometric analysis using Image Lab 4.0.153407 software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). This experiment was
repeated 3 times.
Combination index (CI) analyses
The multiple drug effect analysis developed by Chou
and Talalay (16), which is based on
a median-effect principle, was used to calculate combined drug
effects; results are presented as a CI. Synergism, additivity and
antagonism are indicated by CI<1, CI=1, and CI>1,
respectively.
Statistical analysis
Data were analyzed using SPSS 17 (SPSS, Inc.,
Chicago, IL, USA). Quantitative data are expressed as the mean ±
standard deviation. Statistical analysis was performed using a
paired Student's t-test and one-way analysis of variance. Fisher's
Least Significant Difference test was used as a post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Celastrol acts synergistically with
cisplatin to inhibit the growth of U-2OS cells
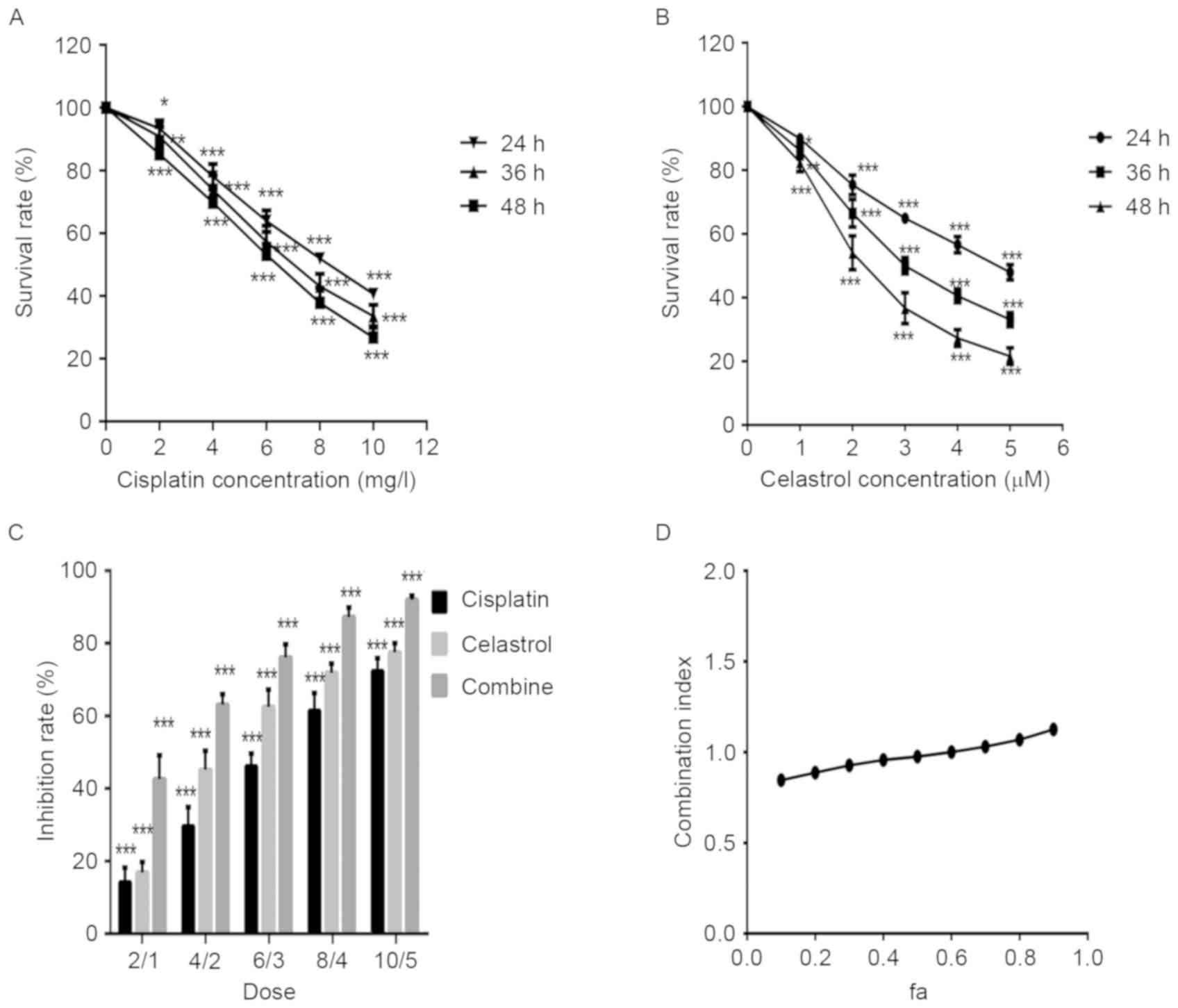
The effect of celastrol and cisplatin on the
viability of U-2OS cells was determined by an MTT assay. U-2OS
cells were treated with various concentrations of celastrol and
cisplatin for 24, 36, and 48 h. As demonstrated in Fig. 1A and B, celastrol and cisplatin
inhibited the growth of U-2OS cells in a dose-dependent manner. The
IC50 value for U-2OS cells was 2.6 µM at 48 h for
celastrol and 6.1 mg/l for cisplatin.
The effect of a combination of celastrol and
cisplatin on the growth of U-2OS cells was also examined, to
investigate potential synergistic effects. Combined treatment with
celastrol/cisplatin markedly decreased U-2OS cell growth, compared
with celastrol or cisplatin alone (Fig.
1C). CI values, based on the Chou-Talalay equation (12), were calculated to estimate the
efficacy of celastrol/cisplatin. The CI-‘fraction affected’ curve
suggested that combined treatment with celastrol/cisplatin
exhibited a synergistic effect on cell growth inhibition, with CI
values ranging from 0.80 to 0.97 at effect levels from
IC10 to IC70 (Fig.
1D).
Celastrol acts synergistically with
cisplatin to induce the apoptosis of U-2OS cells
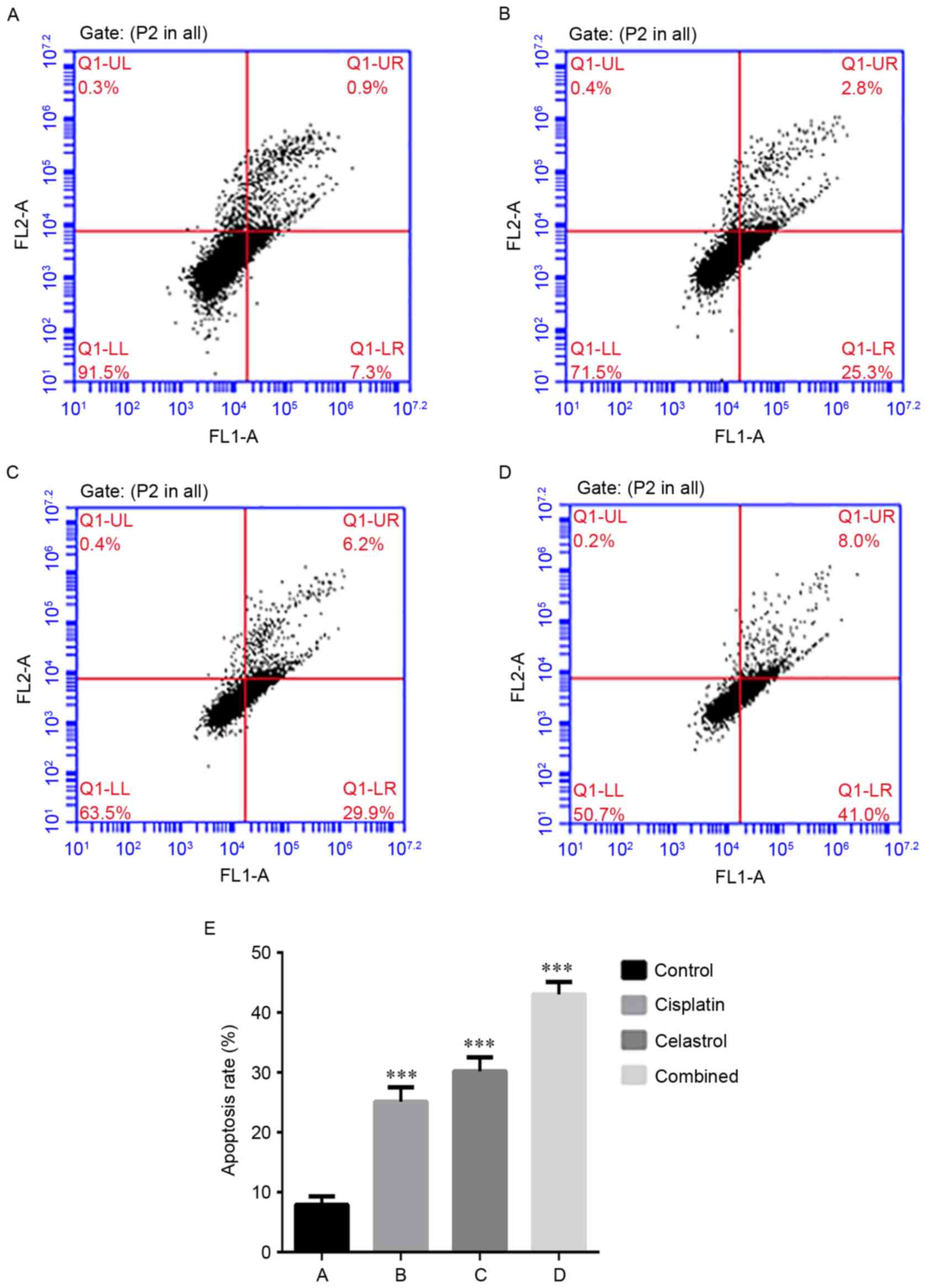
Annexin V-FITC/PI double staining-based FACS
analysis was used to investigate the effect of celastrol/cisplatin
on apoptosis of U-2OS cells. The apoptosis rate was 7.9±1.4% in the
control group, 30.2±2.3% in cells treated with celastrol (2.6
µmol/l), 25.1±2.4% in cells treated with cisplatin (6.1 mg/l) and
43.0±2.1% in cells treated with celastrol/cisplatin (Fig. 2). This result suggests that
celastrol/cisplatin in combination exhibit an enhanced ability to
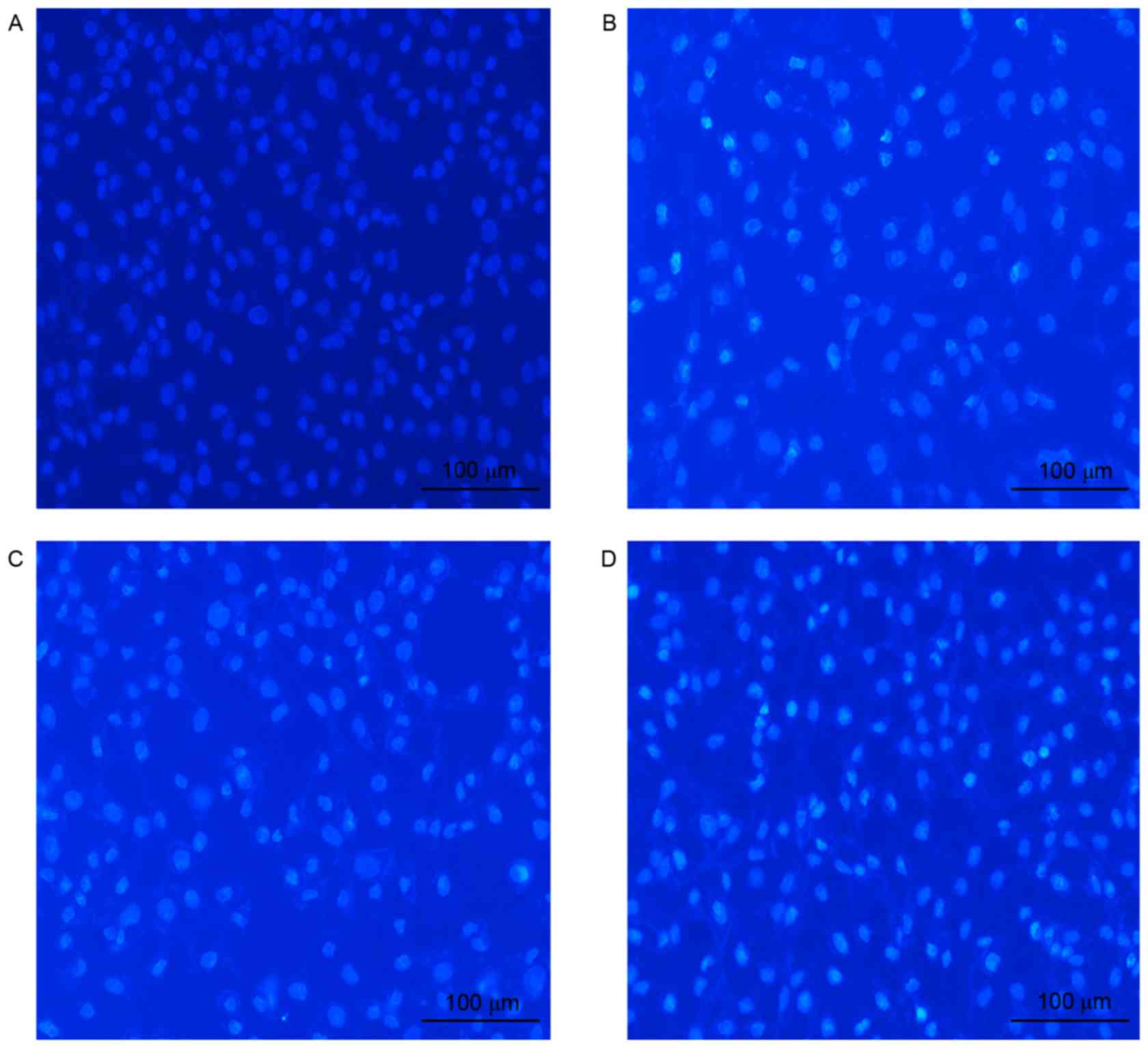
induce apoptosis. In addition, cell morphology analysis performed
subsequent to the staining of U-2OS cells with the fluorescent
DNA-binding dye Hoechst 33258 supported the observation that the
number of apoptotic cells was higher following celastrol/cisplatin
treatment compared with either treatment alone; no morphological
signs of apoptosis were observed in untreated cells, in contrast to
the treated cells (Fig. 3).
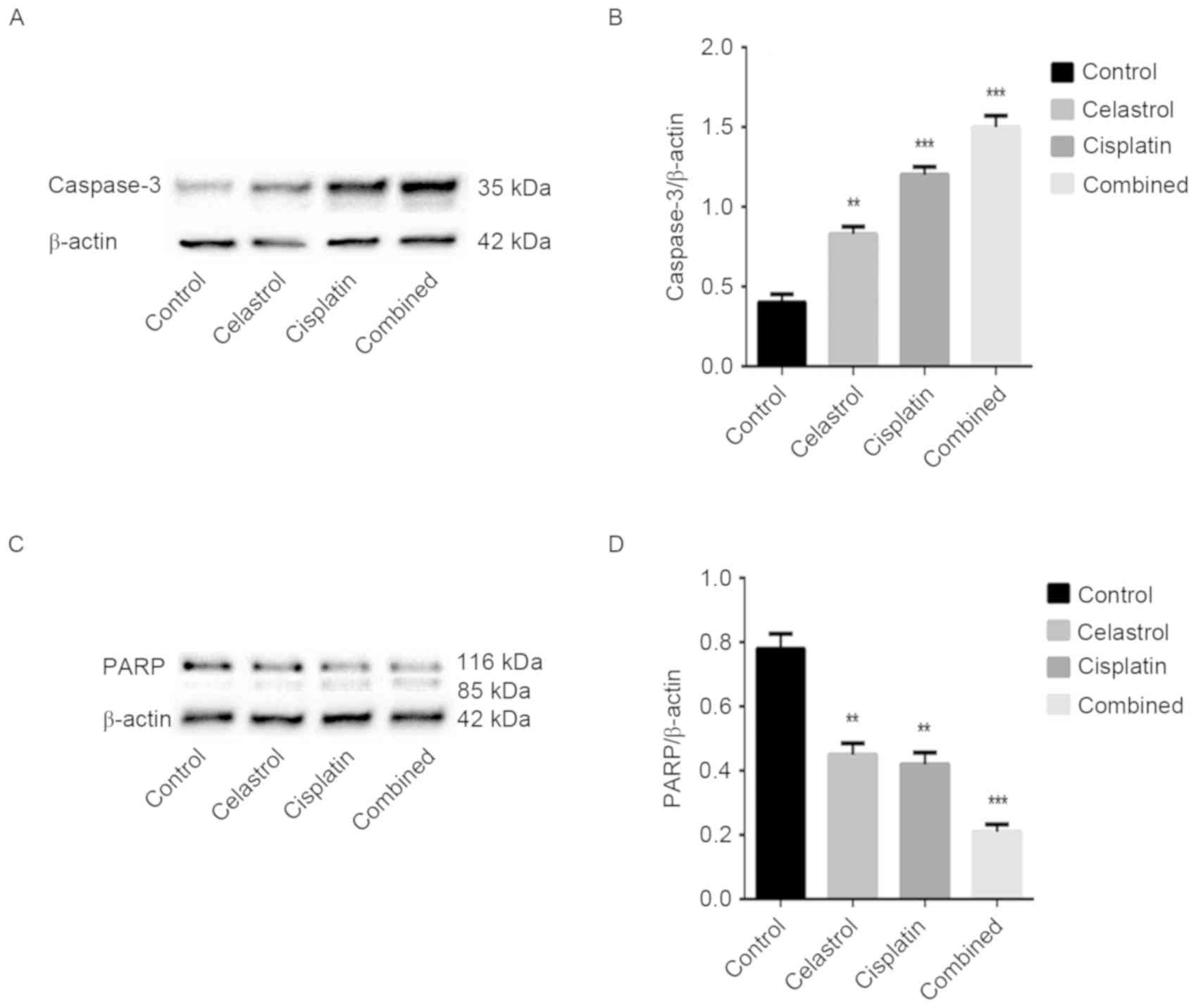
Effect of celastrol/cisplatin on the
expression of caspase-family proteins
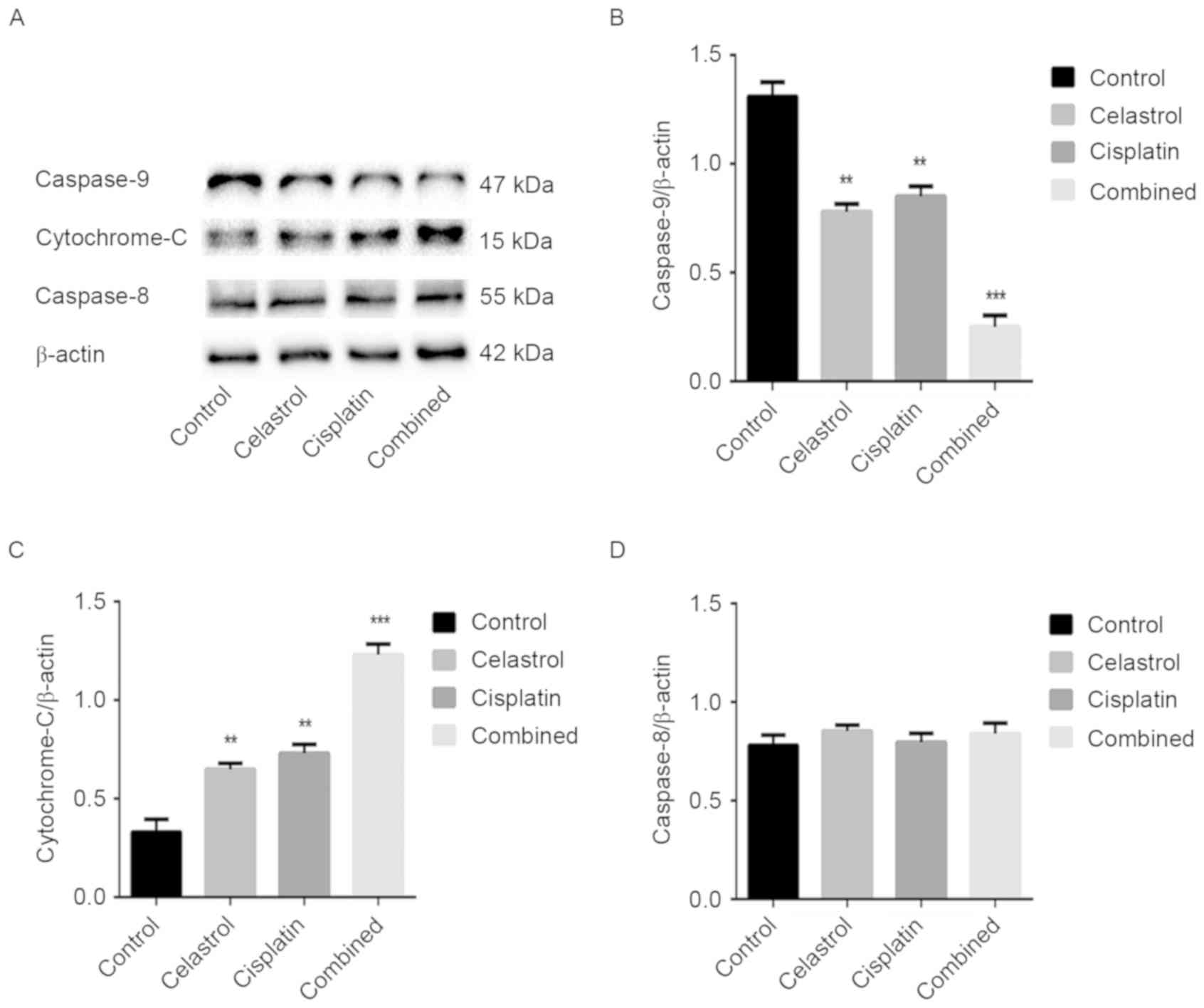
The caspase cascade reaction is critical in the
process of apoptosis; therefore, the expression levels of
caspase-3, −8 and −9 were assessed. Caspase-3 cleavage was
observed, and the expression level of caspase-9 was downregulated
in the treatment groups, particularly following celastrol/cisplatin
treatment. However, the expression levels of caspase-8 remained
unchanged in cells treated with celastrol/cisplatin. The cleavage
of PARP, a key cellular substrate of caspases, was observed; the
relative expression of intact PARP was significantly reduced by all
treatments, particularly the combination treatment. The results
suggest that apoptosis induced by celastrol/cisplatin was
associated with the caspase cascade (P<0.001; Figs. 4 and 5).
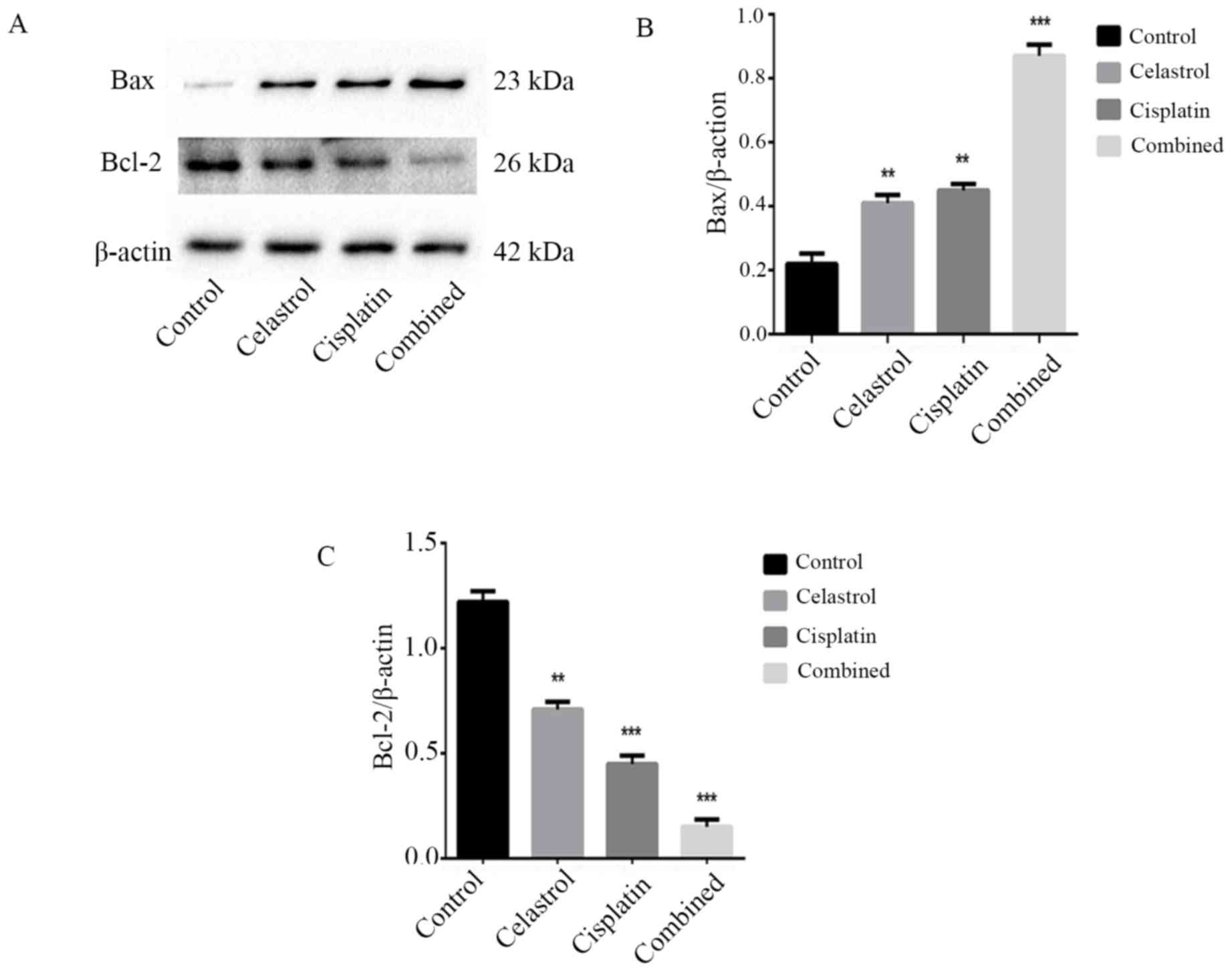
Effects of celastrol/cisplatin on the
expression of proteins associated with mitochondrial apoptosis,
Bcl-2, Bax, and cytochrome c
To investigate the molecular mechanism by which
celastrol/cisplatin induces apoptosis in U-2OS cells, the
expression levels of proteins associated with the mitochondrial
pathway of apoptosis, including Bcl-2, Bax, and cytochrome
c, were assessed. Western blot analysis revealed that
following celastrol/cisplatin treatment, the expression levels of
cytochrome c and Bax were upregulated, but that the
expression level of Bcl-2 was downregulated, compared with either
treatment alone or the control (Figs.
5 and 6; P<0.001). This
confirmed that celastrol/cisplatin may regulate the expression of
the Bcl-2 family proteins to activate the mitochondrial apoptotic
pathway in U-2OS cells.
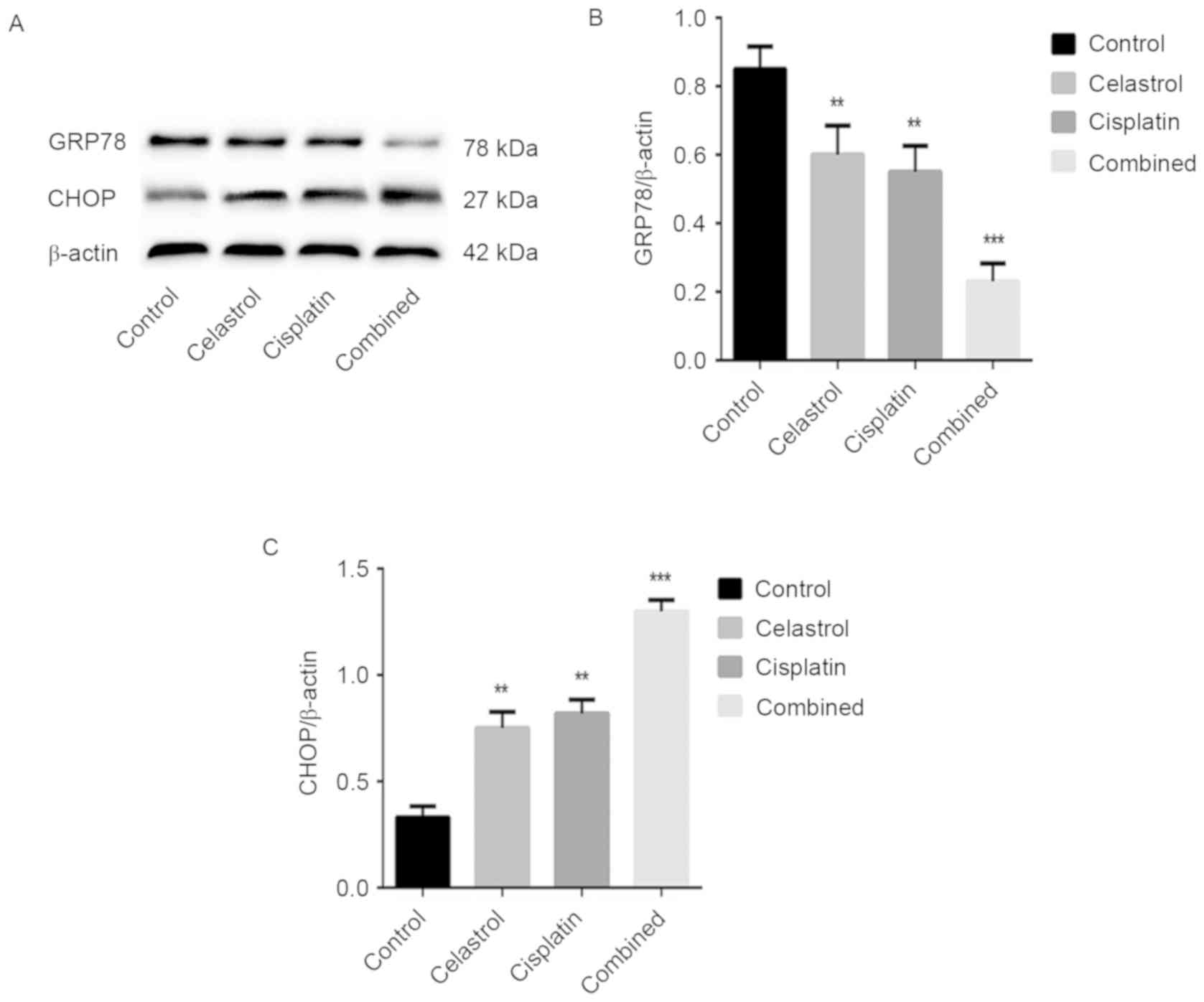
Effects of celastrol/cisplatin on the
expression of proteins associated with the endoplasmic reticulum
pathway of apoptosis
It was previously demonstrated that cisplatin
treatment may induce endoplasmic reticulum stress (17); therefore, we hypothesized that
celastrol/cisplatin activates the endoplasmic reticulum pathway of
apoptosis in U-2OS cells. Consequently, the expression levels of
the endoplasmic reticulum stress-associated protein GRP78 and the
endoplasmic reticulum stress-apoptosis-associated protein CHOP were
assessed by western blot analysis. The expression level of GRP78
was downregulated, whereas CHOP was upregulated following
celastrol/cisplatin treatment, compared with either treatment alone
or the control (P<0.001; Fig. 7).
These data demonstrated that celastrol/cisplatin treatment may
induce endoplasmic reticulum stress and induce the progress of
apoptosis via the endoplasmic reticulum apoptotic pathway.
Discussion
Apoptosis, genetically controlled cell suicide, is
an important mechanism by which numerous anticancer drugs elicit
their effects. Apoptosis is an innate mammalian cellular response
for eliminating abnormal or redundant cells. The two classical
pathways for the induction of apoptosis are the cell death receptor
pathway and the mitochondrial pathway (18–21); in
addition, several studies have identified an additional pathway,
the endoplasmic reticulum pathway (22,23).
In the mitochondrial pathway, downstream caspase
activation is regulated by members of the Bcl-2 family (24–26). The
apoptosis-associated mitochondrial outer membrane permeabilization
requires pro-apoptotic Bax-like proteins, which regulate
mitochondrial pore formation; the anti-apoptotic Bcl-2-like
proteins are functionally distinct in their role in apoptosis. The
ratio of Bax to Bcl-2 is important for determining the release of
apoptogenic proteins from the mitochondrial intermembrane space,
including cytochrome c, which activates caspase-9 (24–26).
Firstly, caspase-9 is activated, following which it activates the
downstream caspase-3, which causes the cleavage or degradation of
various key cellular substrates, including PARP, leading to
apoptosis (24–26). In the cell death receptor pathway,
also termed the extrinsic pathway, the interaction between the Fas
cell surface receptor and the Fas ligand promotes caspase-8
activation (27,28). Therefore, the present study
quantified the expression of Bcl-2, Bax, caspase-3, −8, −9 and PARP
in U-2OS cells (29,30).
The data of the present study indicated that
celastrol/cisplatin-induced apoptosis was affected by the
alteration of the Bax/Bcl-2 ratio and activation of caspase-3 and
−9, but not caspase-8. Cleavage of PARP was also observed. These
results suggested that celastrol/cisplatin-induced apoptosis is
induced via the mitochondrial pathway in U-2OS cells.
In the endoplasm reticulum pathway, apoptosis is
induced by endoplasmic reticulum stress (22,23). The
endoplasmic reticulum stress-associated protein GRP78 and the
endoplasmic reticulum stress apoptosis-associated protein CHOP are
transcription factors specific to the process of endoplasmic
reticulum stress (31,32). A previous study revealed that CHOP
upregulated the expression of pro-apoptotic Bax-like proteins and
downregulated anti-apoptotic members, including Bcl-2 (33–35). In
the present study, western blot analysis indicated that levels of
GRP78 were downregulated and those of CHOP were upregulated,
accompanied by an increase in the Bax/Bcl-2 ratio, following
celastrol/cisplatin treatment. These data indicate that
celastrol/cisplatin-induced apoptosis is triggered via the
endoplasm reticulum pathway in U-2OS cells.
The present study demonstrated that celastrol acts
synergistically with cisplatin to inhibit the growth of U-2OS cells
through the induction of apoptosis. CI analyses revealed that
celastrol/cisplatin exhibit synergy in U-2OS cells, with CIs
ranging from 0.80 to 0.97 at effect levels ranging from
IC10 to IC70. Celastrol/cisplatin-induced
apoptosis was triggered via the mitochondrial and endoplasmic
reticulum pathways in U-2OS cells, particularly the former.
Therefore, celastrol/cisplatin exhibits potential as a novel
therapeutic agent for the treatment of osteosarcoma. Additional
studies are required to improve the understanding of the
synergistic effects of these drugs.
Acknowledgements
The authors thank Xuqiang Liu and Lifang Huang for
their help during the current study.
Funding
The present study was supported by The Support Plan
of Science and Technology Department of Jiangxi Province (grant no.
20112BBG70020) and the Gan-Po Talents Project 555 of Jiangxi
Province.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MD, BZ and HL contributed to the experimental
design. QW, XY and FL contributed to experiments and manuscript
writing. XL, XF, HG and JL contributed to data analysis and
interpretation. All authors approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Raymond AK and Jaffe N: Osteosarcoma
multidisciplinary approach to the management from the pathologist's
perspective. Cancer Treat Res. 152:63–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: State
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ando K, Heymann MF, Stresing V, Mori K,
Rédini F and Heymann D: Current therapeutic strategies and novel
approaches in osteosarcoma. Cancers (Basel). 5:591–616. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arai K, Sakamoto R, Kubota D and Kondo T:
Proteomic approach toward molecular backgrounds of drug resistance
of osteosarcoma cells in spheroid culture system. Proteomics.
13:2351–2360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Robert RS, Ottaviani G, Huh WW, Palla S
and Jaffe N: Psychosocial and functional outcomes in long-term
survivors of osteosarcoma: A comparison of limb-salvage surgery and
amputation. Pediatr Blood Cancer. 54:990–999. 2010.PubMed/NCBI
|
|
6
|
Meyers PA, Heller G, Healey JH, Huvos A,
Applewhite A, Sun M and LaQuaglia M: Osteogenic sarcoma with
clinically detectable metastasis at initial presentation. J Clin
Oncol. 11:449–453. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harting MT, Blakely ML, Jaffe N, Cox CS
Jr, Hayes-Jordan A, Benjamin RS, Raymond AK, Andrassy RJ and Lally
KP: Long-term survival after aggressive resection of pulmonary
metastases among children and adolescents with osteosarcoma. J
Pediatr Surg. 41:194–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Picci P, Mercuri M, Ferrari S, Alberghini
M, Briccoli A, Ferrari C, Pignotti E and Bacci G: Survival in
high-grade osteosarcoma: Improvement over 21 years at a single
institution. Ann Oncol. 21:1366–1373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ta HT, Dass CR, Choong PF and Dunstan DE:
Osteosarcoma treatment: State of the art. Cancer Metastasis Rev.
28:247–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li PP, He W, Yuan PF, Song SS, Lu JT and
Wei W: Celastrol induces mitochondria-mediated apoptosis in
hepatocellular carcinoma Bel-7402 cells. Am J Chin Med. 43:137–148.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mi C, Shi H, Ma J, Han LZ, Lee JJ and Jin
X: Celastrol induces the apoptosis of breast cancer cells and
inhibits their invasion via downregulation of MMP-9. Oncol Rep.
32:2527–2532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ni H, Zhao W, Kong X, Li H and Ouyang J:
NF-kappa B modulation is involved in celastrol induced human
multiple myeloma cell apoptosis. PLoS One. 9:e958462014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao X, Gao S, Ren H, Huang H, Ji W and
Hao J: Inhibition of autophagy strengthens celastrol-induced
apoptosis in human pancreatic cancer in vitro and in vivo models.
Curr Mol Med. 14:555–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HW, Jang KS, Choi HJ, Jo A, Cheong JH
and Chun KH: Celastrol inhibits gastric cancer growth by induction
of apoptosis and autophagy. BMB Rep. 47:697–702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu X, Zhou X, Fu C, Wang Q, Nie T, Zou F,
Guo R, Liu H, Zhang B and Dai M: Celastrol induces apoptosis of
human osteosarcoma cells via the mitochondrial apoptotic pathway.
Oncol Rep. 34:1129–1136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzym Regul. 22:27–55.
1984. View Article : Google Scholar
|
|
17
|
Mandic A, Hansson J, Linder S and Shoshan
MC: Cisplatin induces endoplasmic reticulum stress and
nucleus-independent apoptotic signaling. J Biol Chem.
278:9100–9106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Song J, Wu D, Wang J and Dong W:
Hesperetin induces the apoptosis of hepatocellular carcinoma cells
via mitochondrial pathway mediated by the increased intracellular
reactive oxygen species, ATP and calcium. Med Oncol. 32:1012015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang K, Wang X, Wang C, Zheng H, Li T,
Xiao S, Wang M, Fei C, Zhang L and Xue F: Investigation of
quinocetone-induced mitochondrial damage and apoptosis in HepG2
cells and compared with its metabolites. Environ Toxicol Pharmacol.
39:555–567. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Spierings D, McStay G, Saleh M, Bender C,
Chipuk J, Maurer U and Green DR: Connected to death: The
(unexpurgated) mitochondrial pathway of apoptosis. Science.
310:66–67. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gordon N and Kleinerman ES: Aerosol
therapy for the treatment of osteosarcoma lung metastases:
Targeting the Fas/FasL pathway and rationale for the use of
gemcitabine. J Aerosol Med Pulm Drug Deliv. 23:189–196. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Liu H, Zheng ZM, Zhang KB, Wang
TP, Sribastav SS, Liu WS and Liu T: Role of death receptor,
mitochondrial and endoplasmic reticulum pathways in different
stages of degenerative human lumbar disc. Apoptosis. 16:990–1003.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jang JH, Kim YJ, Kim H, Kim SC and Cho JH:
Buforin IIb induces endoplasmic reticulum stress-mediated apoptosis
in HeLa cells. Peptides. 69:144–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Manfredi G, Kwong JQ, Oca-Cossio JA,
Woischnik M, Gajewski CD, Martushova K, D'Aurelio M, Friedlich AL
and Moraes CT: BCL-2 improves oxidative phosphorylation and
modulates adenine nucleotide translocation in mitochondria of cells
harboring mutant mtDNA. J Biol Chem. 278:5639–5645. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rengarajan T, Nandakumar N, Rajendran P,
Haribabu L, Nishigaki I and Balasubramanian MP: D-pinitol promotes
apoptosis in MCF-7 cells via induction of p53 and Bax and
inhibition of Bcl-2 and NF-κB. Asian Pac J Cancer Prev.
15:1757–1762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gordon N and Kleinerman ES: Aerosol
therapy for the treatment of osteosarcoma lung metastases:
Targeting the Fas/FasL pathway and rationale for the use of
gemcitabine. J Aerosol Med Pulm Drug Deliv. 23:189–196. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Villa-Morales M and Fernández-Piqueras J:
Targeting the Fas/FasL signaling pathway in cancer therapy. Expert
opin Ther Targets. 16:85–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang HY and Yang X: Proteases for cell
suicide: Functions and regulation of caspases. Microbiol Mol Biol
Rev. 64:821–846. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gillies LA and Kuwana T: Apoptosis
regulation at the mitochondrial outer membrane. J Cell Biochem.
115:632–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lamothe B, Wierda WG, Keating MJ and
Gandhi V: Carfilzomib triggers cell death in chronic lymphocytic
leukemia by inducing proapoptotic and endoplasmic reticulum stress
responses. Clin Cancer Res. 22:4712–4726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zeng Y, Sun H, Li Y, Shao M, Han P, Yu X,
He L, Xu Y and Li S: Exposure to triptolide affects follicle
development in NIH mice: Role of endoplasmic reticulum stress in
granulosa cell apoptosis. Hum Exp Toxicol. Mar 27–2016.(Epub ahead
of print).
|
|
33
|
Chen PM, Cheng YW, Wu TC, Chen CY and Lee
H: MnSOD overexpression confers cisplatin resistance in lung
adenocarcinoma via the NF-κB/Snail/Bcl-2 pathway. Free Radic Biol
Med. 79:127–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|