Introduction
Inverted papilloma (IP) is a rare lesion of the
urinary tract system, which was first described in 1927 as adenomas
in the bladder (1) before it was
described in the 1960s as IP (2).
IP is a tumor formed by proliferating urothelium arranged as
inverting cords, which nest in continuity with an overlying intact
urothelium. They account for approximately 2.2% of all urothelial
neoplasms (3) and the majority of
IP cases occur in the bladder, while upper urinary tract IP cases
are extremely rare. IP tumors are generally regarded as benign
lesions, however, certain controversies remain. At present, few
studies have reported the IP occurrence in the upper urinary tract.
The present study collected 10 upper urinary tract IP cases treated
at The First Affiliated Hospital of Zhejiang University, China, and
reviewed the clinical syndromes, diagnostic procedures, treatment
approaches and follow-up study results of each patient.
Patients and methods
The present study included 10 patients who had been
hospitalized in our Department of Urology between 1995 and 2010 as
a result of IP within the upper urinary tract. Of the 10 patients,
9 were male and 1 was female (age range, 61–73 years; median, 67
years). The evaluations included personal data, medical history,
symptoms, localization of the disease, recurrences and malignant
transformations. No patient in this study had a history of
urothelial carcinoma; however, 5 patients had a history of smoking.
In all cases, an intravenous urogram (IVU) and a computed
tomography (CT) scan were performed. Two patients underwent
ureteroscopic evaluation, and biopsies of the lesions were shown to
be consistent with IP. All 10 patients were subject to either a
nephroureterectomy, partial ureterectomy or local resection.
Subsequently, the patients were scheduled for close follow-up
(range, 19–120 months; median, 59 months) with ultrasonic scanning
of the urinary tract and cystoscopy. This study was approved by the
Clinical Research Ethics Committee at the First Affiliated Hospital
of Zhejiang University and written consent was obtained from all
patients.
Results
The clinical features and treatment approaches of
each patient are summarized in Table
I. The initial symptoms of the disease included gross hematuria
and flank pain (on health examination), which were observed in 6
(60%) and 2 (20%) cases, respectively. No symptoms were observed in
2 (20%) cases. The site of development was the ureter in 6 cases
(60%) and the renal pelvis in 4 cases (40%). In addition to this, 7
cases (70%) occurred on the left side and 3 cases (30%) on the
right side of the ureter and renal pelvis, respectively. Following
IVU and CT examination, a filling defect was observed in all 10
patients, with hydronephrosis observed in 9 cases and a kidney
stone observed in 1 case. Retrograde ureter pyelography confirmed
the filling defects in 3 cases and negative urine cytology results
were obtained in 3 cases.
 | Table IClinical characteristics of 10
patients with IP. |
Table I
Clinical characteristics of 10
patients with IP.
| Patient | Age (years) | Gender | Location | Chief complaint | Multiplicity | Treatment | Recurrence |
|---|
| 1 | 62 | Male | Right renal
pelvis | Asymptomatic | Single |
Nephroureterectomy | None |
| 2 | 66 | Male | Left renal
pelvis | Hematuria | Single |
Nephroureterectomy | None |
| 3 | 70 | Male | Right ureter | Hematuria | Single |
Nephroureterectomy | None |
| 4 | 64 | Male | Left renal
pelvis | Hematuria | Single |
Nephroureterectomy | None |
| 5 | 61 | Male | Left ureter | Loin pain | Single | Partial
ureterectomy | None |
| 6 | 67 | Male | Left ureter | Asymptomatic | Multiple |
Nephroureterectomy | None |
| 7 | 67 | Male | Left ureter | Hematuria | Multiple | Local resection | None |
| 8 | 73 | Male | Left ureter | Hematuria | Single | Partial
ureterectomy | None |
| 9 | 73 | Female | Right renal
pelvis | Loin pain | Single |
Nephroureterectomy | None |
| 10 | 68 | Male | Left ureter | Hematuria | Single | Partial
ureterectomy | None |
All 10 patients underwent a surgical procedure. A
nephroureterectomy was performed in 6 patients, a partial
ureterectomy was performed in 3 patients with IP in the ureter,
where two patients had a positive biopsy for IP preoperatively. A
local resection of a nodular polypoid lesion protruding in the
lumen of the ureter, was performed in 1 patient endoscopically
using a holmium laser (Table I).
The pathological examination of the frozen IP cell sections
confirmed the diagnosis of IP. In one case, association with
transitional cell carcinoma (TCC) (Grade 1, stage Ta) was found
adjacent to the IP. In this case, a partial ureterectomy was
performed since the renal function on the contralateral side
appeared to be interrupted from IVU results.
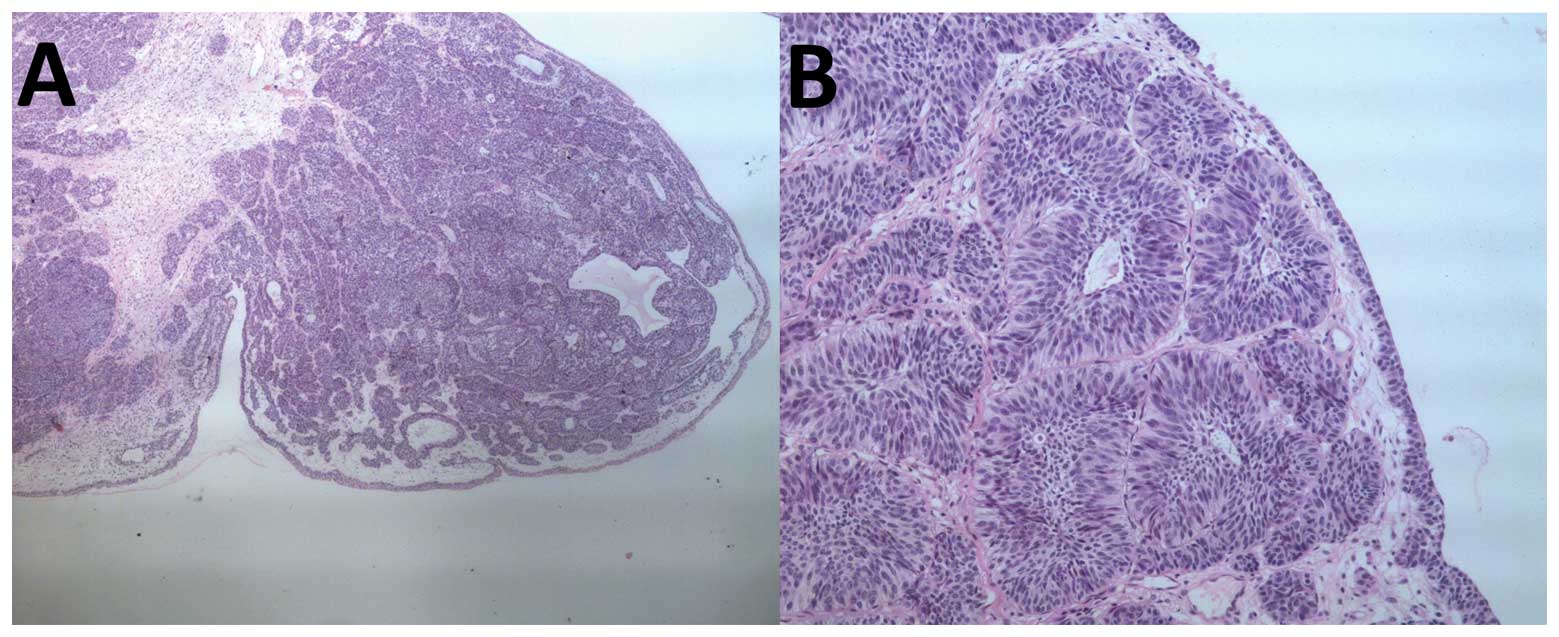
All but 2 tumors were solitary, ranging from 5 to 30
mm in diameter. The frozen sections demonstrated a pedunculated
nodule arising from the transitional cell epithelium. The tumor was
found to be covered by normal urothelium and the tumor cells only
demonstrated a slight degree of nuclear polymorphism. There was an
endophytic proliferation of transitional cells arranged in broad
cords and islands. The cells at the periphery of certain islands
demonstrated a tendency to palisade, and few central gland-like
spaces were present. No significant atypia or mitotic activity was
noted. The typical histological appearances of IP cells are shown
in Fig. 1. During the follow-up
period, no recurrence of IP or subsequent TCC was observed.
Discussion
IP is a rare tumor that develops within the bladder
in 90% of cases (4). It is defined
by its distinctive gross and microscopic appearance, as well as its
benign clinical course, characterised by a lack of invasive growth
and metastases, low incidence of multiplicity and low incidence of
local recurrence. IP of the upper urinary tract is extremely
uncommon as the majority of cases develop within the trigone of the
bladder, neck or prostatic urethra. However, the occurrence of
upper urinary tract localization is not surprising when considering
how the urothelial-lined tissue behaves as a single
pathophysiological unit. It is usually found in patients within the
6th or 7th decade of life, although a wide age range (26 to 85
years) (1) and a mean age of 67
years was observed in our patients. Upper urinary tract IP has been
demonstrated to develop more in males than females, with a
male:female ratio of 9:1 (1). The
same results were obtained from our cases. Usually, the IP lesion
occurs as a solitary lesion, although 3.6–6% appear to be bilateral
or multicentric (5). IP lesions are
twice as common in the ureter in comparison to the renal pelvis
(6) and the lesions range from
approximately 5 to 30 mm up to 3–4 cm in diameter (7).
The associated clinical symptoms of upper urinary
tract IP do not differ from those in other urothelial neoplasias,
with hematuria and renal colic being the most common clinical
manifestations of all upper urinary tract lesions. In the present
study, 6 patients (60%) and 2 patients (20%) complained primarily
of gross hematuria and loin pain, respectively. However, this
disease can also be asymptomatic, which was demonstrated in 2 of
our patients, and may be diagnosed during unrelated clinical
investigations. Preoperative diagnosis of IP is difficult. An IVU
is used to identify filling defects or signs of obstruction,
however, these findings are non-specific. Due to the intact layer
of histologically normal urothelium that covers the IP lesion, it
is not surprising that the cytological morphology falls within the
normal or mild atypia range. In a literature review regarding IP,
it was reported that urine cytology was specified in 8% of cases
(22 of 277) (8). In our experience,
cytology does not appear to be useful in diagnosis and an accurate
diagnosis requires a biopsy and visualization through endoscopic
examination, which we consider is more sensitive than indirect
radiographic studies. In this study, 2 patients underwent
ureteroscopic evaluation and biopsies made the nature of the
lesions evident preoperatively. With the development of flexible
ureteropyeloscopy, it is now possible to examine the entire upper
urinary tract. Certain authors have noted that IP lesions have a
gross appearance described as ‘broad stalked’, ‘more solid’ or
‘less papillary’ in comparison to transitional papillary tumors.
The endoscopic procedure with biopsy provides a preoperative
diagnosis and therapeutic indications, which are able to free the
patient from unnecessary nephroureterectomy.
Treatment of upper urinary tract IP remains
controversial. Various surgical procedures are used, such as total
nephroureterectomy, partial resection of the ureter and endoscopic
surgery. In the past, numerous cases have been treated aggressively
with nephroureterectomy, which was performed in 60% of the cases in
this study. With the development of endoscopy, endoscopical local
excision of ureteral IP is considered adequate treatment by certain
experts (9), as well as partial
resection of the ureter, providing that the IP is positively
diagnosed prior to or during surgery. However, differentiating
ureteral IP from urothelial carcinoma and coexistence of
malignancy, still complicate the preoperative diagnosis.
Laparoscopy is also a minimally invasive approach in comparison
with open surgery. It maintains the ability to obtain pathological
diagnosis and intraoperative assessment and provides a definitive
treatment, such as primary excision, segmental ureterectomy or
nephroureterectomy.
The etiology of IP is not yet clearly understood.
Several studies have argued the importance of inflammatory
causative factors, which is further supported by its close
histological resemblance to cystitis cystica, proliferative
cystitis and glandularis (10).
Sung et al also suggested that the correlation between IP
and smoking required further investigation (11). In their study, 61% (28 of 46) of
patients, had a history of smoking (11). In our study, 50% (5 of 10) of
patients had a history of smoking. Kunze et al subdivided IP
into two morphologically distinct types. Firstly, a glandular type,
which is composed of nests of urothelium with either
pseudoglandular spaces or true glandular elements containing
mucicarminophilic secretions and mucous-secreting cells. Secondly,
a trabecular type, which is composed of anastomosing cords and
trabeculae of urothelial cells invaginating the lamina propria
(4). Although marked cytological
atypia favors a diagnosis of inverted urothelial carcinoma, focal
mild cytological atypia is considered acceptable in IP. Broussard
reported that cases harboring focal cytologic atypia (less than 5%)
did not demonstrate significant cell proliferation characteristics,
and there was no tumor recurrence or progression to urothelial
carcinoma found during the follow-up (12).
A debate over the appropriate classification of IP
as either a true benign neoplasm or a urinary malignant precursor
lesion, has existed since its first description. The histological
appearance, its rare multiplicity (observed in 15 of 277, 5.4%, of
patients) and its low recurrence rate (approximately 1–7% of
cases), provide evidence to favor the benign classification of IP.
Certain authors have concluded that IP does not appear to be a risk
factor for TCC and to the best of our knowledge, there are no
reported cases of invasion or metastasis by IP. However, sporadic
cases of IP with concurrent urothelial carcinoma or malignant
features, have also been recently documented, rendering the
clinical image of this entity controversial. Cheng et al
have reported that the incidence of associated synchronous
urothelial carcinoma and subsequent urothelial carcinoma in the
lower urinary tract IP was 6 and 3%, respectively (13). More notably, in a group of 73
patients, 16 (22%) appeared to have concomitant previous TCC
(8). Therefore, there is a strong
association of IP and urothelial malignancy in the upper tract. The
frequency of synchronous malignancy in ureteric IP was reported to
be three times the frequency of that found in similar lesions in
the bladder. Spevack et al have reported that 7 of 30 (23%)
cases of upper urinary tract IP were complicated with TCC at a
different location or time (14).
Complication with TCC was also found in 1 patient in this study.
Concordance with or a history of TCC in patients with an IP
suggests that the two types of tumors share certain causative
factors. It was also recommended that the possibility of TCC
complication should be taken into consideration when the lesion in
the upper urinary tract is larger than 20 mm, even with a diagnosis
made by biopsy prior to and during surgery. Therefore, there is a
correlation between IP and TCC to some extent, although IP is
generally considered to be a benign lesion. The malignant potential
of IP lesions remain ambiguous due to the unknown etiology and low
incidence rate.
Since the recognition of IP as a distinct lesion of
the urinary tract, clinical follow-up appears to be necessary
following excision, despite the uncertainty regarding the method
and length. Certain authors have argued that surveillance
protocols, as rigorous as those employed in the management of
urothelial carcinoma, appear unnecessary for this benign entity due
to its low incidence of recurrence and markedly favorable prognosis
during follow-up (8,11). However, it is accepted by the
majority of authors that the patients should be followed with
endoscopy and radiographic studies similar to those observed in
patients with low-grade TCC (15),
particularly in upper urinary tract IP cases. As a surveillance
protocol of the upper urinary tract IP, we recommend cystoscopy and
ultrasonic scans every 6 months for the first 2 years, and then
annually. This is due to the fact that the time to recurrence in
the majority of cases is no more than 2 years following
surgery.
In conclusion, IP of the upper urinary tract is a
rare and benign lesion. The 10 cases presented in this study
support previous findings regarding male predominance, multiplicity
and difficulty with preoperative diagnosis. We have reason to
consider that certain cases of the upper urinary tract IP are able
to recur and have a malignant potential. The finding of an IP in
any part of the urinary tract should alert the urologist to conduct
an investigation of the entire urinary tract. They should also
emphasize to the patient the necessity for close clinical
follow-up, particularly when concurrent TCC presents.
References
|
1
|
Paschkis R: Über Adenoma der Harnblase. Z
Urol Chir. 21:315–325. 1927.(In German).
|
|
2
|
Potts IF and Hirst E: Inverted papilloma
of the bladder. J Urol. 90:175–179. 1963.PubMed/NCBI
|
|
3
|
Isaac J, Lowichik A, Cartwright P and Rohr
R: Inverted papilloma of the urinary bladder in children: case
report and review of prognostic significance and biological
potential behavior. J Pediatr Surg. 35:1514–1516. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kunze E, Schauer A and Schmitt M:
Histology and histogenesis of two different types of inverted
urothelial papillomas. Cancer. 51:348–358. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rozanski TA: Inverted papilloma: an
unusual recurrent, multiple and multifocal lesion. J Urol.
155:13911996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Knijff DW, Theunissen PH and Delaere
KP: Inverted papilloma of the ureter with subsequent invasive
bladder cancer. Acta Urol Belg. 65:45–46. 1997.PubMed/NCBI
|
|
7
|
Kyriakos M and Royce RK: Multiple
simultaneous inverted papillomas of the upper urinary tract. A case
report with a review of ureteral and renal pelvic inverted
papillomas. Cancer. 63:368–380. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Witjes JA, van Balken MR and van de Kaa
CA: The prognostic value of a primary inverted papilloma of the
urinary tract. J Urol. 158:1500–1505. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bagley DH, McCue P and Blackstone AS:
Inverted papilloma of renal pelvis: flexible ureteroscopic
diagnosis and treatment. Urology. 36:336–338. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chiura AN, Wirtschafter A and Bagley DH:
Upper urinary tract inverted papillomas. Urology. 52:514–516. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sung MT, Maclennan GT, Lopez-Beltran A,
Montironi R and Cheng L: Natural history of urothelial inverted
papilloma. Cancer. 107:2622–2627. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Broussard JN, Tan PH and Epstein JI:
Atypia in inverted urothelial papillomas: pathology and prognostic
significance. Hum Pathol. 35:1499–1504. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng CW, Chan LW, Chan CK, Ng CF, Cheung
HY, Chan SY, Wong WS and To KF: Is surveillance necessary for
inverted papilloma in the urinary bladder and urethra? ANZ J Surg.
75:213–217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spevack L, Herschorn S and Srigley J:
Inverted papilloma of the upper urinary tract. J Urol.
153:1202–1204. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kilciler M, Bedir S, Erdemir F, Ors O,
Kibar Y and Dayanc M: Evaluation of urinary inverted papillomas: a
report of 13 cases and literature review. Kaohsiung J Med Sci.
24:25–30. 2008. View Article : Google Scholar : PubMed/NCBI
|