Introduction
Platelets are known to enhance the hematogenous
metastasis of tumor cells through various mechanisms. Platelets are
capable of forming a protective cloak by adhering to tumor cells to
protect them from natural killer (NK) cytotoxicity (1,2),
enhancing tumor angiogenesis by forming growth factors 3–5) and
promoting the adhesion of tumor cells on the endothelium and the
invasion of tumor cells into the surrounding tissues (6–8). The
common basis for these carcinogenic effects is the interaction of
platelets and tumor cells. P-selectin and αIIbβ3 integrin,
expressed in platelets, and their heparan sulfate-like proteoglycan
(HSPG) ligands, expressed in tumor cells, have been reported to be
crucial for this interaction (9–12). A
number of studies have focused on inhibitors of adhesive molecules
or their ligands, including blocking antibodies, oligosugars,
polysugars and peptites (13,14).
Among these inhibitors, a traditional clinical reagent, heparin,
may be one of the most valuable candidates in blocking the adhesion
of platelets and tumor cells (15,16).
However, the hemorrhagic dangers caused by the marked anticoagulant
effect of heparin has limited its application as an anti-metastatic
drug. Previously, we demonstrated that certain chemically modified
heparins with low anticoagulant activity were capable of blocking
the P-selectin and αIIbβ3 integrin-mediated adhesion of platelets
and tumor cells (17–19).
Since the adhesion of platelets and tumor cells
occurs in the blood, the effect of plasma proteins cannot be
ignored. In a recent study, we found that fibrinogen strongly
bridged and enhanced the adhesion of platelets and tumor cells
under various shear forces (20).
Fibrinogen is a major component of the plasma; thus, the
identification of blockers to the adhesion of platelets and tumor
cells bridged by fibrinogen is necessary, as identifying such
blockers may have greater significance for clinical tumor
treatment. In the present study, we aimed to detect the effects of
8 chemically modified heparins on the binding of fibrinogen to
platelets or tumor cells using flow cytometry assays, as well as
the fibrinogen-bridged adhesion of platelets and tumor cells using
flow chamber assays. The results revealed that borohydride-reduced
(RO)-, carboxyl-reduced (CR)- and 2-O, 3-O-desulfated
(2/3ODS)-heparins inhibited the binding of fibrinogen to platelets
or tumor cells, and that fibrinogen bridged indirect adhesion
between platelets and tumor cells.
Materials and methods
Cells
B16F10, a murine melanoma cell line, A375, a human
melanoma cell line, and CHO cells were obtained from the Cell Bank
of Type Culture Collection of the Chinese Academy of Science
(Shanghai, China). The cells were grown in Iscove’s Modified
Dulbecco’s Media (IMDM; Gibco; Invitrogen Life Technologies,
Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal
bovine serum in the presence of 100 U/ml penicillin and 0.1 mg/ml
streptomycin (Invitrogen Life Technologies) in a humidified
atmosphere containing 5% CO2 at 37°C. Cells were
passaged by mild trypsinization (0.25% trypsin) and harvested with
2 mM ethylenediaminetetraacetic acid (EDTA) in phosphate-buffered
saline (PBS). Tumor cells were washed twice with PBS containing 1
mM CaCl2, 1 mM MgCl2 and 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH
7.4), resuspended in serum-free culture medium containing 0.1%
bovine serum albumin (BSA), and stored at 4°C for no longer than 5
h prior to use.
Antibodies and reagents
The monoclonal antibodies (mAbs) LM609 recognizing
human β3 integrins (blocking mAb) and 2C9.G2 recognizing murine β3
integrins (blocking mAb) were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). HMb1-1 recognizing
murine β1 integrins (blocking mAb) was purchased from BioLegend,
Inc. (San Diego, CA, USA). Armenian hamster IgG isotype control and
fluorescein isothiocyanate (FITC)-conjugated antibody against
Armenian hamster IgG were purchased from Santa Cruz Biotechnology,
Inc. Human IgG and mouse IgG isotype control, FITC-labeled goat
anti-human IgG and anti-mouse IgG were purchased from Jackson
Immune Research Laboratories, Inc. (West Grove, PA, USA). Human
fibrinogen, 3-aminopropyltriethoxylsilane (APES) and porcine
intestinal heparin were purchased from Sigma-Aldrich Inc. (St.
Louis, MO, USA). Periodate-oxidized, RO-, CR-, 2/3ODS-,
N-desulfated, 2/3ODS- (N/2/3DS-), carboxyl-reduced sulfated-
(CRS-), N-desulfated/N-acetylated- (NDS-), 6-O-desulfated- (6ODS-)
and N-desulfated 6-O- desulfated-heparin (N/6DS-heparin) were
prepared in our laboratory (18).
Preparation of platelets
Specific pathogen-free C57BL/6 J mice (male, 6–8
weeks old) were obtained from the animal center of Jilin University
(Changchun, China). Blood of healthy mice injected with
prostaglandin E1 (PGE-1) was collected retro-orbitally, and then
immediately mixed with sodium citrate anticoagulant buffer (0.38%
w/v). Platelet-rich plasma (PRP) was prepared by centrifugation of
whole blood at 300 × g for 10 min, and platelet-poor plasma (PPP)
and platelets were separated by centrifugation of PRP at 1,300 × g
for 10 min. The density of platelets was adjusted to
2×108/ml by the HEPES Tyrode buffer (134 mM NaCl, 12 mM
NaHCO3, 2.9 mM KCl, 0.34 mM
NaH2PO4, 5 mM HEPES, 5 mM glucose, 1% BSA, pH
7.4) for further use. To avoid any unwanted activation of
platelets, 2 μM PGE-1 was added to the buffers, and PGE-1 was
washed off by HEPES Tyrode buffer prior to the use of platelets
(21). The study protocols were
approved by the Animal Care and Use Committee of Northeast Normal
University, (Changchun, China).
Flow cytometry assay
To assess the adhesion of fibrinogen to tumor cells,
flow cytometry was performed using a flow cytometer (Coulter Epics
XL; Beckman-Coulter, FL, USA). In brief, tumor cells were harvested
and washed as previously described, counted and resuspended in
culture medium at a final concentration of 5×106
cells/ml. For the fibrinogen binding assay, 5×105 cells
were incubated in 100 μl of culture medium containing 0.02 mg of
fibrinogen conjugated with Alexa Fluor488 green (no. F13191;
Molecular Probes, Invitrogen, OR, USA) at room temperature for 30
min. Subsequently, cells were washed twice, and 10,000 cells were
collected for flow cytometry. For the adhesion inhibition
experiments, the cells were preincubated with 1 μg/ml of 2C9.G2,
HMB1-1 or LM609, or with 1 mg/ml of chemically modified heparins
for 30 min.
Static adhesion assay
To assess the adhesion of fibrinogen to platelets,
human and murine platelets were stained with calcein acetoxymethyl
ester (AM) (no. 32805; Molecular Probes, Invitrogen, OR, USA), and
the stained platelets were added into the wells, which were
pre-coated with fibrinogen following washing twice with HEPES
Tyrode buffer. Adhesions were performed for 30 min, non-specific
adhered platelets were removed by mild rinsing, and the
fluorescence density of each well was measured using a microplate
reader (MD VersaMax, Hamilton-Molecular Devices driver; Hamilton
Company, Höchst, Germany), which reflected the number of stably
adhered platelets. For the inhibition assay, platelets were
preincubated with various antibodies or chemically modified
heparins for 30 min prior to addition into the wells.
Flow chamber adhesion assay
Platelet monolayers were prepared as previously
described (18). Non-specific
binding was blocked with 0.1% BSA at 37°C for 10 min. Cell adhesion
to platelets under flow conditions was measured as previously
reported (21). In brief, cells
were washed, resuspended and adjusted to 1×106 cells/ml
in serum-free medium containing 0.1% BSA. Surface-adhered platelets
were incubated with 100 μl of thrombin (1 U/ml in PBS/0.1% BSA) at
37°C for 1 min. Circular glass slides coated with platelets were
assembled in a flow chamber and mounted on the stage of an inverted
microscope (Olympus Optical, Tokyo, Japan) equipped with a camera
(Panasonic; Yokohama, Japan) connected to a personal computer by a
TV monitor card. After washing the platelet layer with PBS/0.1% BSA
for approximately 2 min, tumor cells were perfused through the
chamber for 3 min at appropriate flow rates to obtain wall shear
stresses of 0.3 to 1.2 dyn/cm2 at 22°C using a syringe
pump (Cole-Parmer Instrument Co.; Montreal, Canada), thereby
mimicking the flow mechanical environment of microcirculation in
postcapillary venules. Interactions between tumor cells and
surface-adherent platelets (or fibrinogen) were visualized in
real-time by phase-contrast video microscopy. The number of bound
cells was quantified from the digital recordings of 10 random
fields of the views obtained.
Statistics
Data are shown as the means ± standard deviation
(SD). Statistical significance of differences between the means was
determined by one-way ANOVA. If the means were revealed to be
significantly different, multiple comparisons using pairs were
performed using the Tukey test. Probability values of P<0.01
were considered to indicate statistically significant
differences.
Results
Fibrinogen binds to tumor cells and
platelets in a β3 integrin-dependent manner
Although a number of studies (6,9) have
demonstrated that platelets directly adhere to tumor cells, the
effects of plasma protein cannot be ignored as the adhesion that
occurs in blood and fibrinogen is one of the major components of
the plasma. In a recent study, we found that fibrinogen bridged and
enhanced the adhesion of platelets and tumor cells under
physiological conditions (20). In
the present study, flow cytometry and static adhesion assays were
performed to assess the adhesive ability of fibrinogen to tumor
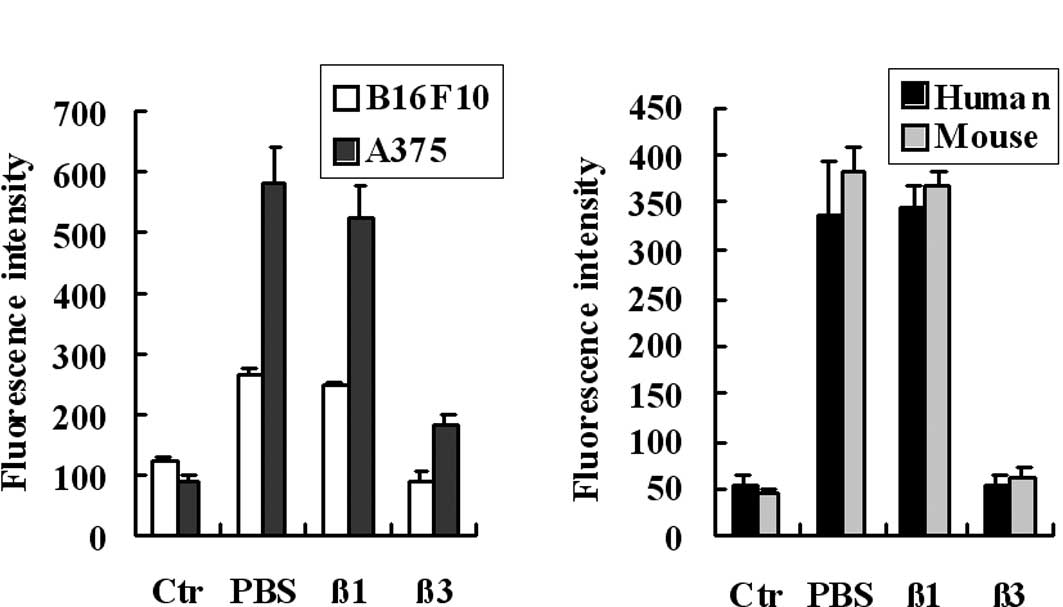
cells and platelets. As shown in Fig.
1, fibrinogen bound to tumor cells (A) and platelets (B). As
previously reported the receptors of fibrinogen on melanoma cells
are the αvβ3 and α5β1 integrins, we assessed these integrins to
determine whether they play crucial roles in the binding process by
using blocking antibodies. The results showed that the binding of
fibrinogen to B16F10 and A375 cells was blocked by the antibody
against the β3 integrin, but not the β1 integrin (Fig. 1A). We also assessed the adhesion of
fibrinogen to platelets using a static adhesion assay. The results
showed that the adhesion of fibrinogen to human and murine
platelets were blocked by the blocking antibody against the β3
integrin (Fig. 1B). These results
indicate that fibrinogen binds to tumor cells and platelets, and
that β3 integrins, expressed in tumor cells and platelets, are the
major receptors of fibrinogen.
Fibrinogen bridges the indirect adhesion
between tumor cells and platelets in a β3 integrin-dependent
manner
To confirm the role of fibrinogen in the adhesion of
tumor cells and platelets, melanoma cells were preincubated with
various concentrations of fibrinogen for 30 min prior to perfusion
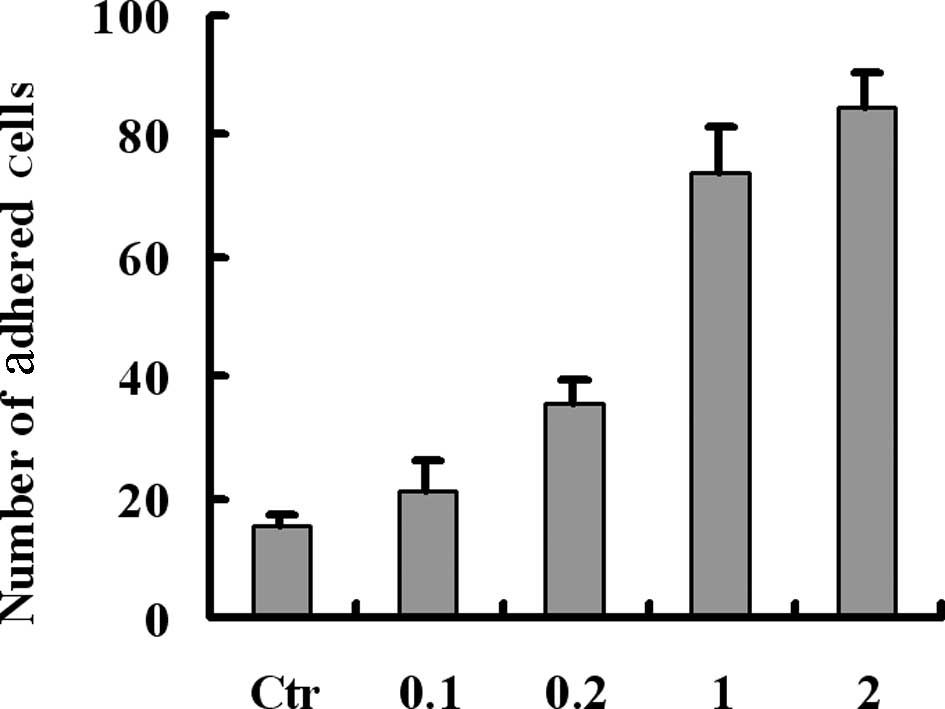
over the platelet monolayers. Results revealed that fibrinogen
mediated and enhanced the adhesion between tumor cells and
platelets in a concentration-dependent manner (Fig. 2). Previously, we demonstrated that
the preincubation of tumor cells with a β3 integrin-blocking
antibody inhibited the indirect adhesion between tumor cells and
platelets (19). In the present
study, we investigated the contribution of β3 integrins, expressed
in platelets, as well as β3 and β1 integrins, expressed in tumor
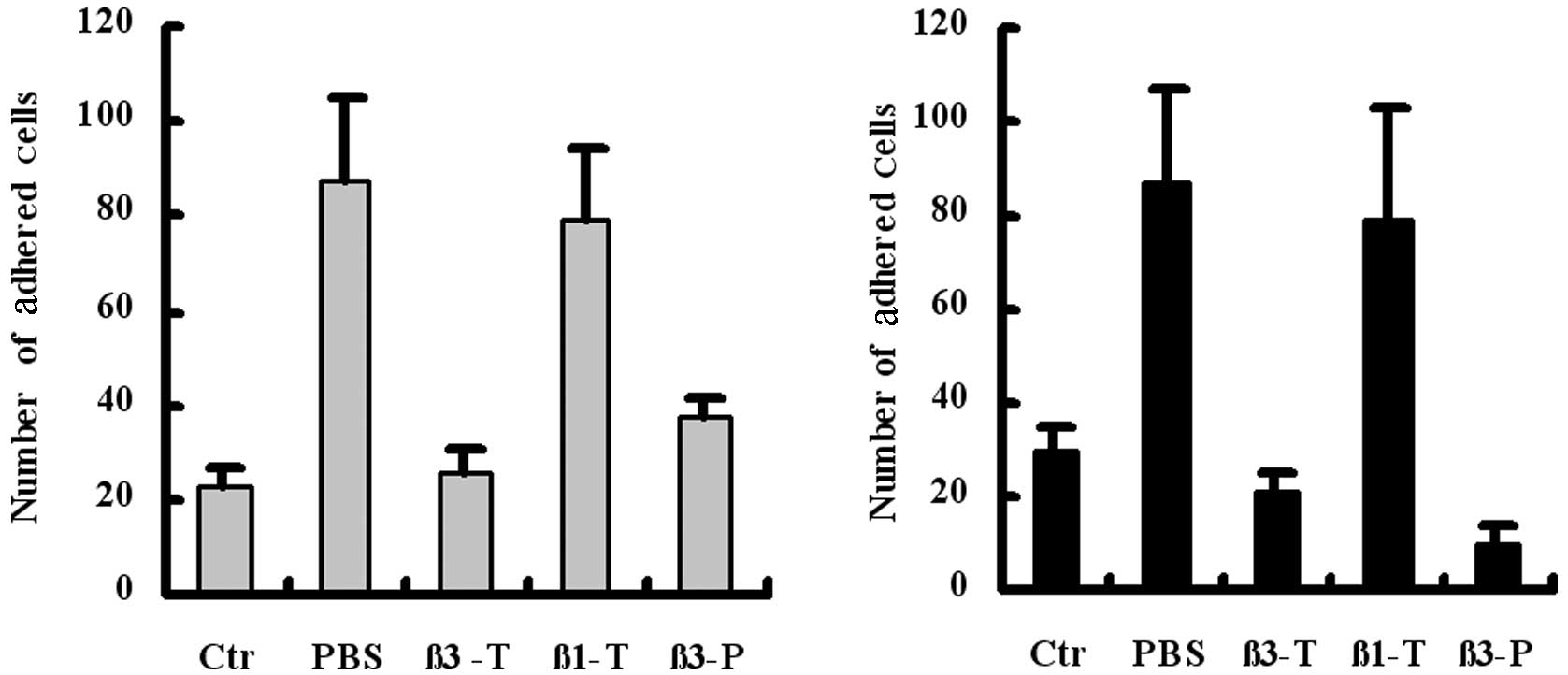
cells. Results from the flow chamber assay revealed that the
preincubation of platelets (β3-P) or tumor cells (β3-T) with β3
integrin-blocking antibodies blocked the indirect adhesion of
B16F10 (Fig. 3A) or A375 (Fig. 3B) to the platelet monolayer under
flow conditions. The results confirm the bridging role of
fibrinogen in mediating the adhesion of tumor cells and platelets,
and indicate that β3 integrins, expressed in tumor cells or
platelets, are involved in indirect adhesion.
Chemically modified heparins inhibit the
direct binding of fibrinogen to tumor cells and platelets
Heparin has been reported to be a powerful inhibitor
of the adhesions between cells and cells, or between cells and
fibrinogen, and the adhesive molecules involved in the adhesion
include selectins and integrins. Fibrinogen is capable of binding
to tumor cells and platelets through β3 integrin and bridging the
adhesion of tumor cells and platelets. In the present study, we
examined the blocking effects of 8 chemically modified heparins (1
mg/ml) on the binding of fibrinogen to tumor cells and platelets.
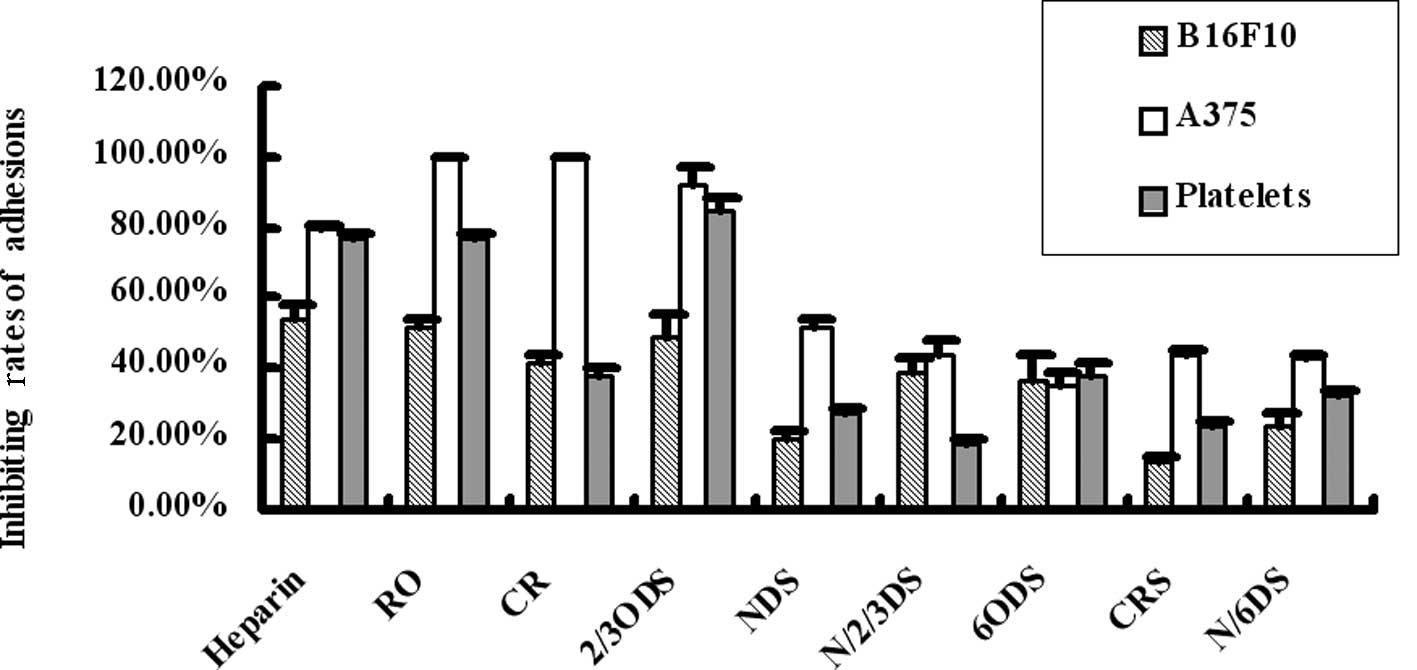
As shown in Fig. 4, heparin, RO-,
CR- or 2,3ODS-heparins strongly blocked the binding of fibrinogen
to A375 cells (inhibition rate, >75%). RO- and 2/3ODS-heparin
blocked the binding of fibrinogen to platelets to the levels of
heparin (inhibition rate, >75%). In addition, RO- and
2/3ODS-heparin blocked the binding of fibrinogen to B16F10 cells
(inhibition rate, >50%). These data suggest that certain types
of chemically modified heparins, including RO-, CR- or
2,3ODS-heparins, act as effective blockers for direct
adhesions.
Chemically modified heparins inhibit the
fibrinogen-bridged indirect adhesion of tumor cells and
platelets
As demonstrated previously, fibrinogen adhered to
tumor cells and platelets and bridged their adhesion, and certain
chemically modified heparins blocked the direct binding of
fibrinogen to tumor cells or platelets. The aim of the present
study was to detect whether the chemically modified heparins were
capable of blocking the fibrinogen-mediated indirect adhesion of
tumor cells and platelets using flow chamber assays. As shown in
Fig. 5, compared to the direct
adhesion (Ctr), pre-incubation of tumor cells with fibrinogen
notably enhanced the adhesion of tumor cells and platelets (PBS),
and this indirect adhesion was inhibited by certain types of
chemically modified heparins. RO-, CR- and 2/3ODS-heparins have
been found to be more effective than other types of heparins in
that their inhibitory abilities were similar to, or even greater
than, heparin and low molecular weight heparin (LMWH), the
well-known clinical reagents. Moreover, the inhibitory effects of
RO-, CR- and 2/3ODS-heparins were concentration-dependent. These
data suggest that RO-, CR- and 2/3ODS-heparins have a greater
potential for cancer treatments due to their limited anticoagulant
activity.
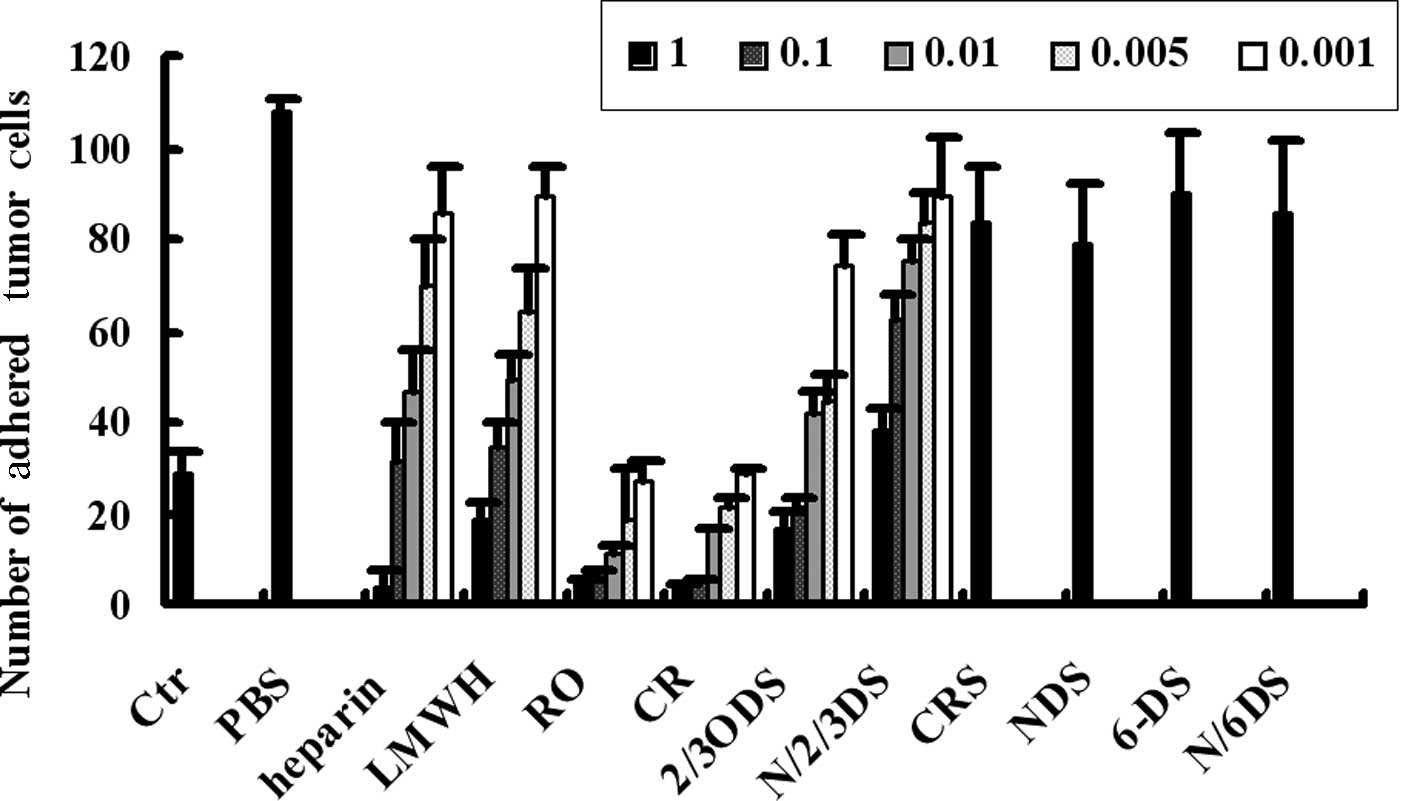 | Figure 5Chemically modified heparins inhibit
the fibrinogen-mediated indirect adhesion of tumor cells and
platelets. The indirect adhesion of A375 cells to platelet
monolayers mediated by fibrinogen were assessed using flow chamber
assays. Prior to incubation with fibrinogen, tumor cells were
preincubated with PBS or varying concentrations of heparin, LMWH or
chemically modified heparins. The adhesions of tumor cells to
platelet monolayers were carried out following incubations, and the
number of adhered tumor cells was quantified. Ctr were the control
samples of direct adhesion of tumor cells and platelets, without
the addition of fibrinogen or heparins. LMWH, low molecular weight
heparin; Ctr, control; PBS, phosphate-buffered saline; NDS,
N-desulfated/N-acetylated; CRS, carboxyl-reduced sulfated; CR,
carboxyl-reduced; RO, borohydride-reduced. |
Discussion
The adhesion between platelets and tumor cells is
known to enhance tumor hematogenous metastasis. Studies have
focused on identifying the adhesive mechanism and searching for
inhibitory drugs. P-selectin and αIIbβ3 integrin, expressed in
platelets, and HSPGs, expressed in tumor cells, are the major
receptors for the direct adhesion between tumor cells and
platelets, and various inhibitors targeting these adhesive
molecules have been developed (13–17).
Among the inhibitors, heparins and their derivatives have been
reported to be the most powerful candidates for mediating direct
adhesion, in that they markedly inhibit the direct adhesion
mediated by P-selectin and αIIbβ3 integrin (18,19).
In the present study, we demonstrated that fibrinogen adhered to
tumor cells and platelets in a β3 integrin-dependent manner
(Fig. 1), and that the adhesion of
melanoma cells to the platelet monolayer was increased by
fibrinogen in a concentration-dependent manner (Fig. 2). β3 integrin, expressed in tumor
cells and platelets, is essential for this indirect adhesion
(Fig. 3), and fibrinogen mediated
the indirect adhesion between melanoma cells and platelets as a
bridge (20).
Since fibrinogen is rich in blood, developing
inhibitors to the indirect adhesion between tumor cells and
platelets is of significance. In this study, we detected the
effects of heparin and its derivatives on indirect adhesion. We
first examined the effects of heparin derivatives on direct
adhesions between melanoma cells and fibrinogen or platelets and
fibrinogen, and found that RO-, CR- and 2/3ODS-heparins blocked the
direct adhesion of fibrinogen to melanoma cells or platelets
(Fig. 4). The blocking effects to
the indirect adhesion were then detected by flow chamber assay, and
we found that RO-, CR- and 2/3ODS-heparins blocked the indirect
adhesion, and that this blocking effect was almost identical to
that of heparin, and even higher than LMWH (Fig. 5). The blocking effects of heparin
derivatives to the indirect adhesion were multiple, although their
inhibitory rates to the fibrinogen-B16F10 were no more than 60%,
and to the fibrinogen-platelets were no more than 80%. RO-, CR- and
2/3ODS-heparins inhibited the indirect adhesion up to 95% (Fig. 5). These results confirmed the
bridging role of fibrinogen in the indirect adhesion of tumor cells
and platelets, and the stronger inhibitory activity of these
heparin derivatives. In this study, a structure comparison
demonstrated that the coexistence of N-sulfate and 6-sulfate was
crucial for the blocking activity of chemically modified heparins,
and that desulfation of 2-/3-/O-sulfate and the reduction of
carboxyl has a slight effect, while the sulfation of reduced
carboxyl (-CH2OH) should be avoided (17).
In conclusion, along with their function in
inhibiting the direct adhesion of tumor cells and platelets
mediated by selectins, integrins and HSPGs, these data suggest that
RO-, CR- and 2/3ODS-heparins should be considered as valuable
candidates for blocking direct and indirect adhesions of tumor
cells and platelets, and that the low anticoagulant character of
the chemically modified heparins is likely to benefit their
clinical application in anti-tumor metastasis (18).
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (81071726 and 31101009), the
Specialized Research Fund for the Doctoral Program of Higher
Education (20100043110007).
References
|
1
|
Weiler H: A platelet cloak for tumor
cells. Blood. 105:5–6. 2005. View Article : Google Scholar
|
|
2
|
Nieswandt B, Hafner M, Echtenacher B and
Männel DN: Lysis of tumor cells by natural killer cells in mice is
impeded by platelets. Cancer Res. 59:1295–1300. 1999.PubMed/NCBI
|
|
3
|
Montrucchio G, Sapino A, Bussolati B,
Ghisolfi G, Rizea-Savu S, Silvestro L, Lupia E and Camussi G:
Potential angiogenic role of platelet-activating factor in human
breast cancer. Am J Pathol. 153:1589–1596. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Manegold PC, Hutter J, Pahernik SA,
Messmer K and Dellian M: Platelet-endothelial interaction in tumor
angiogenesis and microcirculation. Blood. 101:1970–1976. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trikha M and Nakada MT: Platelets and
cancer: implications for antiangiogenic therapy. Semin Thromb
Hemost. 28:39–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McCarty OJ, Mousa SA, Bray PF and
Konstantopoulos K: Immobilized platelets support human colon
carcinoma cell tethering, rolling, and firm adhesion under dynamic
flow conditions. Blood. 96:1789–1797. 2000.PubMed/NCBI
|
|
7
|
Ishai-Michaeli R, Eldor A and Vlodavsky I:
Heparanase activity expressed by platelets, neutrophils, and
lymphoma cells releases active fibroblast growth factor from
extracellular matrix. Cell Regul. 11:833–842. 1990.
|
|
8
|
Suzuki K, Aiura K, Ueda M and Kitajima M:
The influence of platelets on the promotion of invasion by tumor
cells and inhibition by anti-platelet agents. Pancreas. 29:132–140.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jurasz P, Alonso-Escolano D and Radomski
MW: Platelet-cancer interactions: mechanisms and pharmacology of
tumour cell- induced platelet aggregation. Br J Pharmacol.
143:819–826. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma YQ and Geng JG: Heparan sulfate-like
proteoglycans mediate adhesion of human malignant melanoma A375
cells to P-selectin under flow. J Immunol. 165:558–565. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Felding-Habermann B, Habermann R, Saldívar
E and Ruggeri ZM: Role of beta3 integrins in melanoma cell adhesion
to activated platelets under flow. J Biol Chem. 271:5892–5900.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karpatkin S, Pearlstein E, Ambrogio C and
Coller BS: Role of adhesive proteins in platelet tumor interaction
in vitro and metastasis formation in vivo. J Clin Invest.
81:1012–1019. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cominetti MR, Martin AC, Ribeiro JU,
Djaafri I, Fauvel-Lafève F, Crépin M and Selistre-de-Araujo HS:
Inhibition of platelets and tumor cell adhesion by the disintegrin
domain of human ADAM9 to collagen I under dynamic flow conditions.
Biochimie. 91:1045–1052. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li F, Yu G, Li S, Peng S and Fu J:
Antimetastatic effect of integrin IIb/IIIa inhibitors on salivary
adenoid cystic carcinoma. Chin J Cancer Res. 13:198–201. 2010.
View Article : Google Scholar
|
|
15
|
Varki NM and Varki A: Heparin inhibition
of selectin-mediated interactions during the hemorrhagic phase of
carcinoma metastasis: rationale for clinical studies in humans.
Semin Thromb Hemost. 28:53–66. 2002. View Article : Google Scholar
|
|
16
|
Sobel M, Fish WR, Toma N, Luo S, Bird K,
Mori K, Kusumoto S, Blystone SD and Suda Y: Heparin modulates
integrin function in human platelets. J Vasc Surg. 33:587–594.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei M, Tai G, Gao Y, Li N, Huang B, Zhou
Y, Hao S and Zeng X: Modified heparin inhibits P-selectin-mediated
cell adhesion of human colon carcinoma cells to immobilized
platelets under dynamic flow conditions. J Biol Chem.
279:29202–29210. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao Y, Wei M, Zheng S, Ba X, Hao S and
Zeng X: Chemically modified heparin inhibits the in vitro adhesion
of nonsmall cell lung cancer cells to P-selectin. J Cancer Res Clin
Oncol. 132:257–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang C, Liu Y, Gao Y, Shen J, Zheng S,
Wei M and Zeng X: Modified heparins inhibit integrin
alpha(IIb)beta(3) mediated adhesion of melanoma cells to platelets
in vitro and in vivo. Int J Cancer. 125:2058–2065. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng S, Shen S, Jiao Y, Liu Y, Zhang C,
Wei M, Hao S and Zeng X: Platelets and fibrinogen facilitate each
other in protecting tumor cells from NK cytotoxicity. Cancer Sci.
100:859–865. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vestweber D and Blanks JE: Mechanisms that
regulate the function of the selectins and their ligands. Physiol
Rev. 79:181–213. 1999.PubMed/NCBI
|
|
22
|
Diamond MS, Alon R, Parkos CA, Quinn MT
and Springer TA: Heparin is an adhesive ligand for the leukocyte
integrin Mac-1 (CD11b/CD1). J Cell Biol. 130:1473–1482. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gunji Y, Lewis J and Gorelik E: Fibrin
formation inhibits the in vitro cytotoxic activity of human natural
and lymphokine- activated killer cells. Blood Coagul Fibrinolysis.
6:663–672. 1990.PubMed/NCBI
|
|
24
|
Palumbo JS, Talmage KE, Massari JV, La
Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M and Degen JL:
Platelets and fibrin (ogen) increase metastatic potential by
impeding natural killer cell-mediated elimination of tumor cells.
Blood. 105:178–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leung L and Nachman R: Molecular
mechanisms of platelet aggregation. Annu Rev Med. 37:179–186. 1986.
View Article : Google Scholar
|