Introduction
The mechanisms responsible for the appearance of
multiple primary cancers remain unclear. Among the factors most
frequently involved are genetic susceptibility, the immune system
and intensive exposure to carcinogens, including chemical and
biological carcinogens and radiation.
Breast cancer is the most common neoplastic disease
of women in the Western world. In developed countries, more than
200 cases are diagnosed annually per 100,000 women. The etiology
and mechanisms of breast cancer are poorly understood. Well-known
risk factors, including those that affect circulating sex hormones
and genetic background can only explain approximately 50% of all
breast cancer cases. Among the remaining 50% of cases, findings of
numerous studies have suggested that Epstein-Barr virus (EBV)
infection may be a causal factor (1–4).
EBV is a ubiquitous γ herpes virus and infects 90%
of the population. In the majority of individuals, the virus
persists for life in the memory B-cell pool (5) with no adverse health consequences. The
virus is essentially a B-lymphotropic agent and is associated with
malignancies of B-cell origin, including Burkitt’s lymphoma.
However, epithelial cell infection clearly occurs in vivo,
as EBV is also associated with malignancies of epithelial origin,
such as nasopharyngeal carcinoma (NPC). In Burkitt’s lymphoma and
NPC, epidemiological and molecular virological data favor the role
of the virus as a cofactor in tumor initiation and/or development.
The involvement of EBV has been demonstrated by the findings of
EBNA-1 expression in Burkitt’s lymphoma, and EBNA-1, LMP1 and LMP2
expression in NPC (6).
The list of malignancies reportedly associated with
EBV continues to grow and includes Hodgkin’s disease, sino-nasal
T-cell lymphoma, lymphoepithelioma, certain sarcomas and breast
cancers, cancers of the head and neck, and lymphomas arising in
patients with immune dysfunctions. Evidence of a role for EBV in
the pathogenesis of a tumor includes: i) elevated antibody titers
to EBV preceding the development of a neoplasm; ii) the presence of
the viral genome in a large majority (if not all) of tumor cells;
iii) clonality of the viral genome; and iv) expression of viral
genes in neoplastic cells. Elevated antibody titers to EBV have
been found in patients with breast cancer, but the presence of the
EBV genome or its products at the cellular level remains
controversial (4,7). The possible contribution of EBV to the
development and/or progression of breast cancer may be as a
putative ‘non-traditional’ infection, as observed in NPC or
Burkitt’s lymphoma.
We report a rare case of multiple concurrent primary
malignancies of the breast and nasopharynx with EBV infection and
discuss the possible association between EBV infection and breast
cancer. This study was endorsed by the Ethics Committee of the
Guangxi Medical University. The patient received an explanation of
the aims of the study, provided signed informed consent, and
understood that withdrawal from the study was allowed at any time
without influencing her oncological or general medical
treatment.
Case report
Patient history
A 39-year-old female visited our hospital after a
palpable lesion of 1-year duration had been removed from her left
breast at a community hospital. The lump in the patient’s left
breast, which measured 2×2×1 cm according to the report, had been
resected for diagnosis. The histopathological report revealed
invasive ductal adenocarcinoma. The patient refused any treatment
at the community hospital and presented to our breast unit for
further evaluation. Physical examination of the left breast
revealed a 3-cm scar on the lower inner quadrant. The skin of the
breast, including the nipple and areola, was not invaded and was
without redness, erosion or ulcer. Multiple lymph nodes were felt
in the left axilla; the largest node, measuring about 1×1×0.5 cm,
was mobile and non-tender. The right breast yielded negative
findings.
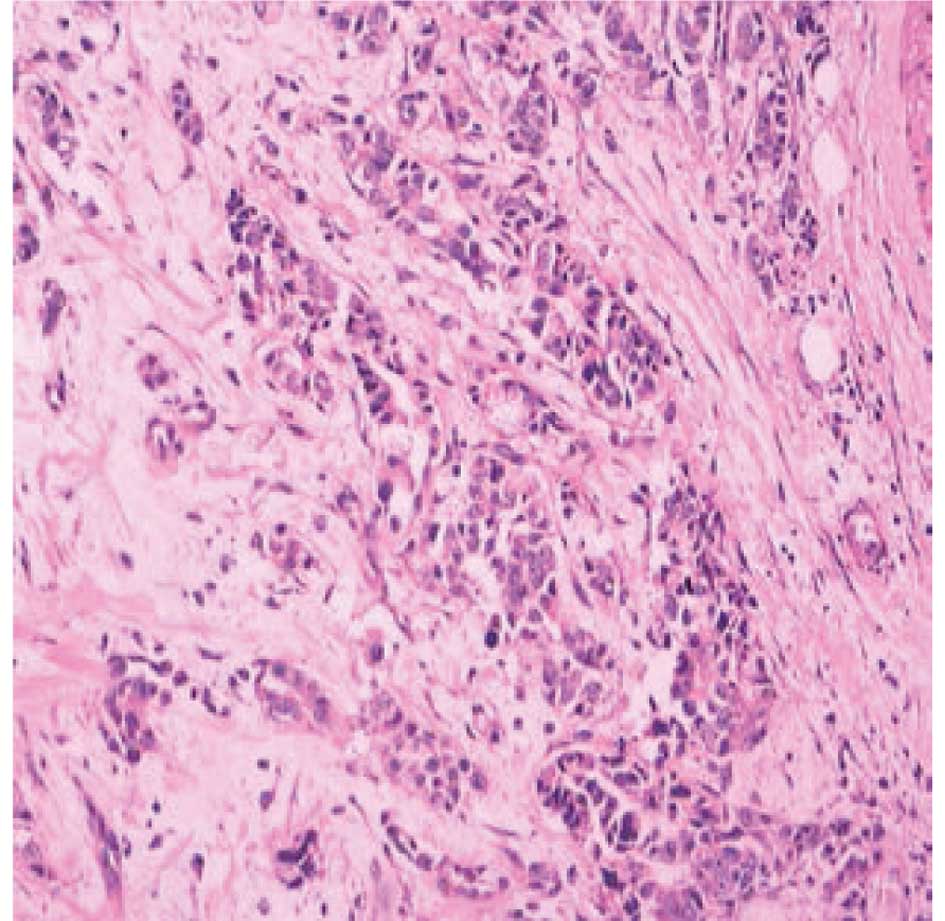
Carcinoma specimen analysis
The pathological specimen provided by the community
hospital showed an invasive ductal adenocarcinoma. According to an
immunohistochemical analysis, the cells were positive for the
estrogen receptor, progesterone receptor and p53 protein, and
negative for vascular endothelial growth factor and epidermal
growth factor receptor. The cells were markedly positive for C-erb
B2 (also known as HER-2/neu), and 30% of the tumor cells were
positive for the Ki-67 antigen (Fig.
1).
Patient examination
Electron beam computed tomography showed a positive
sentinel lymph node located at the upper outer quadrant. A chest
X-ray and ultrasound did not reveal any metastases. Routine blood
test results were within normal limits, except that EBV-CA-IgG was
present and EBV-EA-IgG was reactivated.
Lymph node examination
A simple mastectomy with sentinel lymph node biopsy
and axillary clearance was performed on the left breast. Six
sentinel lymph nodes, including the one positive on electron beam
computed tomography, were found. All presented non-specific
inflammation. Pathology revealed a total of 12 lymph nodes of the
left axilla also had non-specific inflammation. The diagnosis was
pT1N0M0/stage I breast carcinoma (UICC, 2002).
Nasopharyngeal carcinoma
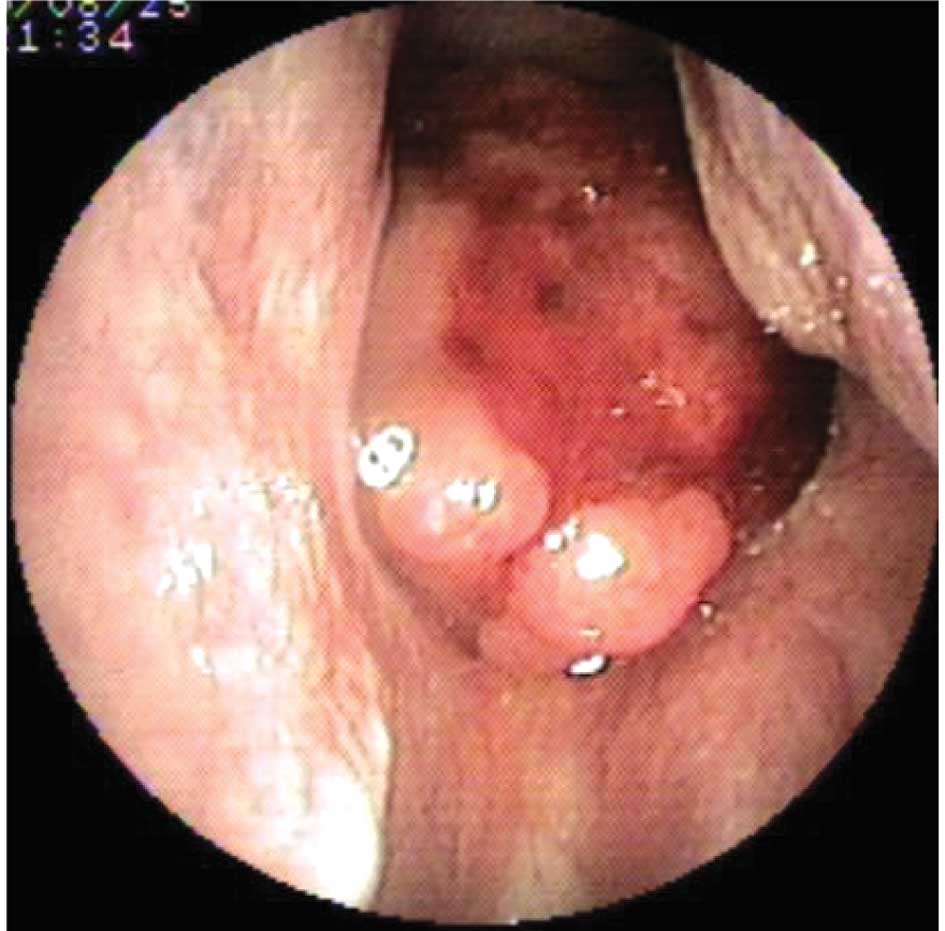
Three days following surgery, the patient
experienced epistaxis and was sent for an endoscopic nasopharyngeal
examination. A mass with active mucosal bleeding was found and
biopsied (Fig. 2). Histopathology
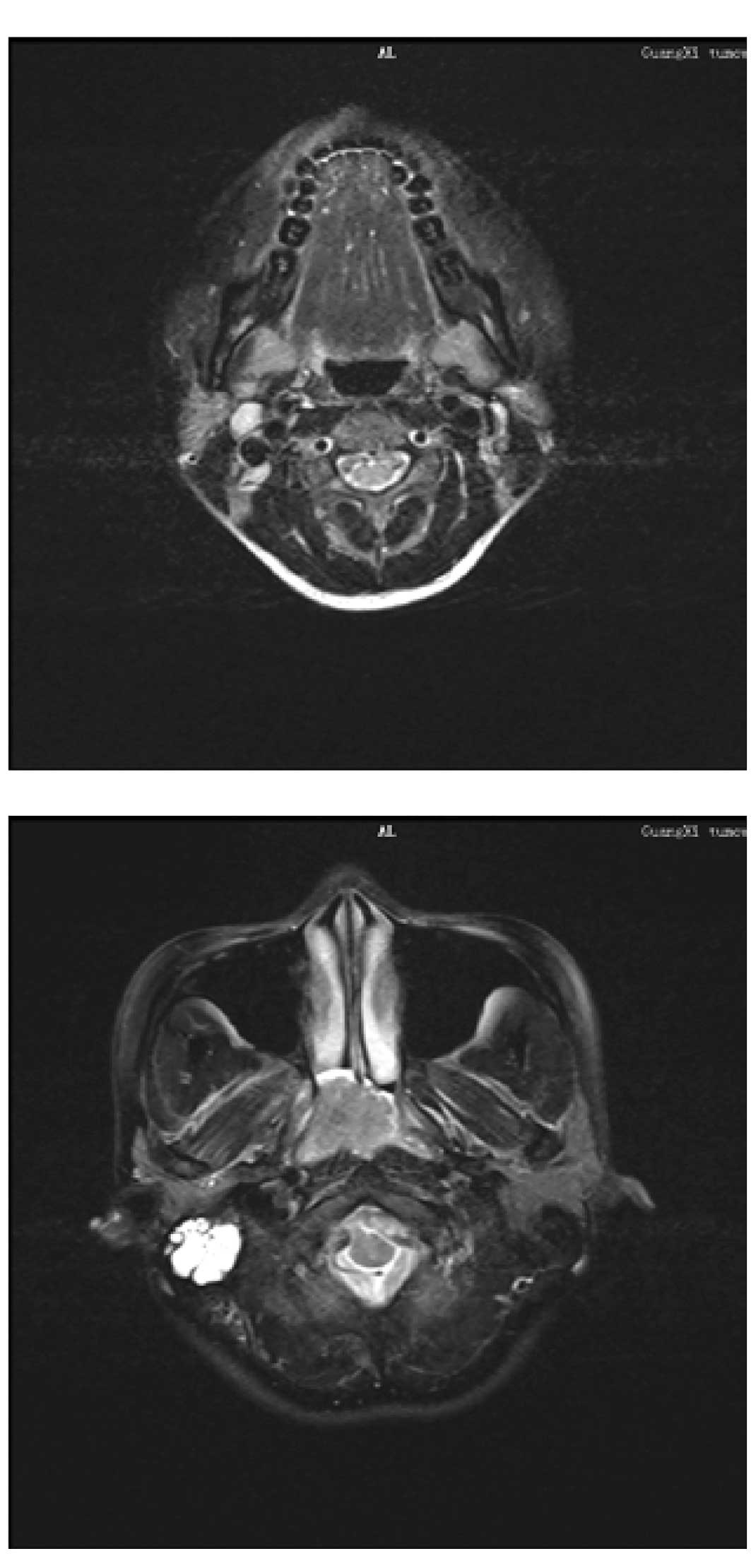
revealed a non-keratinizing undifferentiated carcinoma. MRI showed
an enlarged lymph node in region II of the right neck and an
invasive mass in the parapharyngeal space (Fig. 3A and B). The patient was diagnosed
with nasopharyngeal carcinoma, T2N1M0/stage II (UICC, 2002).
Treatment and follow-up
Two weeks following breast surgery, the patient was
treated with chemoradiotherapy for nasopharyngeal carcinoma. A
planned dose of 70 Gy was to be delivered in 2.0-Gy fractions over
seven weeks to the primary tumor, with 6-MV photons. The neck was
treated with 54 Gy. The positive node was boosted to a total dose
of 64 Gy. The chemotherapy regimen was delivered for three cycles,
on days 1, 22 and 43 during the course of radiotherapy, as
concurrent chemotherapy. The patient was followed up 12 months
after the completion of treatment. Physical examination, CT of the
chest, MRI of the nasopharynx and neck, ultrasound imaging of the
abdomen and a bone scan were performed. There was no evidence of
relapse or metastasis.
Discussion
Previous studies have shown that individuals with
one malignant neoplasm have a 1.29-fold risk of developing a new
independent primary tumor, compared with individuals who have no
cancer (8). Additional primary
cancers occurring simultaneously with breast cancer have been
reported in the opposite breast, salivary glands, uterine corpus,
ovary and thyroid (9). To the best
of our knowledge, the present case is the first report of
concurring multiple primary malignancies of the breast and
nasopharynx.
Epidemiological and molecular virological data have
shown that EBV is a cofactor in tumor initiation and/or development
in NPC. Almost 100% of anaplastic or poorly differentiated
nasopharyngeal carcinomas contain EBV genomes and express EBV
proteins (10). Although the
etiology of breast cancer is poorly understood, genetic background
and hormonal effects are believed to be important in its
development. Our patient did not have a family history for breast
cancer or NPC, suggesting that genetic susceptibility was a weak
factor in breast cancer pathogenesis in this case. However,
epidemiological data on breast cancer indicate that delayed
infection with EBV may be a risk factor for breast cancer (2). Our patient was positive for EBV-CA-IgG
and EBV-EA-IgG, showing that the patient was infected with EBV.
Thus, we suggest that EBV infection causes NPC and may
simultaneously be the cause of breast cancer.
No definitive consensus has yet been achieved
regarding an association between EBV and breast cancer (11,12).
Detection of the EBV genome or its products in breast cancer cells
would provide strong evidence of this association. In 2001, Fina
et al (4)reported the
frequency and genome load of EVB in 509 breast cancers from
different geographical areas. EBER-1 has been identified in 31.8%
of frozen sections from different breast cancers (4). By contrast, a study conducted in 2002
found no evidence of EBV infection in breast cancer, as no EBERs,
EBNa1, LMP1 or LMP2A was detected in 43 frozen sections of breast
cancer (7).
A few studies have demonstrated a new way in which
EBV infects breast epithelial cells (13,14).
This finding may lead to an explanation for the pathogenesis of
breast cancer caused by EBV. Contact between lymphoid and
epithelial cells appears to provide a mechanism for EBV transfer
from lymphoid to epithelial cells. This idea is supported by Imai
et al, who showed that co-culturing target cells with
semi-permissive B-cells leads to the infection of epithelial cells
(13). As further evidence, a study
by Speck et al suggested that breast epithelial cells became
infected with EBV following direct contact with lymphoid cells
(14).
A model for the putative role of EBV in the
progression of breast cancer has been provided by Hippocrate et
al (15). A limited number of
previously transformed epithelial cells may become infected with
EBV via direct contact with infiltrating EBV-positive B cells. The
inflammatory milieu of the tumor may activate the virus, leading to
an increased expression of factors involved in angiogenesis and
cell invasion, which favors tumor progression. Alternatively,
EBV-positive infiltrating B cells may provide EBV-induced or
EBV-encoded products such as cytokines and microRNA, which could
alter the cellular environment to influence the growth of
transformed epithelial cells.
There are no established therapeutic rules for
multiple primary cancers. The tumor type, location, stage and
progression, as well as the patient’s general health status should
be considered. The treatment of choice involves curative surgical
resection of each cancer, followed by radiotherapy and chemotherapy
(16–18). The prognosis should be determined
independently as a function of the tumor stage and treatment
results for each cancer. When the two cancers present the
possibility for a cure, radical therapy is indicated. However, when
radical therapy of the primary cancer is impossible, conservative
therapy is indicated for the second cancer. In the present case,
radical surgery for the breast cancer and radical concurrent
chemoradiotherapy for the nasopharyngeal carcinoma were performed,
in anticipation of a better prognosis.
In conclusion, EBV infection may be involved in the
pathogenesis of breast cancer, as observed in nasopharyngeal
carcinoma. This possibility must be taken into account, even though
this association may not reflect a ‘traditional infection’ as
observed in EBV-associated tumors.
Acknowledgements
We are grateful to Yi Jiang of the Breast Unit,
Cancer Hospital of Guangxi Medical University for her assistance
during the preparation of the manuscript.
References
|
1
|
Richardson A: Is breast cancer caused by
late exposure to a common virus? Med Hypotheses. 48:491–497. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yasui Y, Potter JD, Stanford JL, Rossing
MA, Winget MD, Bronner M and Daling J: Breast cancer risk and
‘delayed’ primary Epstein-Barr virus infection. Cancer Epidemiol
Biomarkers Prev. 10:9–16. 2001.
|
|
3
|
Labrecque LG, Barnes DM, Fentiman IS and
Griffin BE: Epstein-Barr virus in epithelial cell tumors: a breast
cancer study. Cancer Res. 55:39–45. 1995.PubMed/NCBI
|
|
4
|
Fina F, Romain S, Ouafik L, Palmari J, Ben
Ayed F, Benharkat S, Bonnier P, Spyratos F, Foekens JA, Rose C,
Buisson M, et al: Frequency and genome load of Epstein-Barr virus
in 509 breast cancers from different geographical areas. Br J
Cancer. 84:783–790. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Babcock GJ, Decker LL, Volk M and
Thorley-Lawson DA: EBV persistence in memory B cells in vivo.
Immunity. 9:395–404. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rickinson AB and Kieff E: Epstein-Barr
virus. Fields’ Virology. Howley DKP: Lippincott William &
Wilkins; Philadelphia: pp. 2655–2700. 2007
|
|
7
|
Deshpande CG, Badve S, Kidwai N and
Longnecker R: Lack of expression of the Epstein-Barr virus (EBV)
gene products, EBERs, EBNA1, LMP1, and LMP2A, in breast cancer
cells. Lab Invest. 82:1193–1199. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schoenberg BS: Multiple primary malignant
neoplasms. The Connecticut experience, 1935–1964 Recent Results.
Cancer Res. 58:1–173. 1977.
|
|
9
|
Schenker JG, Levinsky R and Ohel G:
Multiple primary malignant neoplasms in breast cancer patients in
Israel. Cancer. 54:145–150. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cohen JI: Epstein-Barr virus infection. N
Engl J Med. 343:481–492. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Glaser SL, Hsu JL and Gulley ML:
Epstein-Barr virus and breast cancer: state of the evidence for
viral carcinogenesis. Cancer Epidemiol Biomarkers Prev. 13:688–697.
2004.PubMed/NCBI
|
|
12
|
Arbach H and Joab I: EBV and breast
cancer: questions and implications. Epstein-Barr Virus. Robertson
ES: Caister, Norfolk: pp. 139–155. 2005
|
|
13
|
Imai S, Nishikawa J and Takada K:
Cell-to-cell contact as an efficient mode of Epstein-Barr virus
infection of diverse human epithelial cells. J Virol. 72:4371–4378.
1998.PubMed/NCBI
|
|
14
|
Speck P and Longnecker R: Infection of
breast epithelial cells with Epstein-Barr virus via cell-to-cell
contact. J Natl Cancer Inst. 92:1849–1851. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hippocrate A, Oussaief L and Joab I:
Possible role of EBV in breast cancer and other unusually
EBV-associated cancers. Cancer Lett. 305:144–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Passman MA, Pommier RF and Vetto JT:
Synchronous colon primaries have the same prognosis as solitary
colon cancers. Dis Colon Rectum. 39:329–334. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tamura M, Shinagawa M and Funaki Y:
Synchronous triple early cancers occurring in the stomach, colon
and gallbladder. Asian J Surg. 26:46–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Van Dalen R, Church J, McGannon E, Fay S,
Burke C and Clark B: Patterns of surgery in patients belonging to
amsterdam-positive families. Dis Colon Rectum. 46:617–620.
2003.PubMed/NCBI
|