Introduction
Some patients who undergo surgical treatment for
soft tissue tumors arising from the thoracic wall and lung tumors
invading the thoracic wall require thoracic cage reconstruction. In
this procedure, fixation using sutures and mesh, metal
implantation, and autologous tissue are used, and a
musculocutaneous flap is required if the skin defect is large
(1–13). The reconstruction method is preferred
with consideration given to the size and site of the defect as well
as the patient's health condition and prognosis (14). The purpose of reconstruction is to
achieve thoracic cage stability as well as thoracic cavity air and
water tightness. Moreover, this procedure is performed for
aesthetic purposes (14). At our
institution, suture fixation and non- or semi-rigid fixation using
a mesh are proactively performed, and have achieved good
outcomes.
Complications after thoracic wall resection include
respiratory problems, skin necrosis, and infection. When a lung
tumor invades the thoracic wall, combined resection of the lung and
thoracic wall is required. Therefore, respiratory complications can
readily occur. In contrast, in patients with musculoskeletal
tumors, the tumor is excised along with surrounding bone and soft
tissue, such as muscle and skin, which causes skin and soft tissue
defects. To date, there are few studies that have compared patients
with lung tumors and those with musculoskeletal tumors (9,15).
Furthermore, there is a limited number of studies on the changes in
pulmonary function before and after combined thoracic wall
resection (16). There is no evidence
regarding the effects of reconstruction, non-reconstruction, and
type of reconstruction on the functional outcomes after the
procedure (9). Therefore, the present
study aimed to retrospectively examine patients who required
thoracic wall resection at the time of surgery for malignant
tumors. The survival rates, disease-free survival period, and
incidence of complications were compared between the lung tumor
group and the musculoskeletal tumor group to identify the
associated risk factors, and the differences between the pre- and
postoperative pulmonary functions of the two groups were
examined.
Patients and methods
This retrospective study included 68 patients who
underwent thoracic reconstruction during surgical treatment of a
tumor at our institution or an affiliated institution between 2006
and 2016 (Table I). Because of
postoperative complications and respiratory function changes were
expected to be different as compared with cases with simple ribs
resection and/or sternotomy, patients who underwent diaphragm or
vertebral resection were excluded for a simpler analysis. And
patients with recurrence were also excluded. Surgical treatment was
performed at the Department of Respiratory Surgery or Department of
Orthopedic Surgery. During surgery, the patients were under general
anesthesia with differential lung ventilation, and postoperative
management was performed in the intensive care unit. The criteria
for thoracic wall reconstruction using mesh included the following:
i) Thoracic wall defect ≥6 cm, ii) costectomy of ≥3 ribs, or iii)
suspected thoracic cage instability despite a defect in one or two
ribs. In addition, reconstruction was performed by mattress sutures
(suture reconstruction). COMPOSIX® Mesh (C.R.BARD) or
DUALMESH® (GORE) was used, which was strongly sutured to
the ribs and surrounding soft tissues to achieve water tightness. A
drainage tube was inserted into the thoracic cavity of all
patients. Although the wound could be covered by the skin, when the
artificial objects (mesh and sutures) and lung could not be covered
by the muscle, reconstruction was performed by a musculocutaneous
flap. For the postoperative follow-up, computed tomography scan
were performed every 3 months.
 | Table I.Patient characteristics, operation
data and outcomes. |
Table I.
Patient characteristics, operation
data and outcomes.
| Characteristics | Total, n (%) | Lung tumor, n
(%) | Bone and soft tissue
tumor, n (%) | P-value |
|---|
| No. of patients | 68 | 50 | 18 |
|
| Sex |
| Male | 58 (85.2) | 46 (92.0) | 12 (66.7) | 0.02 |
|
Female | 10 (14.7) | 4
(8.0) | 6
(33.3) |
|
| Average age at
surgery (range), years | 62 | 66 (43–79) | 51.2 (13–69) | <0.01 |
| Average follow-up
period (range), months | 42.4 | 35.4 (1–113) | 62.5 (4–164) | <0.01 |
| Average blood loss
(range), ml | 454 | 480 (35–2,500) | 381 (10–1,100) | 0.45 |
| Average operation
time (range), min | 330 | 308 (118–598) | 395 (125–625) | 0.02 |
| Outcome |
|
Continuously disease free | 34 (52.2) | 23 (46.0) | 11 (61.1) |
|
| Alive
with disease | 7
(11.6) | 4
(8.0) | 3
(16.7) |
|
| Mortality
(from disease) | 26 (34.8) | 22 (44.0) | 4 (22.2) |
|
| Mortality
(from another disease) | 1
(1.4) | 1
(2.0) | 0 |
|
In the lung tumor group, the overall survival and
disease-free survival period were classified by lymph node
metastasis (N factor) and free margin (R factor) and examined. In
the musculoskeletal tumor group, the overall survival and
disease-free survival period were classified by the R factor and
examined.
The present study was approved by the ethics review
board of Aichi Cancer Center Hospital (approval no. 2017-285). The
requirement for written informed consent was waived due to the
retrospective nature of the study.
Complications
The incidence of complications observed within 90
days after surgery was examined. Potential predictors (age, sex,
operative duration, estimated volume of intraoperative blood loss,
number of resected ribs, reconstruction method, histological type,
and history of resection and musculocutaneous flap reconstruction)
of severe complications (grade ≥3) were analyzed using the Common
Terminology Criteria for Adverse Effects (CTCAE) version 4.03
(17).
Pre- and postoperative pulmonary functions were
compared among 16 patients (Table
II) who consented to undergo the testing, which was performed
within 2 years postoperatively.
 | Table II.Details of cases with pulmonary
function test. |
Table II.
Details of cases with pulmonary
function test.
| Patient
characteristics | No. of patients
(%) |
|---|
| Histopathological
type |
|
| Lung
tumor | 13 (81.3) |
| Bone
and soft tissue tumor | 3 (19.0) |
| Postoperative
observation period, average number of months (range) | 12.0 (3–24) |
|
Sex |
|
|
Male | 13 (81.3) |
| Female | 3 (19.0) |
| Average age at
surgery (range) | 66 (43–78) |
| Reconstruction
method |
|
| No
reconstruction | 2 (12.5) |
| Suture
reconstruction | 10 (62.5) |
| Mesh
reconstruction | 4 (25.0) |
| Number of resected
ribs |
|
| 1 | 3 (19.0) |
| 2 | 5 (31.3) |
| 3 | 5 (31.3) |
| 4 | 2 (12.5) |
| Sternum | 1 (6.3) |
Statistical analysis
For statistical analysis on patient background,
Fisher's exact test was used for sex, Mann-Whitney U test was used
for age at surgery, follow-up period, blood loss, and operation
time. Fisher's exact test was used to analyze the relationship
between the number of resected ribs and the reconstruction method.
The survival rate and disease-free survival period (in months) of
the lung tumor and musculoskeletal tumor groups were compared. The
overall survival rate and disease-free survival period were
calculated using the Kaplan-Meier method and compared using the
log-rank test. The risk factors associated with the complications,
including age, sex, operative duration, estimated volume of
intraoperative blood loss, number of resected ribs, reconstruction
method, presence or absence of musculocutaneous flap
reconstruction, histological type (lung tumor vs. musculoskeletal
tumor), and the presence or absence of combined lung resection,
were examined via univariate analysis. The following statistical
tests were used: Mann-Whitney U test, Fisher's exact test, and
t-test. Pre- and postoperative pulmonary function were compared in
terms of percent vital capacity (%VC) and forced expiratory volume
within 1 sec (FEV1.0%) using a paired t-test.
All statistical analyses were performed with EZR
(Saitama Medical Center, Jichi Medical University, Saitama, Japan),
which is a graphical user interface for R (The R Foundation for
Statistical Computing, Vienna, Austria). More precisely, it is a
modified version of the R commander designed to add statistical
functions frequently used in biostatistics (18).
Results
The study sample included 50 patients with lung
tumors and 18 patients with musculoskeletal tumors (Table I). The patients were mostly male (58,
85.3%), with a mean age of 61.9 (range, 13–79) years at the time of
surgery. Compared with the musculoskeletal tumor group, the lung
tumor group mostly consisted of male patients (P=0.02) who were
older at the time of surgery (P<0.01). The mean observation
period was 42.4 (range, 1–164) months. No significant differences
were observed in the estimated blood loss. The operation time was
significantly longer in the musculoskeletal tumor group than in the
lung tumor group.
Squamous cell lung cancer was the most common tumor
histological type (20 patients, 29.4%), followed by pulmonary
adenocarcinoma (nine patients), and large cell lung cancer (eight
patients; Table III). In the
musculoskeletal tumor group, chondrosarcoma was most common (three
patients, 4.4%). Metastatic tumors were observed in patients with
pulmonary metastasis. Two patients presented with colorectal
cancer, and one patient presented with pharyngeal cancer. Regarding
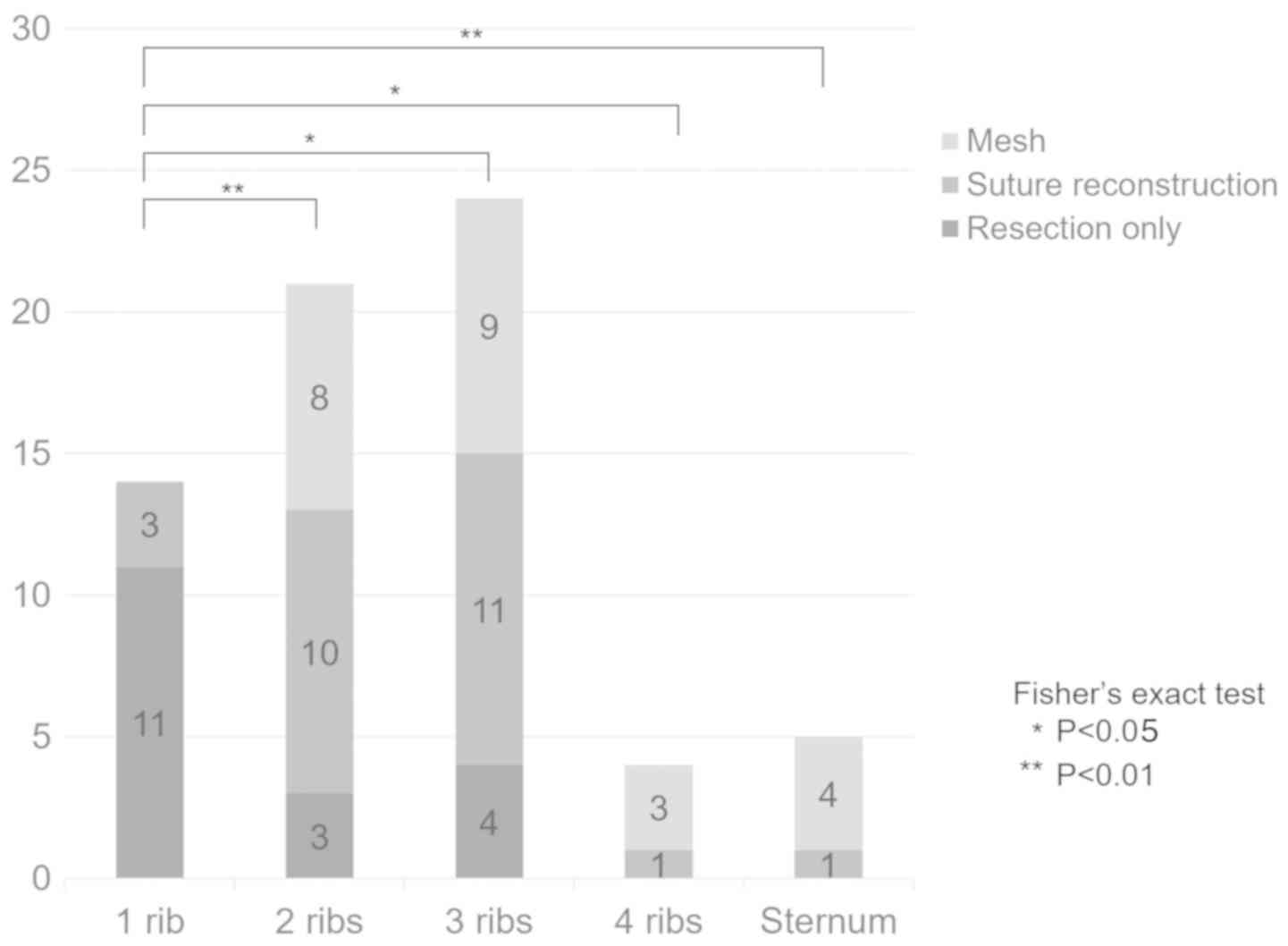
to the extent of resection, one, two, three, and four ribs were
resected in 14, 21, 24, and four patients, respectively, and five
patients underwent costectomy and sternum resection (Fig. 1). Thoracic cage reconstruction was
performed in 50 (73.5%) patients. When there was only one rib
resection, the reconstruction rate was significantly lower than ≥2
ribs or sternum resection. All patients with ≥4 resected ribs and
those who also underwent sternum resection underwent thoracic cage
reconstruction. In total, 52 (76.5%) patients also underwent
resection of the lungs, and all patients in the lung tumor group
also underwent lung resection. Musculocutaneous flap reconstruction
was performed in 11 patients, all of whom were in the
musculoskeletal tumor group. Free margins were observed in 57
(83.8%) patients (R0), microscopically positive margins (R1) were
observed in 11 (16.2%) patients, and no R2 margin was observed. In
the lung tumor group, the R0 margin was observed in 41 (82.0%)
patients, and R1 was observed in nine (18.0%) patients. In the
musculoskeletal tumor group, the R0 margin was observed in 16
(88.9%) patients, and R1 was observed in two (11.1%) patients. In
the lung tumor group, excluding patients with metastatic tumors, 35
(74.5%) patients were N0, eight (17.0%) patients were N1, and four
(8.5%) patients were N2.
 | Table III.Histopathologic distribution. |
Table III.
Histopathologic distribution.
| Lung tumor | Total n (n=50) | Bone and soft
tissue tumor | Total n (n=18) |
|---|
| Squamous cell
carcinoma | 20 | Chondrosarcoma | 3 |
| Adenocarcinoma | 9 | Ewing sarcoma | 2 |
| Large cell
carcinoma | 8 | Myxofibro
sarcoma | 2 |
| Neuro endocrine
carcinoma | 4 | Solitary fibrous
tumor | 1 |
| Adenosquamous
carcinoma | 2 | Osteosarcoma | 1 |
| Small cell
carcinoma | 1 | Angiosarcoma | 1 |
| Non-small cell lung
cancer | 1 | Synovial
sarcoma | 1 |
| Sarcomatoid
carcinoma | 1 | Chondroid
syringoma | 1 |
| Pleomorphic
carcinoma | 1 | WDLS | 1 |
| Metastatic
carcinoma | 3 | UPS | 1 |
|
|
| Malignant phyllodes
tumor | 1 |
|
|
| Fibrosarcoma | 1 |
|
|
| Inflammatory pseudo
tumor | 1 |
|
|
| Myofibroblastic
sarcoma | 1 |
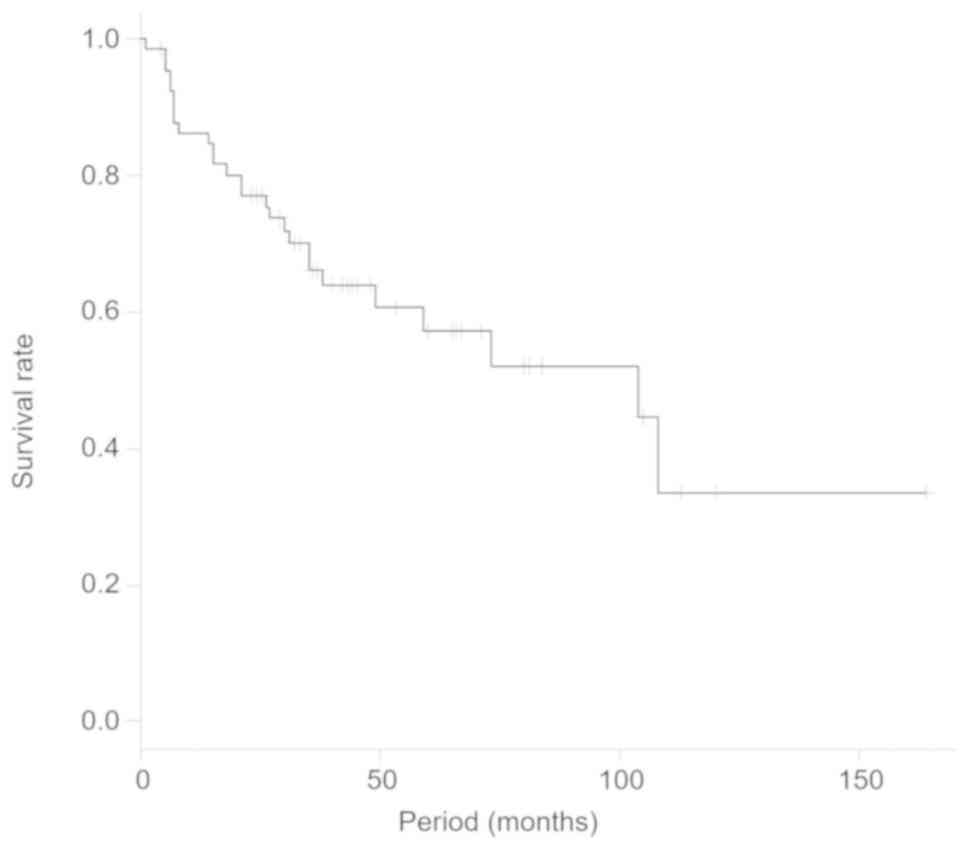
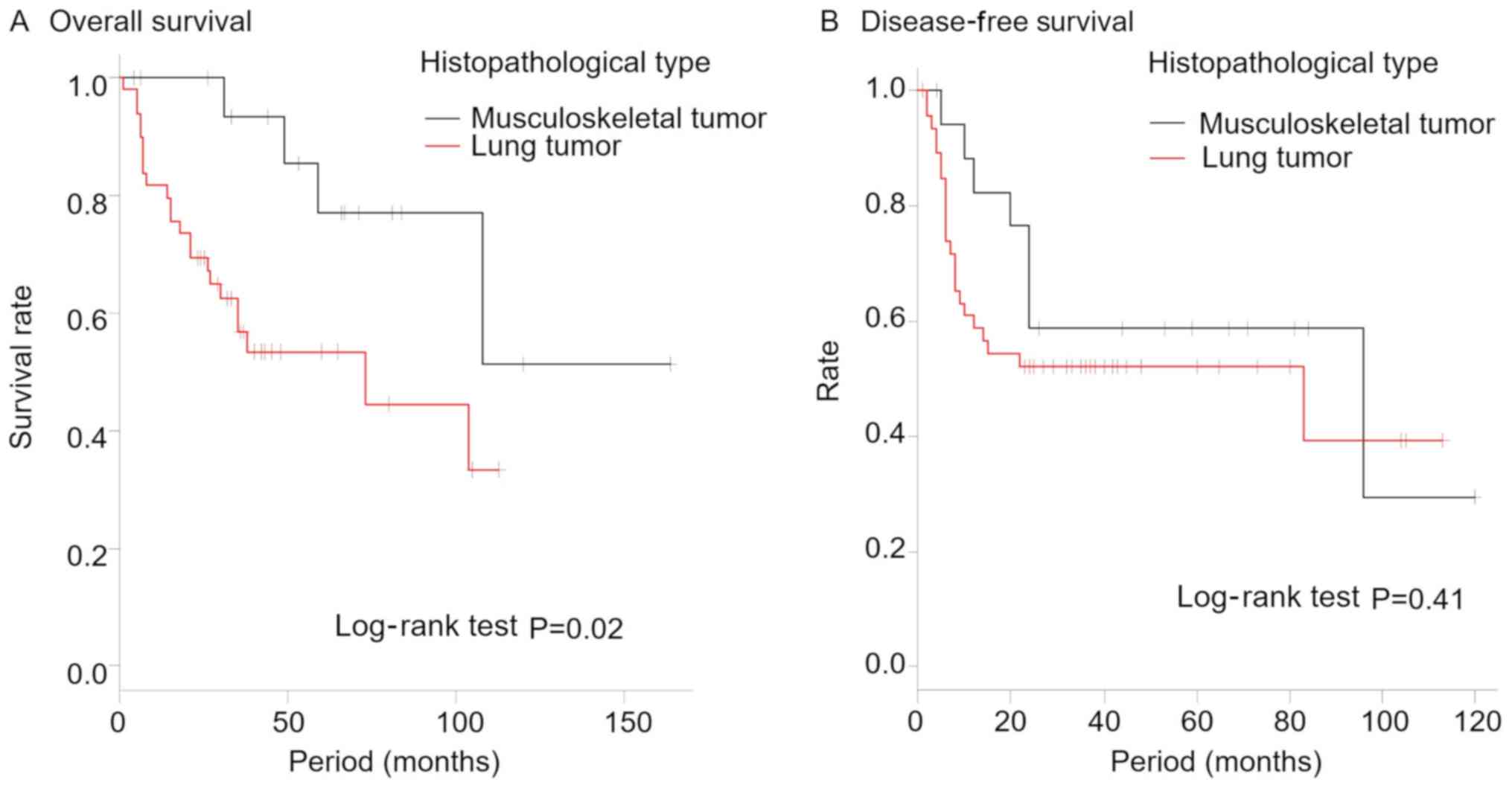
The overall 5-year survival rate of the patients was
57.4% (Fig. 2). The 5-year survival
rates were 53.4% [95% confidence interval (CI), 37.3–67.1] in the
lung tumor group and was significantly lower than the rate of 77.0%
(95% CI, 43.9–92.0) in the musculoskeletal tumor group (P=0.02;
Fig. 3A). There was no statistically
significant difference in the disease-free survival between the two
groups (P=0.41; Fig. 3B). Localized
recurrence was observed in 14 patients (20.6%) [lung tumor group:
10 (20.0%) patients; musculoskeletal tumor group: four (22.2%)
patients], and postoperative distal metastasis was observed in 17
(25.0%) patients [lung tumor group: 12 (25.5%) patients;
musculoskeletal tumor group: five (27.8%) patients]. The outcomes
at the time of the final observation were continuous disease-free
in 33 patients, alive with disease in eight patients, dead from
disease in 26 patients, and dead from other diseases in one patient
(Table I). No statistically
significant differences were observed in the postoperative survival
according to the reconstruction method or number of resected
ribs.
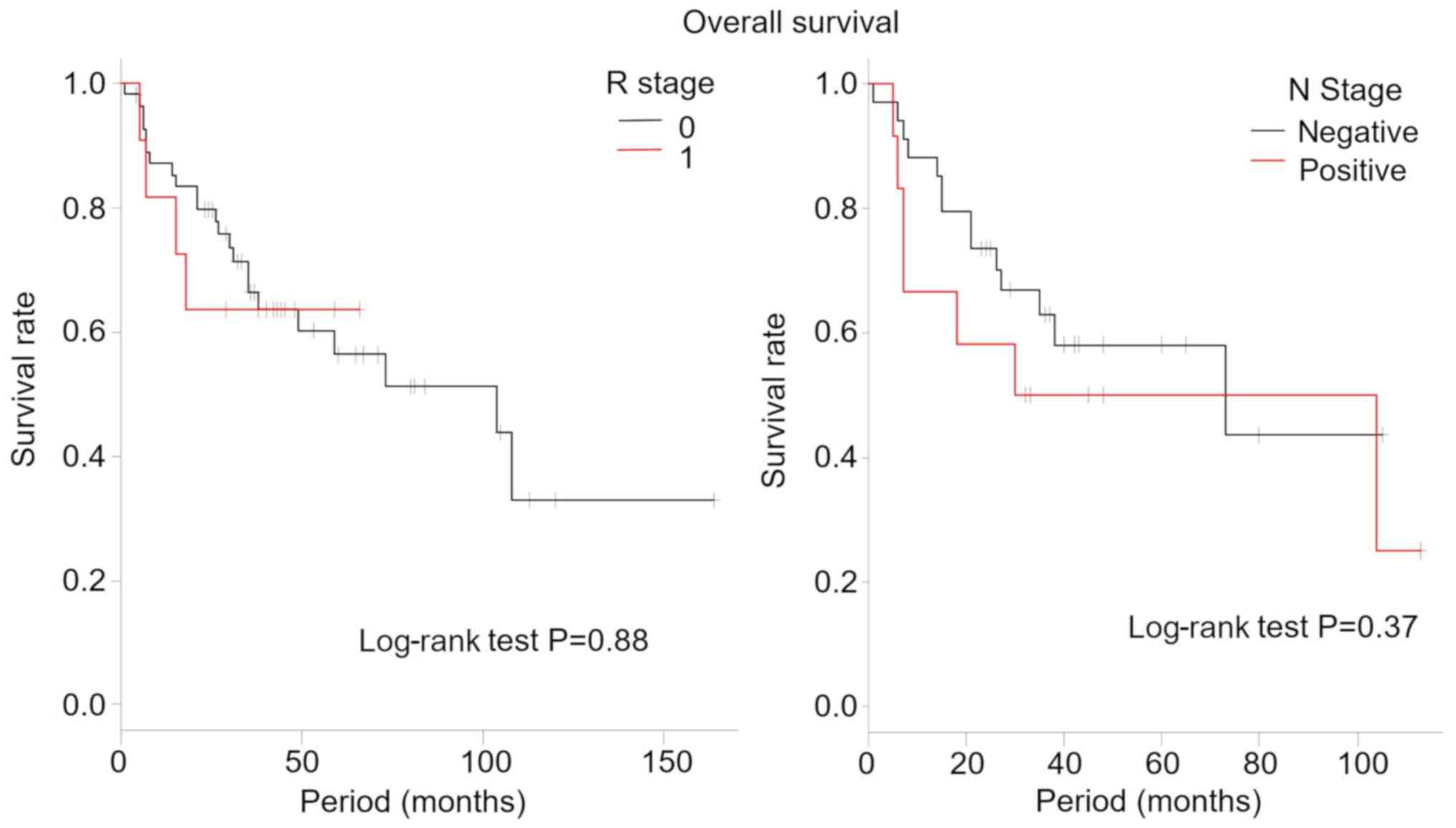
The overall survival and disease-free survival rates
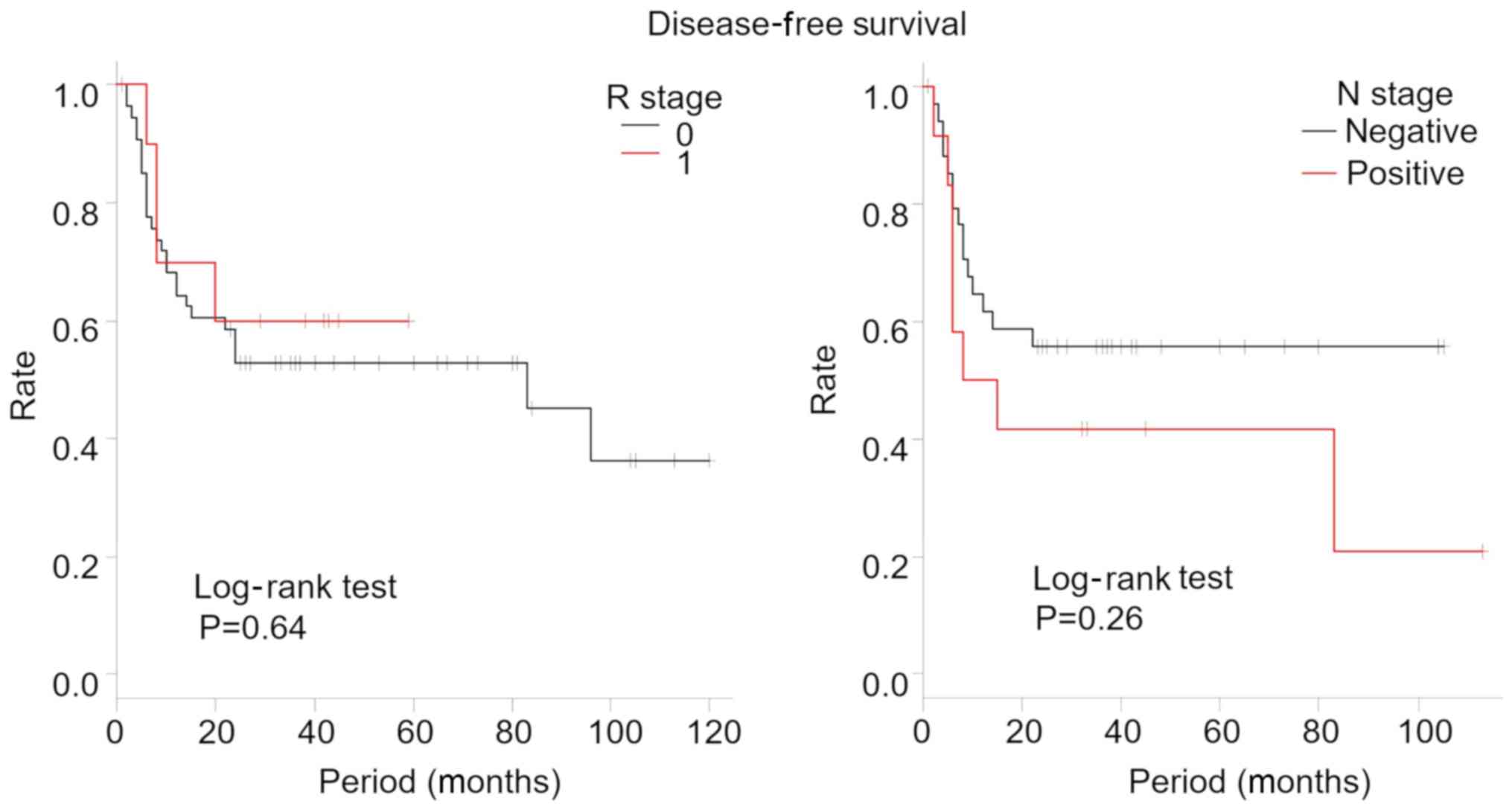
by R stage and N stage are shown in Figs.
4 and 5, respectively. In our
study, there were no significant differences between the overall
survival and the disease-free survival rates for R and N stage.
Complications were observed in 30 (44.1%) patients,
including air leak in 10 patients and atelectasis in eight
patients; and other complications are presented in Table IV. The incidence of complications was
52.0% (26 patients) in the lung tumor group and 22.2% (four
patients) in the musculoskeletal tumor group. Respiratory
complications, such as air leak and atelectasis, were common in the
lung tumor group. In the musculoskeletal tumor group, skin necrosis
was the most common complication, affecting three patients. Only
one patient died from postoperative pneumonitis. Thus, the
perioperative mortality rate was 1.5%. The significant risk factors
associated with severe complications (grade ≥3) included a large
volume of estimated blood loss (P=0.01), lung cancer as the
histological type (P=0.01), and also undergoing lung resection
(P=0.03; Table V).
 | Table IV.Postoperative complications. |
Table IV.
Postoperative complications.
| Complication | Total, n (%) | Lung tumor, n | Bone and soft
tissue tumor, n |
|---|
| Total no. of
patients | 68 | 50 | 18 |
| Air leak | 10 (14.7) | 10 | − |
| Atelectasis | 8
(11.8) | 6 | 2 |
| Bronchial
obstruction | 5
(7.4) | 5 | − |
| Pain | 5
(7.4) | 5 | − |
| Pneumothorax | 4
(5.9) | 4 | − |
| Brachial
neuralgia | 3
(4.4) | 3 | − |
| Skin necrosis | 3
(4.4) | − | 3 |
| Surgical site
infection | 3
(4.4) | 2 | 1 |
| Arrhythmia | 2
(2.9) | 2 | − |
|
Aerodermectasia | 2
(2.9) | 2 | − |
| Postoperative
pneumonia | 2
(2.9) | 2 | − |
| Bleeding | 2
(2.9) | 1 | 1 |
| Pleural
effusions | 1
(1.5) | 1 | − |
| Chylothrax | 1
(1.5) | 1 | − |
| Total with
duplicate, n (%) | 30 (44.1) | 26 (52.0) | 4 (22.2) |
 | Table V.Risk factor of severe complications
(Common Terminology Criteria for Adverse Effects grade ≥3). |
Table V.
Risk factor of severe complications
(Common Terminology Criteria for Adverse Effects grade ≥3).
|
| Severe
complications |
|
|---|
|
|
|
|
|---|
| Factors | Present (total
n=54), n (%) | None (total n=14),
n (%) | P-value |
|---|
| Agec (average), years | 61.0 | 66 | 0.68 |
| Sexd (male) | 46 (85.2) | 12 (85.7) | 0.67 |
| Operation
timec, min | 335.4 | 313.1 | 0.91 |
| Blood
lossc, ml | 437.4 | 516.1 | 0.01b |
| Number of resected
ribsd (average) | 2.3+5 sternum | 2.3 | 0.06 |
| Reconstruction
methodd |
|
| 0.11 |
|
Resection only | 18 (33.3) | 4
(28.6) |
|
|
Suture | 16 (29.6) | 5
(35.7) |
|
|
Mesh | 20 (37.0) | 5
(35.7) |
|
| Using muscle
flapd (yes) | 9
(16.7) | 2
(14.3) | 0.10 |
| Histopathologic
typec |
|
| 0.01b |
| Lung
tumor | 38 (70.4) | 12 (85.7) |
|
|
Musculoskeletal tumor | 16 (29.6) | 2
(14.3) |
|
| Pulmonary combined
excisiond (yes) | 41 (75.9) | 12 (85.7) | 0.03a |
The patient who compared pre- and postoperative
pulmonary function, details are shown in Table VI. Postoperative pulmonary function
testing was performed at a mean of 12.0 (range, 3–24) months after
surgery. Of the 16 patients, 13 (81.3%) presented with lung tumor
and three (18.8%) with musculoskeletal tumor, of which 13 (81.3 %)
were male and three (18.8%) were female, with a mean age of 66.1
(range, 43–78) years (Table II). No
reconstruction was performed for two patients (12.5%),
reconstruction by suture fixation was performed for 10 (62.5%)
patients, and mesh reconstruction was performed for four (25.0%)
patients. In total, one, two, three, and four ribs were resected in
three (19.0%), five (31.3%), five (31.3%), and two (12.5%)
patients, respectively, and sternum resection was performed in one
(6.3%) patient. Twelve (75.0%) patients were upper rib resection
and three (18.8%) were lower rib resection. Lung resection was
performed in 13 patients, all of which were in the lung tumor
group. Single lobectomy was performed in nine patients (56.3%),
wedge resection, segmental resection, segmental resection with
lobectomy and double lobectomy were performed in one patient
respectively (Table VI).
 | Table VI.Details of the patient who
participated in the respiratory function test. |
Table VI.
Details of the patient who
participated in the respiratory function test.
| Age/sex | Histo- pathological
type | Pneumo- nectomy
area | Resected ribs | Reconstruction
method | Post-OP month until
examination | Pre-OP %VC | Pre-OP FEV1.0% | Post-OP %VC | Post-OP
FEV1.0% |
|---|
| 73/F | SQ | RLL | #7,8,9 | Suture | 4 | 124.0 | 78.0 | 58.0 | 76.0 |
| 62/M | LA | RML | #8,9,10 | Suture | 5 | 108.0 | 73.0 | 73.0 | 82.0 |
| 65/M | LA | RUL | Sternum | Suture | 9 | 111.1 | 78.3 | 82.2 | 75.8 |
| 74/M | SQ | LUL | #3 | − | 3 | 100.8 | 75.5 | 90.7 | 73.0 |
| 76/M | SQ | RUL | #3 | Suture | 3 | 79.1 | 65.3 | 67.6 | 70.1 |
| 61/M | SQ | RUL+RML wedge | #5,6 | Suture | 24 | 97.2 | 73.1 | 68.0 | 83.3 |
| 63/M | Pleomorphic
carcinoma | Wedge RUL | #3,4,5,6 | Mesh | 12 | 90.4 | 77.9 | 63.5 | 82.7 |
| 58/M | SQ | RUL | #3,4 | − | 24 | 81.4 | 86.7 | 74.4 | 80.4 |
| 70/M | SQ | RUL | #2,3,4 | Suture | 12 | 79.6 | 77.4 | 45.2 | 89.1 |
| 78/M | AS | LUL seg+S6 seg | #5,6 | Suture | 12 | 86.6 | 73.0 | 83.5 | 82.2 |
| 77/M | LA | RUL+S6 seg | #4 | Suture | 12 | 99.4 | 70.0 | 65.9 | 85.4 |
| 65/M | Adenocarcinoma | RUL | #5,6 | Suture | 12 | 69.3 | 74.2 | 71.2 | 71.7 |
| 73/M | SQ | LUL | #1,2,3 | Suture | 12 | 94.3 | 62.9 | 64.5 | 64.6 |
| 59/F | SFT | − | #7,8 | Mesh | 12 | 51.8 | 84.1 | 61.8 | 86.5 |
| 43/F | MPT | − | #3,4,5,6 | Mesh | 24 | 97.3 | 82.6 | 93.4 | 85.3 |
| 61/M | Fibrosarcoma | − | #4,5,6 | Mesh | 12 | 112.1 | 57.4 | 117.8 | 52.5 |
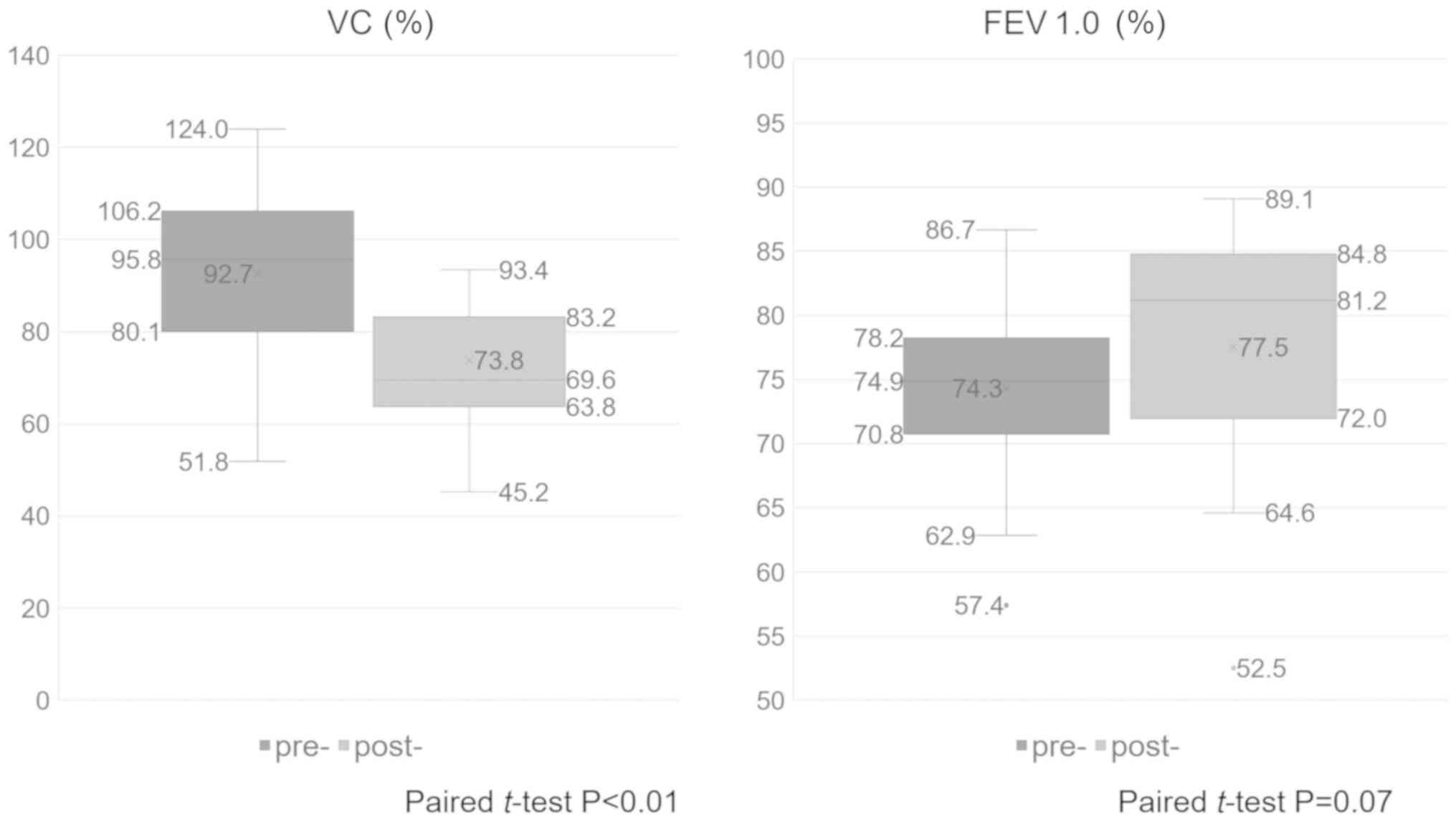
Compared with preoperative pulmonary function
testing, postoperative pulmonary function testing revealed that the
mean %VC decreased from 92.7 to 73.8% (paired t-test, P<0.01)
and the mean FEV1.0% increased from 74.3 to 77.5% (paired t-test,
P=0.07; Fig. 6). Therefore, a
restrictive ventilator impairment-like change was observed
postoperatively compared with the preoperative status. Comparison
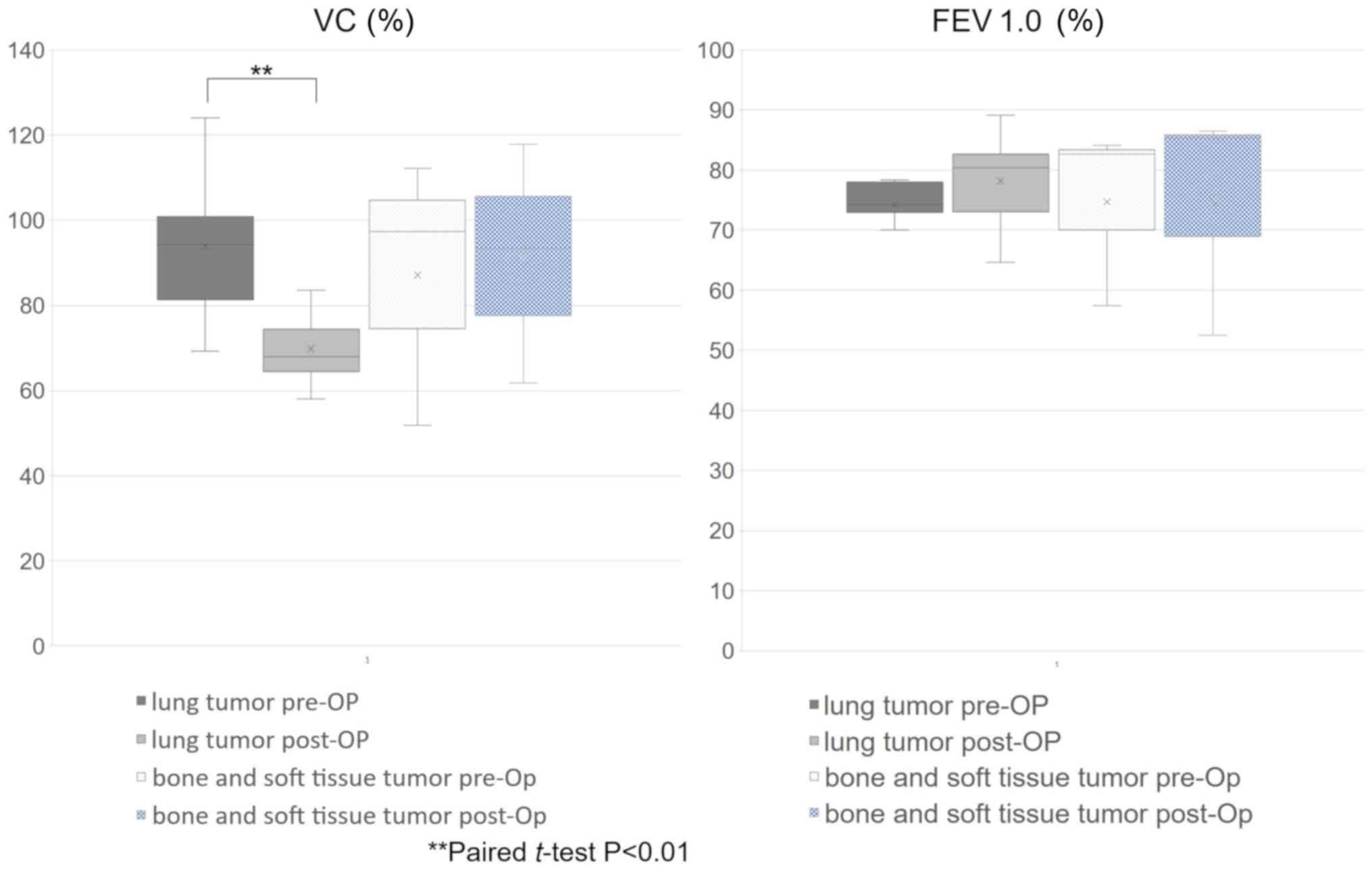
of the patients with lung and musculoskeletal tumors showed that
the mean %VC significantly decreased from 93.9% preoperatively to
69.8% postoperatively in lung tumor patients (paired t-test,
P<0.01) and increased from 87.1 to 91.0% in musculoskeletal
tumor patients, but the difference was not significant (paired
t-test, P=0.44; Fig. 7). The average
postoperative FEV1.0% increased from 74.3 to 78.2% in lung tumor
patients and from 74.7 to 74.8% in musculoskeletal tumor patients,
but differences were not significant in either patient group
(paired t-test, P=0.44 and 0.99, respectively). Compared with the
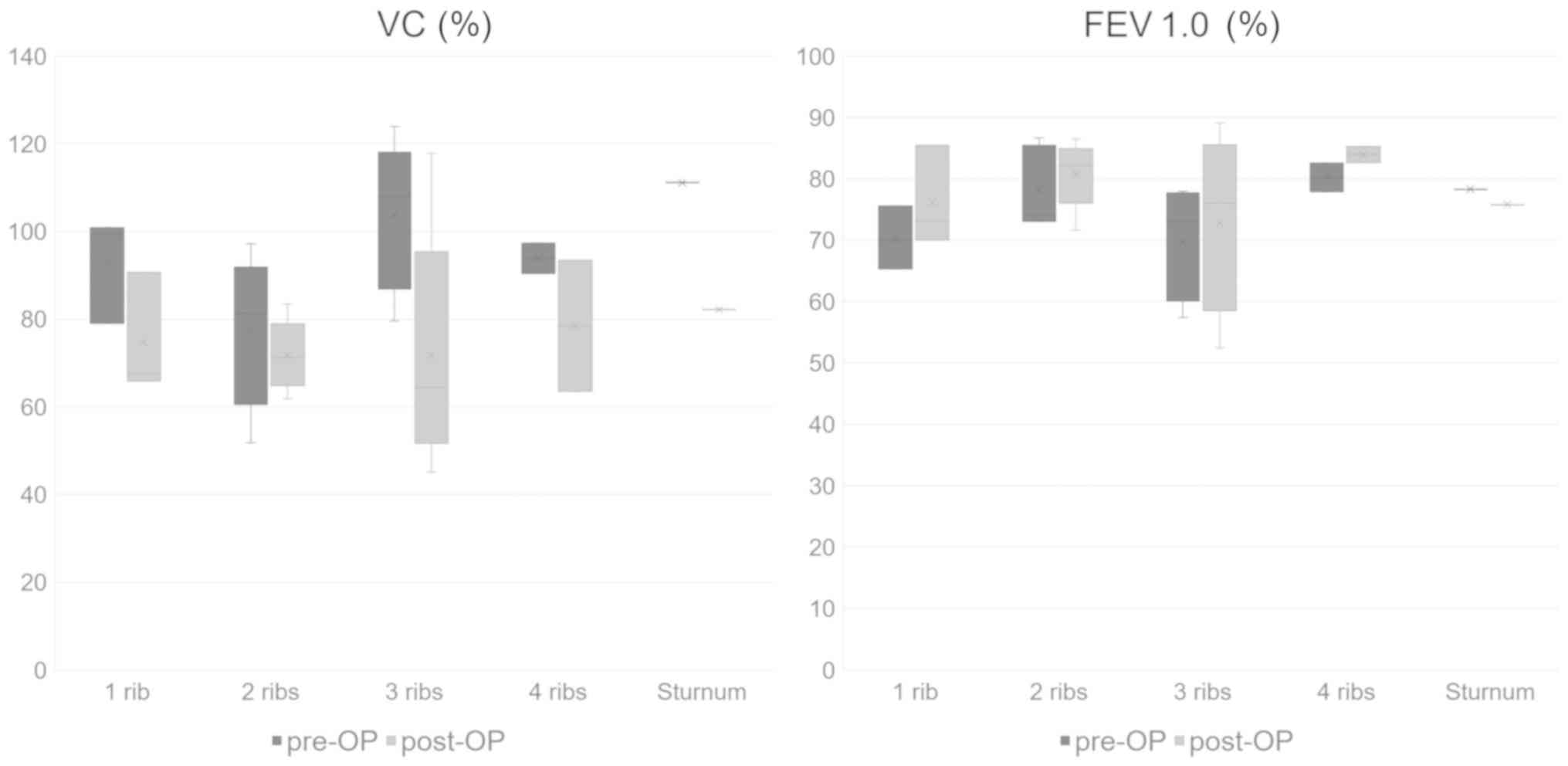
number of resected ribs, the mean %VC decreased in all patients
after the operation (Fig. 8).
Additionally, the mean postoperative FEV1.0% increased in the
patients other than those who underwent sternum resection. Compared
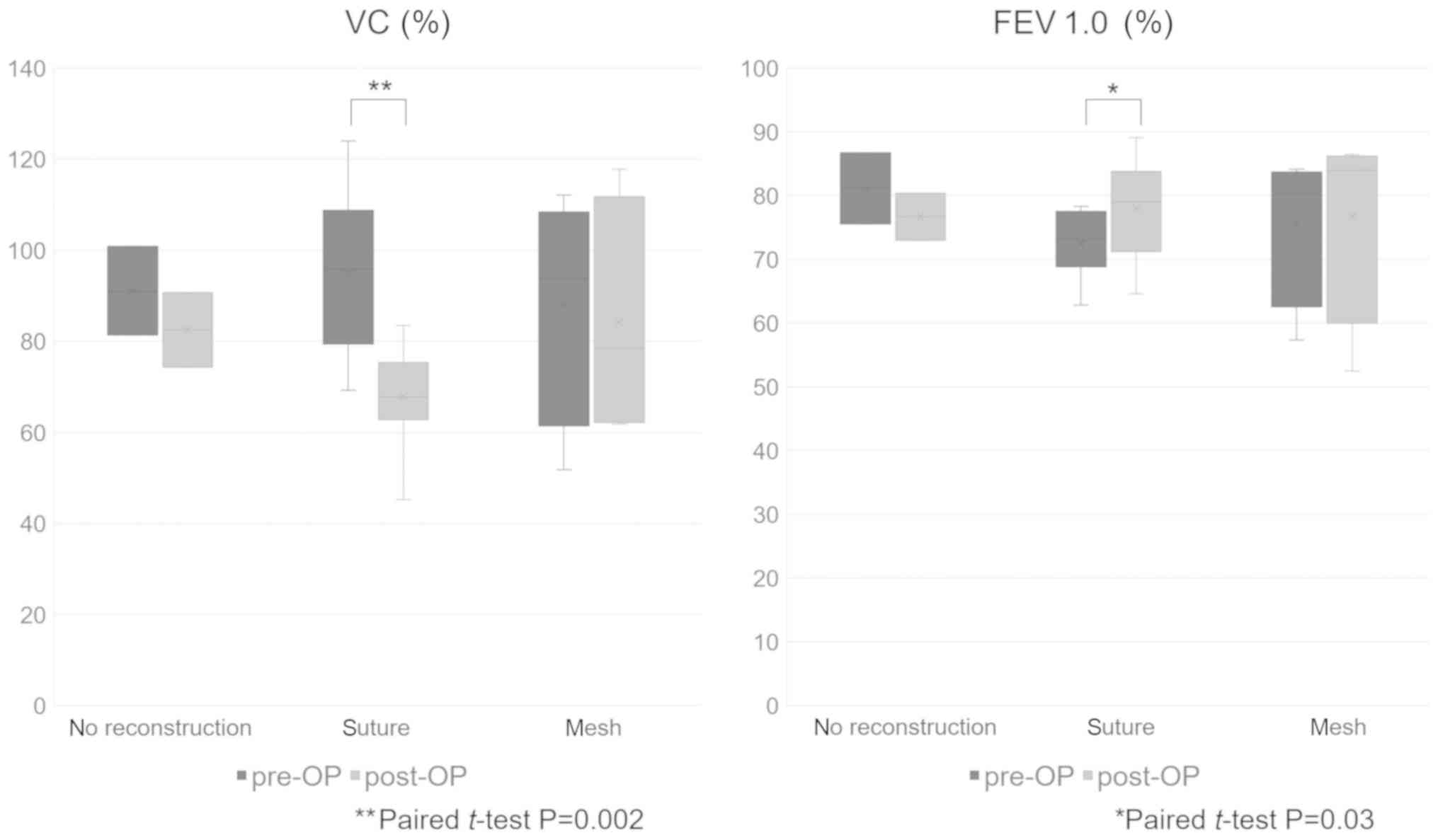
with the reconstruction method, we observed a statistically
significant decrease in the mean postoperative %VC and an increase
in the mean postoperative FEV1.0% only in suture reconstruction
(paired t-test, P=0.002 and 0.03, respectively) (Fig. 9). However, all patients who underwent
pulmonary function testing did not experience problems in
activities of daily living due to changes in pulmonary
function.
Discussion
Thoracic cage reconstruction maintains anatomical
and structural stability, protects vital organs, and helps sustain
the respiratory mechanism. The procedure is required in 40–60% of
individuals who have undergone thoracotomy (1,9,11). Reconstruction is required for
individuals with ≥3 resected ribs and defects that are ≥5 cm in
diameter or even for patients with smaller defects and cases of
suspected thoracic cage instability (9–11,13,19).
Furthermore, because anterior and anterolateral movements are
greater than posterior movements, thoracic cage reconstruction is
often required (19). Rigid
reconstruction has found wide use because it is effective for
stabilizing the thorax and preventing postoperative respiratory
complications, but it can be costly and also induce severe
complications, including deep infections and postoperative pain,
plate exposure, screw loosening (4,13). To
minimize the development of postoperative complications, our group
selected a simpler suture or mesh method rather than rigid
reconstruction (4,20). In the present study, thoracic cage
reconstruction was performed in 73.5% of the patients who underwent
thoracic wall resection. To achieve thoracic cage stability, the
reconstruction was performed using simple methods rather than those
used in previous studies, and stable postoperative outcomes were
observed.
With regard to the postoperative overall survival
rate, the 5-year survival rates were 53.4% in the lung tumor group
and 77.0% in the musculoskeletal tumor group. With regard to the
postoperative outcomes of individuals with lung cancer who
presented with thoracic wall invasion, positive margin (R1,2) and
lymph node metastasis (N1,2) have been reported as poor prognostic
factors (1,9,10). The
number of resected ribs and thoracic wall invasion reportedly did
not affect prognosis (1).
Furthermore, regarding primary thoracic wall tumors, Bagheri et
al (5) reported that the presence
of distal metastasis significantly worsened prognosis, and any
significant effect on survival was regardless of whether
reconstruction was performed. According to previous studies, the
5-year survival rates are 21–61% in patients with lung cancer who
present with thoracic wall invasion (1,9) and
73.9–88.5% in those with tumors arising from the soft tissues of
the thoracic wall (10,19), results that are comparable to those of
our study. However, in our study, neither the R factor nor the N
factor caused statistically significant differences in prognosis.
Since the N factor shows a tendency to worsen prognosis, it is
possible that a significant difference may be obtained by
accumulating a greater number of cases. Our study had a similar
number of patients as in the report by Scarnecchia et al
(9). The conclusion was different
despite the fact that the ratios of R factor and N factor were
equivalent. This can be explained by the fact that in our study,
the 5-year survival rate was approximately 60%, but their study it
was 44%, the thoracic reconstruction methods and reconstruction
rate were different, and the average observation period was 10
months shorter in our study. Further studies are needed to clarify
whether these differences affect prognosis.
Postoperative complications were observed in 44.1%
of the patients. The incidence of complication was 52.0% in the
lung tumor group and 22.1% in the musculoskeletal tumor group.
Previously reported complications included pneumonitis, acute
respiratory distress syndrome, tissue necrosis, and infection
(4). Among these complications,
respiratory complications are commonly associated with
perioperative death, and therefore, postoperative pain and
respiratory management that prevent complications are thought to be
important. However, studies on the risk factors associated with the
onset of complications are limited. We therefore compared the risk
factors associated with severe complications in both the lung tumor
and musculoskeletal tumor groups. As a result, a large volume of
estimated intraoperative blood loss, a high number of resected
ribs, lung tumor, and undergoing lung resection were considered to
be significant risk factors. In contrast, no significant difference
was observed in the incidence of severe complications in terms of
whether musculocutaneous flap and thoracic wall reconstructions
were performed. This result suggested that good clinical outcomes
can be obtained by performing soft tissue reconstruction even in
the event of extensive soft tissue defect.
Among the patients who also underwent lung resection
(n=52), one patient died due to postoperative pneumonitis (1.9%).
Previous studies have indicated that the perioperative mortality
rate of individuals who underwent thoracic wall resection along
with pneumonectomy was relatively high at 6%, which is three-fold
higher than the rate for those who underwent simple lobectomy
(1). Thoracic wall reconstruction was
performed more often in our study than in previous studies.
Although rigid reconstruction was not performed and our
reconstruction method was used, the perioperative mortality rate
did not increase.
Leuzzi et al (15) reported that postoperative respiratory
function of patients with chest wall resection decreased %VC from
94.1 to 82.0 and FEV1.0% from 87.1 to 82.3, but reported no
significant difference. And they also said that the location of
chest wall defect (antero-lateral) and lung resection are
significant as a risk factor for FEV1.0% decline. In the present
study, postoperative pulmonary function testing showed a reduced
%VC but increased FEV1.0%, and a tendency of restrictive impairment
was observed. Since the detail of cases in which respiratory
function tests were conducted in the report of Leuzzi et al
(15) has not been clarified, the
cause of the difference from our study results is not clear. In
contrast, although sternotomy was described, Nishida et al
(16) have reported that no
significant difference in postoperative changes in FEV1.0% was
observed in patients who underwent non-rigid or semi-rigid
fixation, but %VC significantly decreased, and restrictive
impairment was more likely to occur. This phenomenon might be
attributed to reduced lung expansion resulting from thoracotomy and
pneumonectomy. Among the 16 patients who underwent pulmonary
function testing, 13 presented with lung tumor and underwent
combined lung resection. Regarding pulmonary function after
lobectomy, it has been reported that %VC decreased to 97.4% and
FEV1.0% was 83 to 94% of the preoperative value 12 months after
surgery (21,22). In our study, %VC decreased
significantly in the lung tumor group but did not differ between
before and after surgery in the musculoskeletal tumor group. In the
lung tumor group, 11 of 13 patients resected the lungs as
extensively as single lobectomy. Therefore, in this study, the
decrease in vital capacity observed after resection of the chest
wall mainly reflects the pulmonary resection, and it seems that the
expansion capability of the thoracic cage is preserved.
In lung resection, reconstruction prevents the
decline in pulmonary function to the extent of inhibiting
activities of daily living, and this is comparable with the results
of the present study. Regarding the relationship between
postoperative respiratory complications (RPCs) and postoperative
pulmonary function, Haragushi et al (23) reported in a study of elderly
individuals that when the predicted postoperative (ppo) %VC and/or
ppoFEV1.0% was <55%, the rate of RPCs increased. The early
period showed a ppo%VC and/or ppoFEV1.0% of <55%, which is
considered to be the most significant risk factor in elderly
patients (23). In the present study,
pulmonary function did not deteriorate to that extent, and with
regard to the risk of complications, the results were permissible
for our reconstruction method. The evaluation of pulmonary function
according to thoracic wall resection must be validated in future
studies.
The present study had several limitations. This was
a retrospective study with a small sample size. Not all
participants who were included in this study underwent pulmonary
function testing. Moreover, the postoperative pulmonary function
evaluation period slightly varied among the patients, and the
effects of rigid reconstruction were not compared. However, studies
that examined the postoperative vital capacity in individuals with
both musculoskeletal and lung tumors are extremely limited. We
consider it to be of value that the present study compared the
vital capacities of individuals with both musculoskeletal and lung
tumors.
In conclusion, compared with individuals with
primary lung cancers, individuals with musculoskeletal tumors
arising from the thoracic wall had a better prognosis and were more
likely to require musculocutaneous flap reconstruction. Even in the
event of extensive soft tissue defect, good clinical outcomes were
obtained by performing thoracic wall and musculocutaneous flap
reconstructions. When combined thoracic wall and lung resection was
performed, a high rate of postoperative complications was observed.
However, problems with activities of daily living due to
complications were not observed in any patient. In this present
study, the pulmonary function of patients who underwent thoracic
wall resection alone was not thoroughly evaluated and therefore
should be further examined in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TH analyzed the patient data and wrote the
manuscript. NS, DI, EK and MY acquired the data. YS and HY were
involved in the planning of the research plan, provided guidance
throughout the research, and gave final approval of the version to
be published. ST performed data analysis and interpretation. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics review
board of Aichi Cancer Center Hospital (approval no. 2017-285). The
requirement for written informed consent was waived due to the
retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
%VC
|
vital capacity (%)
|
|
FEV1.0%
|
forced expiratory volume within 1 sec
(%)
|
References
|
1
|
Filosso PL, Sandri A, Guerrera F, Solidoro
P, Bora G, Lyberis P, Ruffini E and Oliaro A: Primary lung tumors
invading the chest wall. J Thorac Dis. 8 (Suppl 11):S855–S862.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boerma LM, Bemelman M and Van Dalen T:
Chest wall reconstruction after resection of a chest wall sarcoma
by osteosynthesis with the titanium MatrixRIB (Synthes) system. J
Thorac Cardiovasc Surg. 146:e37–e40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Puviani L, Fazio N, Boriani L, Ruggieri P,
Fornasari PM and Briccoli A: Reconstruction with fascia lata after
extensive chest wall resection: Results. Eur J Cardio-thoracic
Surg. 44:125–129. 2013. View Article : Google Scholar
|
|
4
|
Tsukushi S, Nishida Y, Sugiura H, Yamada
Y, Kamei Y, Toriyama K and Ishiguro N: Non-rigid reconstruction of
chest wall defects after resection of musculoskeletal tumors. Surg
Today. 45:150–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bagheri R, Haghi SZ, Kalantari MR,
Sharifian Attar A, Salehi M, Tabari A and Soudaneh M: Primary
malignant chest wall tumors: Analysis of 40 patients. J
Cardiothorac Surg. 9:1062014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luan A, Galvez MG and Lee GK: flow-through
omental flap to free anterolateral thigh flap for complex chest
wall reconstruction: Case report and review of the literature.
Microsurgery. 36:70–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang H, Tantai J and Zhao H: Clinical
experience with titanium mesh in reconstruction of massive chest
wall defects following oncological resection. J Thorac Dis.
7:1227–1234. 2015.PubMed/NCBI
|
|
8
|
Gonfiotti A, Santini PF, Campanacci D,
Innocenti M, Ferrarello S, Caldarella A and Janni A: Malignant
primary chest-wall tumours: Techniques of reconstruction and
survival. Eur J Cardiothorac Surg. 38:39–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scarnecchia E, Liparulo V, Capozzi R,
Ceccarelli S, Puma F and Vannucci J: Chest wall resection and
reconstruction for tumors: Analysis of oncological and functional
outcome. J Thorac Dis. 10 (Suppl 16):S1855–S1863. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abdel Rahman ARM, Rahouma M, Gaafar R,
Bahaa S, Loay I, Kamel M, Abdelbaki H and Yahia M: Contributing
factors to the outcome of primary malignant chest wall tumors. J
Thorac Dis. 9:5184–5193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ong K, Ong CS, Chua YC, Fazuludeen AA and
Ahmed ADB: The painless combination of anatomically contoured
titanium plates and porcine dermal collagen patch for chest wall
reconstruction. J Thorac Dis. 10:2890–2897. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scarnecchia E, Liparulo V, Pica A, Guarro
G, Alfano C and Puma F: Multidisciplinary approach to chest wall
resection and reconstruction for chest wall tumors, a single center
experience. J Thorac Dis. 9:5093–5100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wen X, Gao S, Feng J, Li S, Gao R and
Zhang G: Chest-wall reconstruction with a customized titanium-alloy
prosthesis fabricated by 3D printing and rapid prototyping. J
Cardiothorac Surg. 13:42018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tukiainen E: Chest wall reconstruction
after oncological resections. Scand J Surg. 102:9–13. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leuzzi G, Nachira D, Cesario A, Novellis
P, Petracca Ciavarella L, Lococo F, Facciolo F, Granone P and
Margaritora S: Chest wall tumors and prosthetic reconstruction: A
comparative analysis on functional outcome. Thorac Cancer.
6:247–254. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nishida Y, Tsukushi S, Urakawa H, Toriyama
K, Kamei Y, Yokoi K and Ishiguro N: Post-operative pulmonary and
shoulder function after sternal reconstruction for patients with
chest wall sarcomas. Int J Clin Oncol. 20:1218–1225. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Institute of Cancer: Common
terminology criteria for adverse events (CTCAE). NIH Publ.
2009:0–71. 2010.
|
|
18
|
Kanda Y: Investigation of the freely
available easy-to-use software “EZR” for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Althubaiti G and Butler CE: Abdominal wall
and chest wall reconstruction. Plast Reconstr Surg. 133:688e–701e.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsukushi S, Nishida Y, Sugiura H,
Nakashima H and Ishiguro N: Soft tissue sarcomas of the chest wall.
J Thorac Oncol. 4:834–837. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kobayashi N, Kobayashi K, Kikuchi S, Goto
Y, Ichimura H, Endo K and Sato Y: Long-term pulmonary function
after surgery for lung cancer. Interact Cardiovasc Thorac Surg.
24:727–732. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim HK, Lee YJ, Han KN and Choi YH:
Pulmonary function changes over 1 year after lobectomy in lung
cancer. Respir Care. 61:376–382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haraguchi S, Koizumi K, Hatori N, Akiyama
H, Mikami I, Kubokura H and Tanaka S: Prediction of the
postoperative pulmonary function and complication rate in elderly
patients. Surg Today. 31:860–865. 2001. View Article : Google Scholar : PubMed/NCBI
|