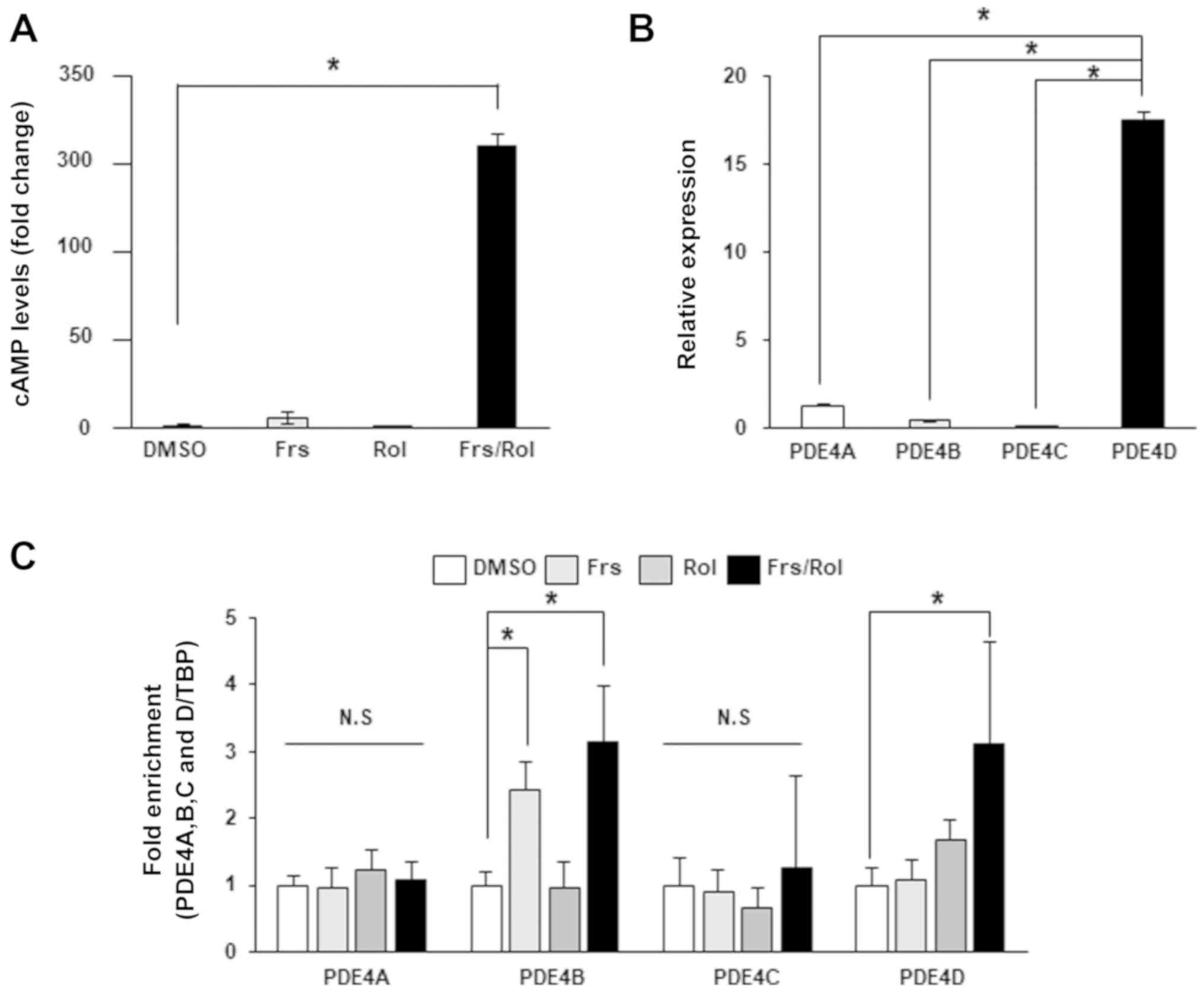
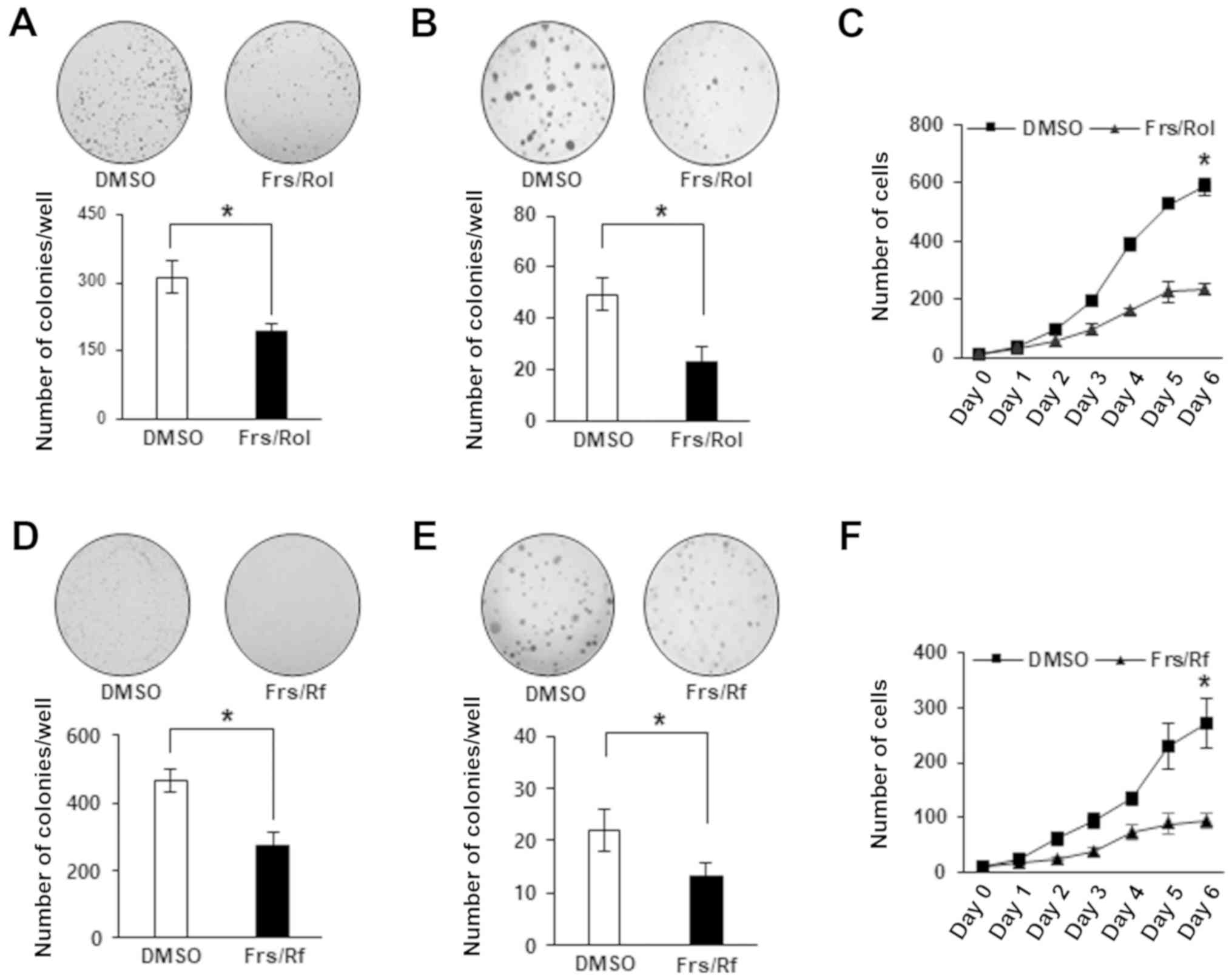
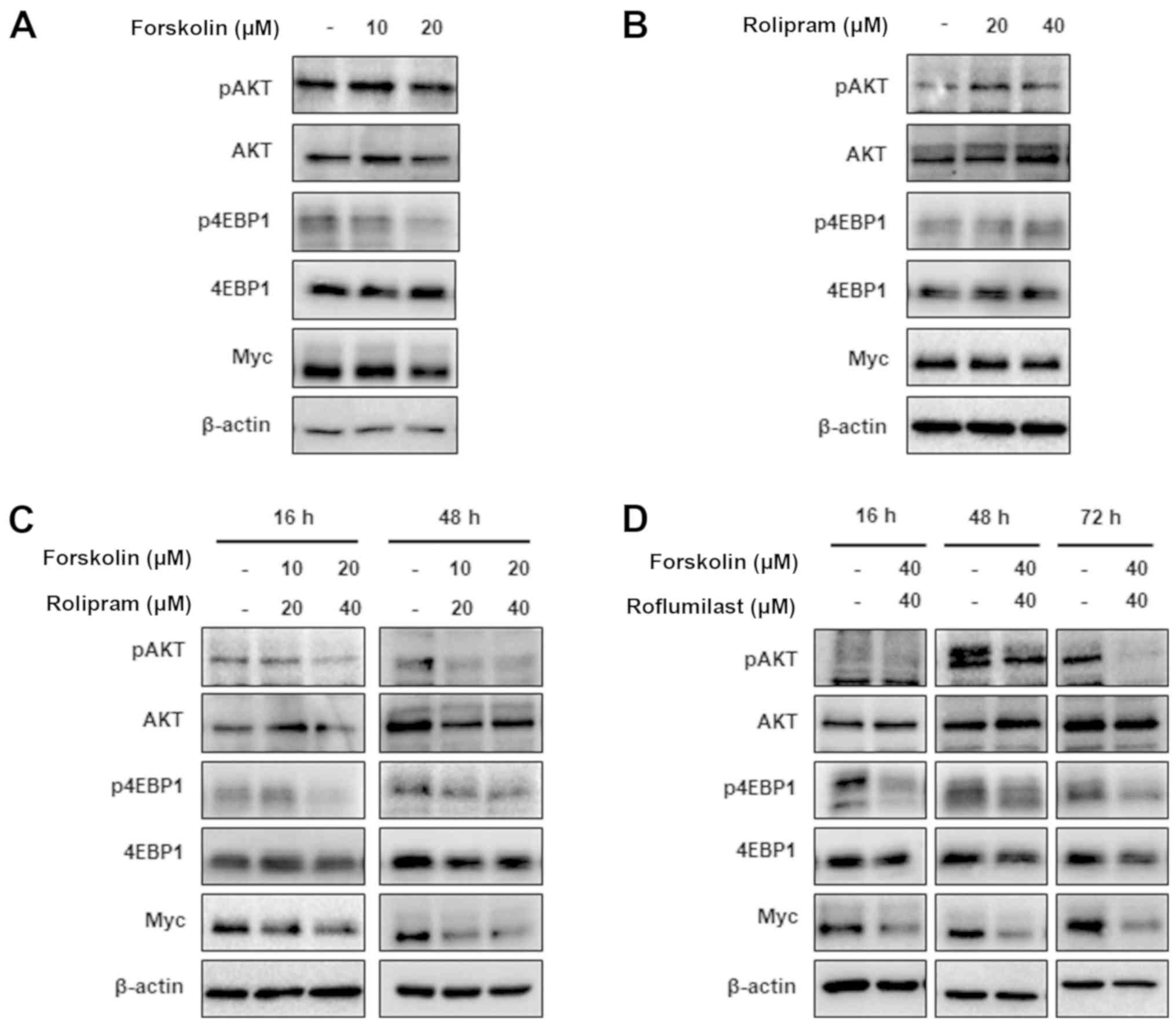
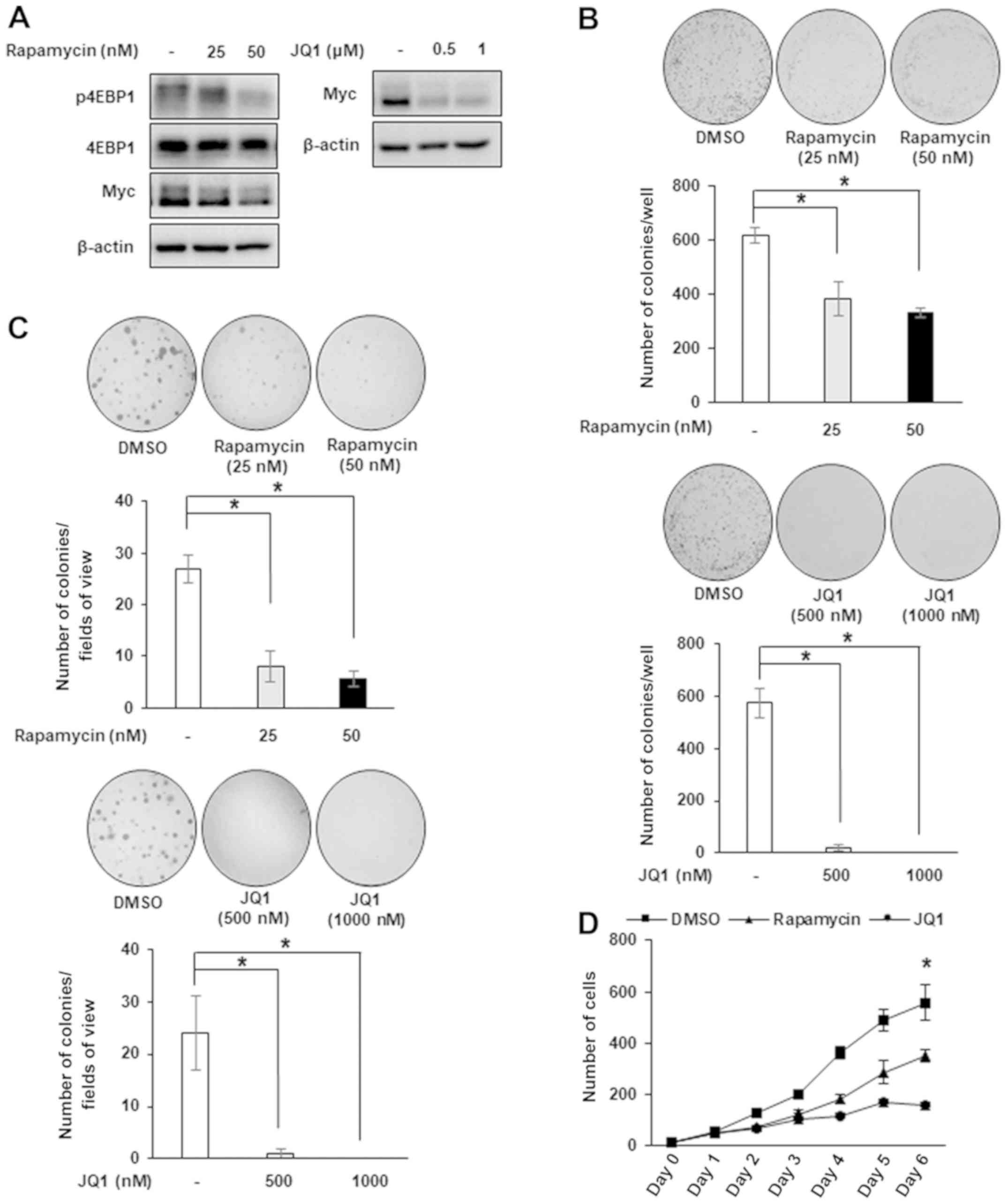
|
1
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A:
Colorectal cancer mortality rates in adults aged 20 to 54 years in
the united states, 1970–2014. JAMA. 318:572–574. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Worthley DL and Leggett BA: Colorectal
cancer: Molecular features and clinical opportunities. Clin Biochem
Rev. 31:31–38. 2010.PubMed/NCBI
|
|
4
|
Hinoue T, Weisenberger DJ, Pan F, Campan
M, Kim M, Young J, Whitehall VL, Leggett BA and Laird PW: Analysis
of the association between CIMP and BRAF in colorectal cancer by
DNA methylation profiling. PLoS One. 4:e83572009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mundade R, Imperiale TF, Prabhu L, Loehrer
PJ and Lu T: Genetic pathways, prevention, and treatment of
sporadic colorectal cancer. Oncoscience. 1:400–406. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Walther A, Johnstone E, Swanton C, Midgley
R, Tomlinson I and Kerr D: Genetic prognostic and predictive
markers in colorectal cancer. Nat Rev Cancer. 9:489–499. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fodde R: The APC gene in colorectal
cancer. Eur J Cancer. 38:867–871. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schneikert J and Behrens J: The canonical
Wnt signalling pathway and its APC partner in colon cancer
development. Gut. 56:417–425. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Easwaran V, Lee SH, Inge L, Guo L,
Goldbeck C, Garrett E, Wiesmann M, Garcia PD, Fuller JH, Chan V, et
al: Beta-Catenin regulates vascular endothelial growth factor
expression in colon cancer. Cancer Res. 63:3145–3153.
2003.PubMed/NCBI
|
|
10
|
Herbst A, Jurinovic V, Krebs S, Thieme SE,
Blum H, Göke B and Kolligs FT: Comprehensive analysis of β-catenin
target genes in colorectal carcinoma cell lines with deregulated
Wnt/β-catenin signaling. BMC Genomics. 15:742014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morin PJ: Beta-catenin signaling and
cancer. Bioessays. 21:1021–1030. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sansom OJ, Meniel VS, Muncan V, Phesse TJ,
Wilkins JA, Reed KR, Vass JK, Athineos D and Clevers H: Myc
deletion rescues Apc deficiency in the small intestine. Nature.
446:676–679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sewastianik T, Prochorec-Sobieszek M,
Chapuy B and Juszczyński P: MYC deregulation in lymphoid tumors:
Molecular mechanisms, clinical consequences and therapeutic
implications. Biochim Biophys Acta. 1846:457–467. 2014.PubMed/NCBI
|
|
14
|
Rall TW and Sutherland EW: Formation of a
cyclic adenine ribonucleotide by tissue particles. J Biol Chem.
232:1065–1076. 1958.PubMed/NCBI
|
|
15
|
Sutherland EW and Rall TW: Fractionation
and characterization of a cyclic adenine ribonucleotide formed by
tissue particles. J Biol Chem. 232:1077–1091. 1958.PubMed/NCBI
|
|
16
|
Daniel PB, Walker WH and Habener JF:
Cyclic AMP signaling and gene regulation. Annu Rev Nutr.
18:353–383. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fimia GM and Sassone-Corsi P: Cyclic AMP
signalling. J Cell Sci. 114:1971–1972. 2001.PubMed/NCBI
|
|
18
|
Sassone-Corsi P: The cyclic AMP pathway.
Cold Spring Harb Perspect Biol. 4(a011148)2012.PubMed/NCBI
|
|
19
|
Kim SW, Rai D and Aguiar RC: Gene set
enrichment analysis unveils the mechanism for the phosphodiesterase
4B control of glucocorticoid response in B-cell lymphoma. Clin
Cancer Res. 17:6723–6732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smith PG, Wang F, Wilkinson KN, Savage KJ,
Klein U, Neuberg DS, Bollag G, Shipp MA and Aguiar RC: The
phosphodiesterase PDE4B limits cAMP-associated PI3K/AKT-dependent
apoptosis in diffuse large B-cell lymphoma. Blood. 105:308–316.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsunoda T, Ota T, Fujimoto T, Doi K,
Tanaka Y, Yoshida Y, Ogawa M, Matsuzaki H, Hamabashiri M, Tyson DR,
et al: Inhibition of phosphodiesterase-4 (PDE4) activity triggers
luminal apoptosis and AKT dephosphorylation in a 3-D colonic-crypt
model. Mol Cancer. 11:462012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao B, Wang K, Liao JM, Zhou X, Liao P,
Zeng SX, He M, Chen L, He Y, Li W and Lu H: Inactivation of
oncogenic cAMP-specific phosphodiesterase 4D by miR-139-5p in
response to p53 activation. Elife. 5(e15978)2016.
|
|
24
|
Lakics V, Karran EH and Boess FG:
Quantitative comparison of phosphodiesterase mRNA distribution in
human brain and peripheral tissues. Neuropharmacology. 59:367–374.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McEwan DG, Brunton VG, Baillie GS, Leslie
NR, Houslay MD and Frame MC: Chemoresistant KM12C colon cancer
cells are addicted to low cyclic AMP levels in a phosphodiesterase
4-regulated compartment via effects on phosphoinositide 3-kinase.
Cancer Res. 67:5248–5257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Le Jeune IR, Shepherd M, Van Heeke G,
Houslay MD and Hall IP: Cyclic AMP-dependent transcriptional
up-regulation of phosphodiesterase 4D5 in human airway smooth
muscle cells. Identification and characterization of a novel PDE4D5
promoter. J Biol Chem. 277:35980–35989. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takahashi M, Terwilliger R, Lane C, Mezes
PS, Conti M and Duman RS: Chronic antidepressant administration
increases the expression of cAMP-specific phosphodiesterase 4A and
4B isoforms. J Neurosci. 19:610–618. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lipworth BJ: Phosphodiesterase-4
inhibitors for asthma and chronic obstructive pulmonary disease.
Lancet. 365:167–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Francipane MG and Lagasse E: mTOR pathway
in colorectal cancer: An update. Oncotarget. 5:49–66. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pandurangan AK: Potential targets for
prevention of colorectal cancer: A focus on PI3K/Akt/mTOR and Wnt
pathways. Asian Pac J Cancer Prev. 14:2201–2205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sipos F, Firneisz G and Műzes G:
Therapeutic aspects of c-MYC signaling in inflammatory and
cancerous colonic diseases. World J Gastroenterol. 22:7938–7950.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pirson I, Coulonval K, Lamy F and Dumont
JE: c-Myc expression is controlled by the mitogenic cAMP-cascade in
thyrocytes. J Cell Physiol. 168:59–70. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Williamson EA, Burgess GS, Eder P,
Litz-Jackson S and Boswell HS: Cyclic AMP negatively controls c-myc
transcription and G1 cell cycle progression in p210 BCR-ABL
transformed cells: Inhibitory activity exerted through cyclin D1
and cdk4. Leukemia. 11:73–85. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Di Costanzo A, Del Gaudio N, Migliaccio A
and Altucci L: Epigenetic drugs against cancer: An evolving
landscape. Arch Toxicol. 88:651–668. 2014. View Article : Google Scholar
|
|
35
|
Park SJ, Ahmad F, Philp A, Baar K,
Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, et al:
Resveratrol ameliorates aging-related metabolic phenotypes by
inhibiting cAMP phosphodiesterases. Cell. 148:421–433. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsunoda T, Ishikura S, Doi K, Matsuzaki H,
Iwaihara Y and Shirasawa S: Resveratrol induces luminal apoptosis
of human colorectal cancer HCT116 cells in three-dimensional
culture. Anticancer Res. 34:4551–4555. 2014.PubMed/NCBI
|
|
37
|
Goldhoff P, Warrington NM, Limbrick DD Jr,
Hope A, Woerner BM, Jackson E, Perry A, Piwnica-Worms D and Rubin
JB: Targeted inhibition of cyclic AMP phosphodiesterase-4 promotes
brain tumor regression. Clin Cancer Res. 14:7717–7725. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ogawa R, Streiff MB, Bugayenko A and Kato
GJ: Inhibition of PDE4 phosphodiesterase activity induces growth
suppression, apoptosis, glucocorticoid sensitivity, p53, and
p21(WAF1/CIP1) proteins in human acute lymphoblastic leukemia
cells. Blood. 99:3390–3397. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Scott PH and Lawrence JC Jr: Attenuation
of mammalian target of rapamycin activity by increased cAMP in
3T3-L1 adipocytes. J Biol Chem. 273:34496–34501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Blancquaert S, Wang L, Paternot S,
Coulonval K, Dumont JE, Harris TE and Roger PP: cAMP-dependent
activation of mammalian target of rapamycin (mTOR) in thyroid
cells. Implication in mitogenesis and activation of CDK4. Mol
Endocrinol. 24:1453–1468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kelly K, Mejia A, Suhasini AN, Lin AP,
Kuhn J, Karnad AB, Weitman S and Aguiar RC: Safety and
pharmacodynamics of the PDE4 inhibitor roflumilast in advanced
B-cell malignancies. Clin Cancer Res. 23:1186–1192. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Delyon J, Servy A, Laugier F, André J,
Ortonne N, Battistella M, Mourah S, Bensussan A, Lebbé C and Dumaz
N: PDE4D promotes FAK-mediated cell invasion in BRAF-mutated
melanoma. Oncogene. 36:3252–3262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ge X, Milenkovic L, Suyama K, Hartl T,
Purzner T, Winans A, Meyer T and Scott MP: Phosphodiesterase 4D
acts downstream of Neuropilin to control Hedgehog signal
transduction and the growth of medulloblastoma. Elife.
4:e070682015. View Article : Google Scholar
|
|
44
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bruno O, Fedele E, Prickaerts J, Parker
LA, Canepa E, Brullo C, Cavallero A, Gardella E, Balbi A,
Domenicotti C, et al: GEBR-7b, a novel PDE4D selective inhibitor
that improves memory in rodents at non-emetic doses. Br J
Pharmacol. 164:2054–2063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ricciarelli R, Brullo C, Prickaerts J,
Arancio O, Villa C, Rebosio C, Calcagno E, Balbi M, van Hagen BT,
Argyrousi EK, et al: Memory-enhancing effects of GEBR-32a, a new
PDE4D inhibitor holding promise for the treatment of Alzheimer's
disease. Sci Rep. 7:463202017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mishra RR, Belder N, Ansari SA, Kayhan M,
Bal H, Raza U, Ersan PG, Tokat ÜM, Eyüpoğlu E, Saatci Ö, et al:
Reactivation of cAMP pathway by PDE4D inhibition represents a novel
druggable axis for overcoming tamoxifen resistance in ER-positive
breast cancer. Clin Cancer Res. 24:1987–2001. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Böttcher R, Dulla K, van Strijp D, Dits N,
Verhoef EI, Baillie GS, van Leenders GJ, Houslay MD, Jenster G and
Hoffmann R: Human PDE4D isoform composition is deregulated in
primary prostate cancer and indicative for disease progression and
development of distant metastases. Oncotarget. 7:70669–70684. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li Q, Liang X, Wang Y, Meng X, Xu Y, Cai
S, Wang Z, Liu J and Cai G: miR-139-5p inhibits the
epithelial-mesenchymal transition and enhances the chemotherapeutic
sensitivity of colorectal cancer cells by downregulating BCL2. Sci
Rep. 6:271572016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Garcia-Morales V, Luaces-Regueira M and
Campos-Toimil M: The cAMP effectors PKA and Epac activate
endothelial NO synthase through PI3K/Akt pathway in human
endothelial cells. Biochem Pharmacol. 145:94–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Miklos W, Heffeter P, Pirker C, Hager S,
Kowol CR, van Schoonhoven S, Stojanovic M, Keppler BK and Berger W:
Loss of phosphodiesterase 4D mediates acquired triapine resistance
via Epac-Rap1-Integrin signaling. Oncotarget. 7:84556–84574. 2016.
View Article : Google Scholar : PubMed/NCBI
|