Introduction
Gastric cancer remains the second leading cause of
cancer-related mortality worldwide. With the development of novel
diagnostic markers and effective treatments, the morbidity and
mortality rates of this disease have significantly decreased
worldwide, particularly in Asian countries. Although an increasing
number of molecules that play critical roles in the development of
gastric cancer have been identified, the pathophysiological
progression of the carcinogenesis is far from clear, and the
relative five-year survival rate of gastric cancer patients remains
low (1). In recent years,
accumulating studies have focused on the contribution of the
metabolism of cancer cells in carcinogenesis.
Tumor cells require steady and sufficient nutrition
to maintain their energy supply and the protein synthesis required
for rapid growth. Amino acid transporters are commonly upregulated
in cancer cells for their supply of amino acids. Large (L)-type
amino acid transporter 1 (LAT1) is encoded by the SLC7A5
gene and belongs to system L, which is an
Na+-independent system. LAT1 mainly transports large
neutral, branched and aromatic amino acids, including leucine,
isoleucine and tyrosine, the majority of which are essential amino
acids (2). LAT1 therefore has a
significant role in cell metabolism (3). LAT1 has been demonstrated to be
upregulated in proliferative tissue, cancer cell lines and numerous
types of human cancer tissue, including lung, colon, breast,
prostate, head and neck, and ovarian cancer, as well as in gliomas
(2–5). In non-small cell lung carcinoma
(NSCLC), the increased expression of LAT1 is not only correlated
with histological type, disease stage and metastasis, but also with
the five-year survival rate (6). In
gliomas, the overexpression of LAT1 is correlated with pathological
grade, proliferation and angiogenesis (7). Recently, Ichinoe et al revealed
that LAT1 was overexpressed in gastric cancer, suggesting that it
may be involved in the oncogenesis of gastric cancer (8).
LAT1 has been demonstrated to promote cell
proliferation, migration and invasion in certain cancer cell lines,
including gliomas and ovarian and oral cancer (7). This protein is involved in cancer
progression and metastasis, and functions by forming a
heterodimeric complex with another glycoprotein, CD98hc. The heavy
chain, CD98hc, recruits the light chain, LAT1, in the plasma
membrane through covalent association (9). LAT1 may be upregulated or activated by
the PI3K/Akt, mTOR, MAPK and c-myc signaling pathways. This
upregulation results in an increase of amino acids transported to
the plasma, and the subsequent activation of the mTOR signaling
pathway, which is important in protein synthesis and supplying
energy (9). CD98hc has been
demonstrated to link to intergrin β in order to regulate the
intergrin signaling pathway that is involved in cell proliferation,
survival, migration and epithelial adhesion/polarity (9). The role of LAT1 and its signaling
pathway in gastric cancer are currently unclear.
In the present study, two plasmids were constructed
with different short (sh)RNAs inserts that targeted LAT1, which
resulted in a LAT1 knockdown. A corresponding control shRNA plasmid
was also constructed. Subsequently, stable SGC7901 cell lines with
a LAT1 knockdown, and the corresponding control cell lines, were
established by transfection with these plasmids. The efficiency of
LAT1 silencing and the expression levels of CD98hc were then
confirmed. The effects of silencing the LAT1 expression on the
proliferation, cell cycle, migration and invasion of these SGC7901
cells was then further investigated. These results suggested that
the down-regulation of LAT1 expression using shRNAs inhibited the
proliferation, migration and invasion of gastric cancer cells.
These findings suggested that LAT1 is important in gastric cancer,
and that it may be developed as a therapeutic target.
Materials and methods
Reagents
Lipofectamine 2000 transfection reagent was
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
The LAT1 antibody was purchased from Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd., (Beijing, China) and the CD98hc
antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). The actin antibody was purchased from Bioworld
Technology, Inc. (St. Louis Park, MN, USA).
Construction of plasmids
Two sets of shRNAs targeting SLC7A5 (GenBank,
NM_003486), which encodes LAT1, were designed according to the
principles of shRNA design. The oligonucleotide sequences of these
two shRNAs were as follows: 5′-GGGAACATTGTGCTGGCATT-3′, targeting
at 793 bp; and 5′-GCATTATACAGCGGCCTCT-3′, targeting at 808 bp. The
sequence of the non-targeting shRNA was 5′-GTTCTCCGAACGTGTCACGT-3′;
this served as the control. The loop and stop sequences used were
TTCAAGAGA and T6, respectively. The digestion site of PstI
(sequence CACC) was added to the 5′ end of the sense strands, and
the digestion site of BamHI (sequence GATC) was added to the
5′ end of the antisense strands. The oligonucleotides were
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). The
sense and antisense strands were annealed and inserted into the
pGPU6/GFP/Neo plasmid using T4 DNA ligase. These were transformed
in DH5α and selected by kanamycin. The constructs were named
LAT1-shRNA1 (targeting at 793 bp) and LAT1-shRNA2 (targeting at 808
bp), confirmed by enzyme digestion and then sequenced by the
Invitrogen Corporation Shanghai Representative Office (Shanghai,
China).
Cell lines and cell culture
The human gastric cancer cell line, SGC7901, was
obtained from the Shanghai Institutes for Biological Sciences,
Chinese Academy of Sciences, China. The SGC7901 cells were divided
into four groups and either transfected with the LAT1-shRNA1,
LAT1-shRNA2 or LAT1-sh NC constructs, or not transfected, for 48 h.
The cells were then selected for two weeks with 400 μg/ml
G418. Subsequently, the cell lines were named SGC7901_shRNA1,
SGC7901_shRNA2, SGC7901_shNC and SGC7901_blank, respectively. The
cells were cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum (Gibco-BRL, Grand Island, NY, USA) at 37°C in a
humidified atmosphere consisting of 5% CO2. The
fluorescence of the green fluorescent protein (GFP) encoded by the
pGPU6/GFP/Neo plasmids was observed and images were captured by a
fluorescence microscope.
RNA isolation and semi-quantitative
RT-PCR
The total RNA was extracted using Trizol reagent
(Gibco, Carlsbad, CA, USA) according to the manufacturer’s
instructions. The reverse transcription was conducted using M-MLV
reverse transcriptase obtained from Promega Corporation (Madison,
WI, USA), according to the standard procedure. The PCR reactions
were conducted using Taq DNA polymerase (Thermo Fisher Scientific
Inc., Rockford, IL, USA) according to the manufacturer’s
instructions. The forward (F) and reverse (R) primers used were as
follows: LAT1 F, 5′-GCATGCGCAGAGGCC AGTTAA-3′ and R,
5′-TATGGTCAGGAGTCCATCGGG-3′; CD98hc F, 5′-CCAGGTTCGGGACATAGAG-3′
and R, 5′-TGGTAGAGTCGGAGAAGTTGAG-3′; GAPDH F, 5′-AGA
AGGCTGGGGCTCATTTG-3′ and R, 5′-AGGGGCCATCCA CAGTCTTC-3′, and were
synthesized by Invitrogen Life Technologies (10). The product lengths of LAT1, CD98hc
and GAPDH were 537, 326 and 258 bp, respectively. The PCR fragments
were separated by 1.5% agarose gel. The quantity of the PCR
products of LAT1 or CD98hc were determined by scanning the density
of the bands, using Quantity One software (Tanon Science and
Technology Co., Shanghai, China) and normalizing to GAPDH.
Western blot analysis
Cell lysis buffer was purchased from Promega
Corporation and stored at 4°C. Protease inhibitors were added
immediately prior to use. The cells were harvested and subjected to
western blot analysis following the standard procedure. Briefly, 50
mg protein was electrophoresed in 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to polyvinylidene difluoride (PVDF) membranes. The
membranes were blocked with 5% skimmed milk in Tris-buffered saline
and Tween-20 (TBST) for 1 h at room temperature. The blots were
incubated with an appropriate dilution of the primary antibody for
2 h at room temperature, then rinsed three times with TBST. The
rinsed blots were incubated with the secondary antibody for 1 h at
room temperature and then rinsed three times with TBST. The signals
were visualized with an enhanced chemiluminescence (ECL) detection
system (cat. no. RPN2132; Amersham Pharmacia Biotech Inc.,
Piscataway, NJ, USA). The quantity of the proteins was determined
by scanning the density of the bands using Quantity One software
and by normalizing to actin.
Cell proliferation assay
Cell proliferation activity was determined by the
3-(4,5-dimethyl- thiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) assay, according to the standard methods. Briefly, cells were
seeded in 96-well plates for 1–4 days. Subsequently, 20 μl
0.5% MTT was added to each well and incubated for 4 h at 37°C. The
MTT was removed and 150 μl DMSO was added. Absorbance was
measured at 490 nm and detected using the Bio-Tek μQuant
Universal Microplate Spectrophotometer (Bio-Tek Instruments, Inc.,
Winooski, VT, USA).
Cell cycle analysis
The cells were seeded in 6-well plates in triplicate
and fixed in 70% ice-cold ethanol for 24 h at 4°C. They were
subsequently washed with phosphate-buffered saline (PBS) solution
and resuspended in 1 ml staining solution (50 μg/ml
propidium iodide and 100 μg/ml RNase A in PBS) for 30 min.
The cell cycle distribution was detected by the FC 500 Series Flow
Cytometer (Beckman Coulter Inc., Brea, CA, USA) and analyzed by BD
CellQuest analysis software (BD, Franklin Lakes, NJ, USA). Each
experiment was repeated three times.
Cell migration assay
Cell migration was measured with the Boyden chamber
(Corning Costar Corp., Cambridge, MA, USA). Briefly,
1×105 cells, in 200 μl RPMI-1640 containing 0.1%
fetal calf serum, were plated on the upper compartment of the
chamber. The conditioned medium, which was obtained from cultured
NIH3T3 cells with serum-free medium, was added to the lower
chambers. After 24 h, non-migratory cells on the upper surface of
the filter were removed completely with a cotton swab. The migrated
cells on the lower surface of the filter were fixed with 95%
alcohol for 30 min, stained with hematoxylin and eosin and then
counted under a microscope. The mean number of migratory cells of
the triplicates for each experimental condition was recorded.
Cell invasion assay
Cell invasion was also measured with the Boyden
chamber (Corning Costar Corp.), but the upper side of the filters
were coated with 100 μl matrigel (1 mg/ml), which was
dissolved in serum-free RPMI-1640 medium. The remaining operations
were the same as those of the migration assay. The invaded cells
were fixed, stained and counted as described previously.
Statistical analysis
Unless otherwise stated, all data are presented as
the mean ± standard deviation (SD). Statistical significance
(P<0.05) was determined by the Student’s t-test or analysis of
variance (ANOVA) followed by the assessment of differences using
the Statistical Package for the Social Sciences (SPSS) for Windows,
Version 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
Establishment of stable gastric cancer
cell lines with low LAT1 expression levels
To identify the role of LAT1 in gastric cancer,
stable cell lines with a LAT1 knockdown were first established
using shRNAs. Two sets of shRNA sequences and one set of control
shRNA sequences were designed. Subsequently, the shRNAs were
inserted into the pGPU6/GFP/Neo plasmids and named LAT1-shRNA1
(targeting at 793 bp), LAT1-shRNA2 (targeting at 808 bp) and
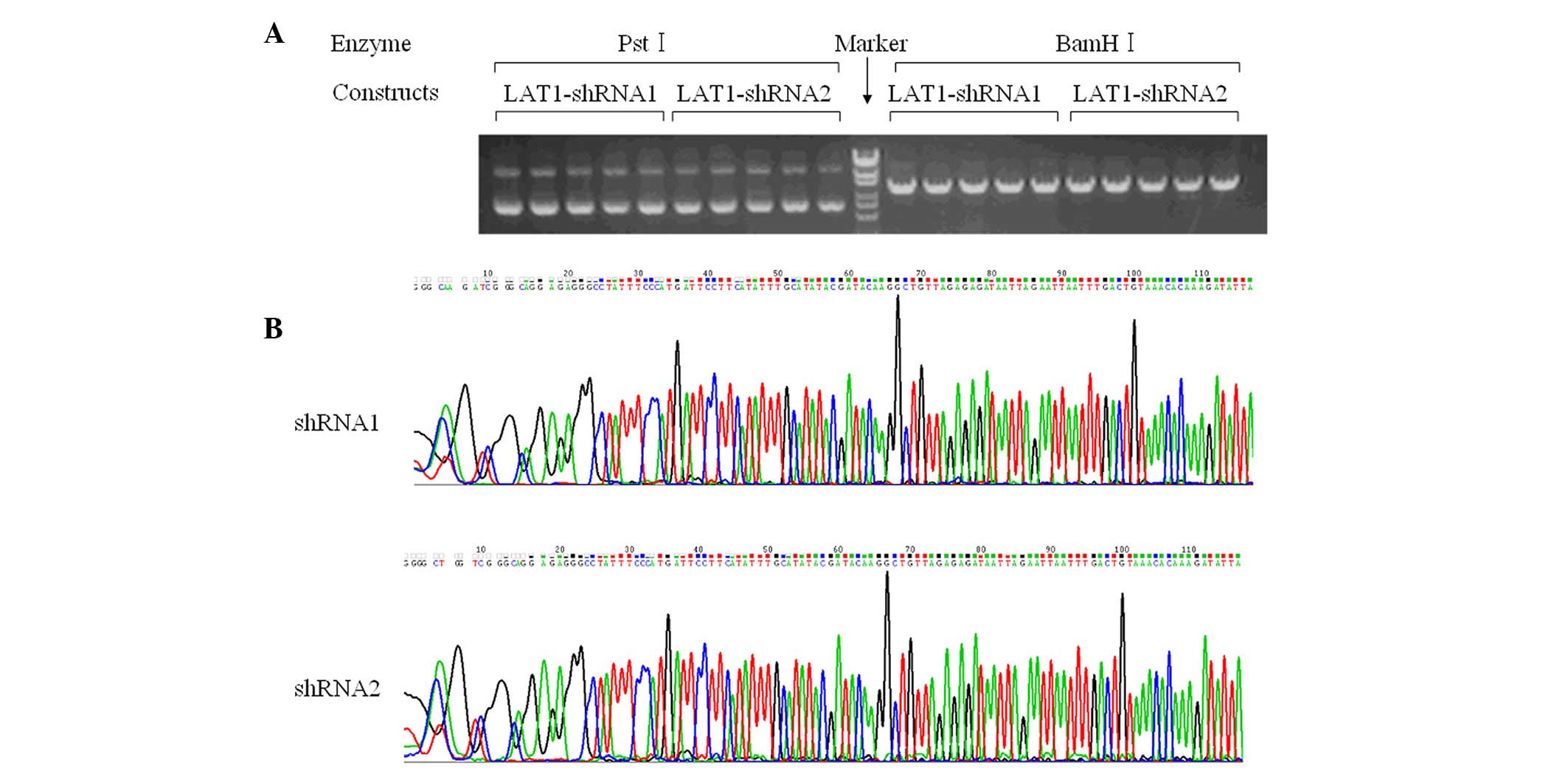
LAT1-shNC (negative control), respectively. Fig. 1A reveals that the enzymes
PstI and BamHI digested the plasmids as predicted.
Moreover, DNA sequencing confirmed that the sequences were correct
(Fig. 1B).
Subsequently, the plasmids were transfected into
SGC7901 cells and further selected the positive colonies using 400
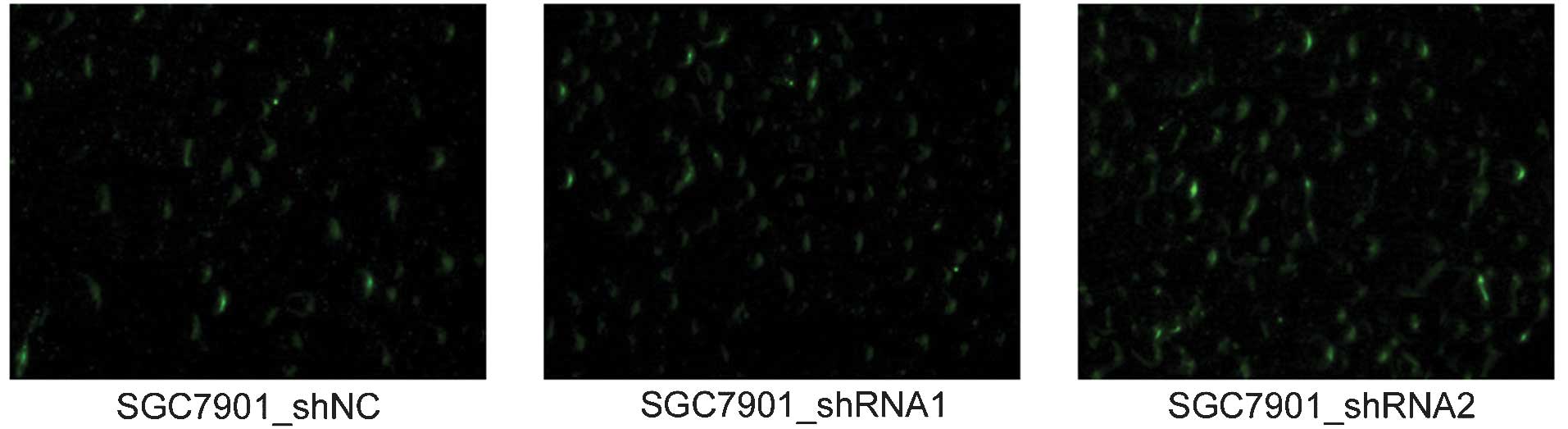
μg/ml G418 for two weeks. As demonstrated in Fig. 2, successful expression of the
plasmids exhibited green fluorescence under a fluorescence
microscope due to the expression of GFP, the coding sequence of
which had been inserted into the backbone of the pGPU6/GFP/Neo
plasmids. The established cell lines were named SGC7901_shRNA1,
SGC7901_shRNA2 and SGC7901_shNC, respectively. SGC7901 cells
without transfection were also cultured, and these served as a
blank control. These results suggested that the constructs were
successfully expressed in the SGC7901 cells.
Expression levels of LAT1 and CD98hc in
the established SGC7901 cells
To further determine the expression levels of LAT1
and its functional partner, CD98hc, in the established SGC7901 cell
lines, their mRNA levels were detected using RT-PCR analysis.
Fig. 3A reveals that the mRNA
levels of LAT1 markedly decreased in the SGC7901_shRNA1 and
SGC7901_shRNA2 cells compared with the SGC7901_shNC and SGC7901
(blank control) cells, while those of CD98hc only minimally
decreased. The density of the mRNA bands was further quantified
(Fig. 3B). The decreases in LAT1 in
the SGC7901_shRNA1 and SGC7901_shRNA2 cells were significant
compared with the SGC7901_shNC cells (P<0.05). Moreover, the
knockdown efficiency of shRNA2 (which decreased the LAT1 mRNA
expression by 62.1%) was greater than that of shRNA1 (which
decreased the LAT1 mRNA expression by 48.9%). In addition, the mRNA
levels of CD98hc did not change significantly (P>0.05).
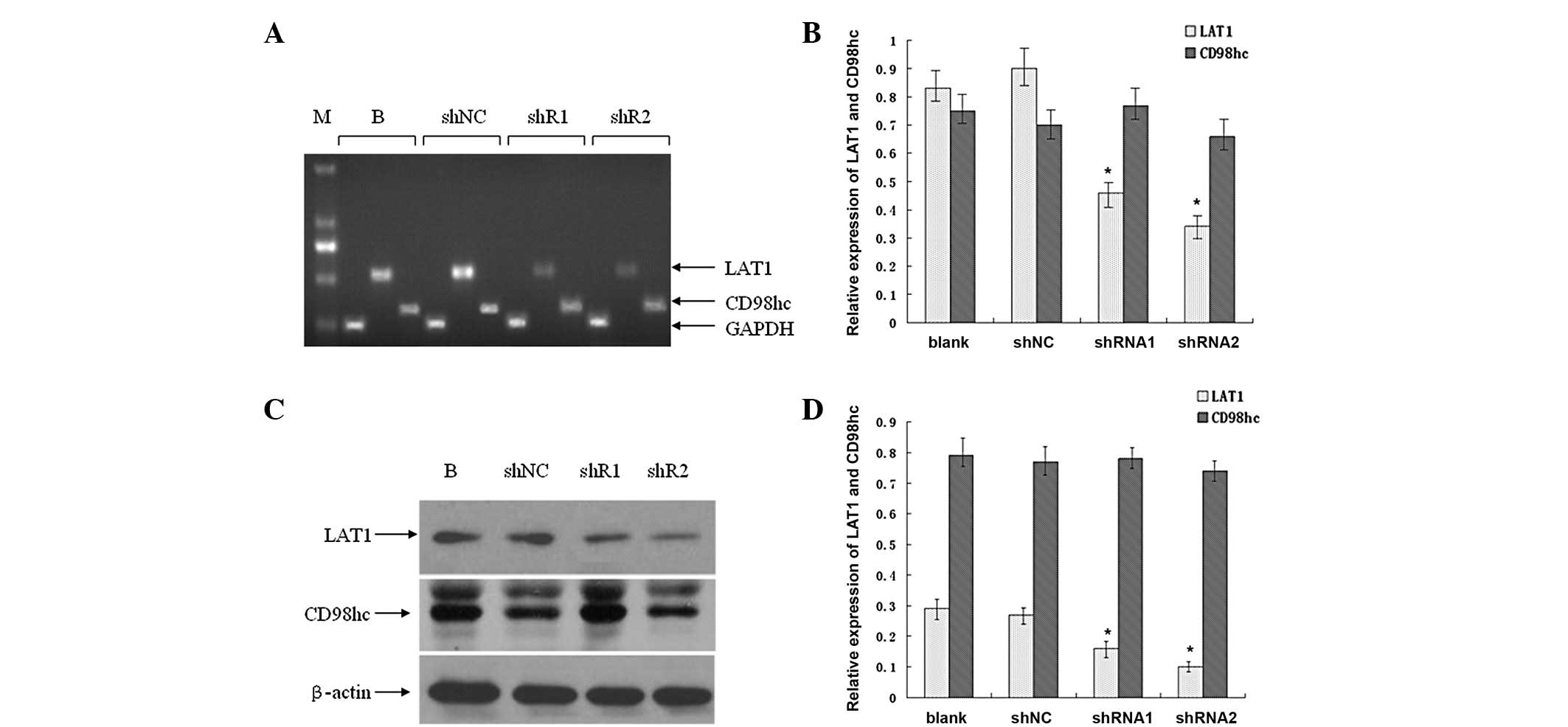 | Figure 3.Expression of large (L)-type amino
acid transporter 1 (LAT1) and CD98hc in established SGC7901 cells.
Total mRNA was extracted from SGC7901_blank, SGC7901_shNC,
SGC7901_shRNA1 and SGC7901_shRNA2 cells, and subjected to RT-PCR
analysis. (A) RT-PCR products were separated in 1.5% agarose gel.
(B) Quantification of the DNA fragments in (A). (C) Whole-cell
protein lysates were purified from the four aforementioned cell
lines and subjected to western blot analysis. (D) Quantification of
the protein bands in (C). Columns, means; bars, SD. *P<0.05 vs.
blank. Representatives of three independent experiments. M, marker;
B, SGC7901_blank; shNC, SGC7901_shNC; shR1, SGC7901_shRNA1; shR2,
SGC7901_shRNA2. |
Furthermore, the protein levels of LAT1 were
detected by western blot analysis. As demonstrated in Fig. 3C, the LAT1 protein levels were
markedly decreased, in the SGC7901_shRNA1 and SGC7901_shRNA2 cells
compared with the SGC7901_shNC and SGC7901 cells, but those of
CD98hc did not appear to be altered. Additionally, following
quantification of the band densities, the present study identified
that the LAT1 protein levels were significantly decreased in the
SGC7901_shRNA1 and SGC7901_shRNA2 cells compared with the
SGC7901_shNC cells (P<0.05; Fig.
3D). Moreover, the knockdown efficiency of shRNA2 was greater
than that of shRNA1. Furthermore, the CD98hc levels did not change
following quantification and analysis.
These results suggested that the expression of LAT1
was downregulated in the established SGC7901_shRNA1 and
SGC7901_shRNA2 cells compared with the SGC7901_shNC and SGC7901
cells, and that the downregulation of LAT1 expression does not
affect the expression of its functional partner, CD98hc.
Downregulation of LAT1 expression
inhibits the growth of gastric cancer cells
To explore the function of LAT1 in gastric cancer,
the present study first examined its effect on cell proliferation.
Cell proliferation was determined by a 3-day MTT assay in the
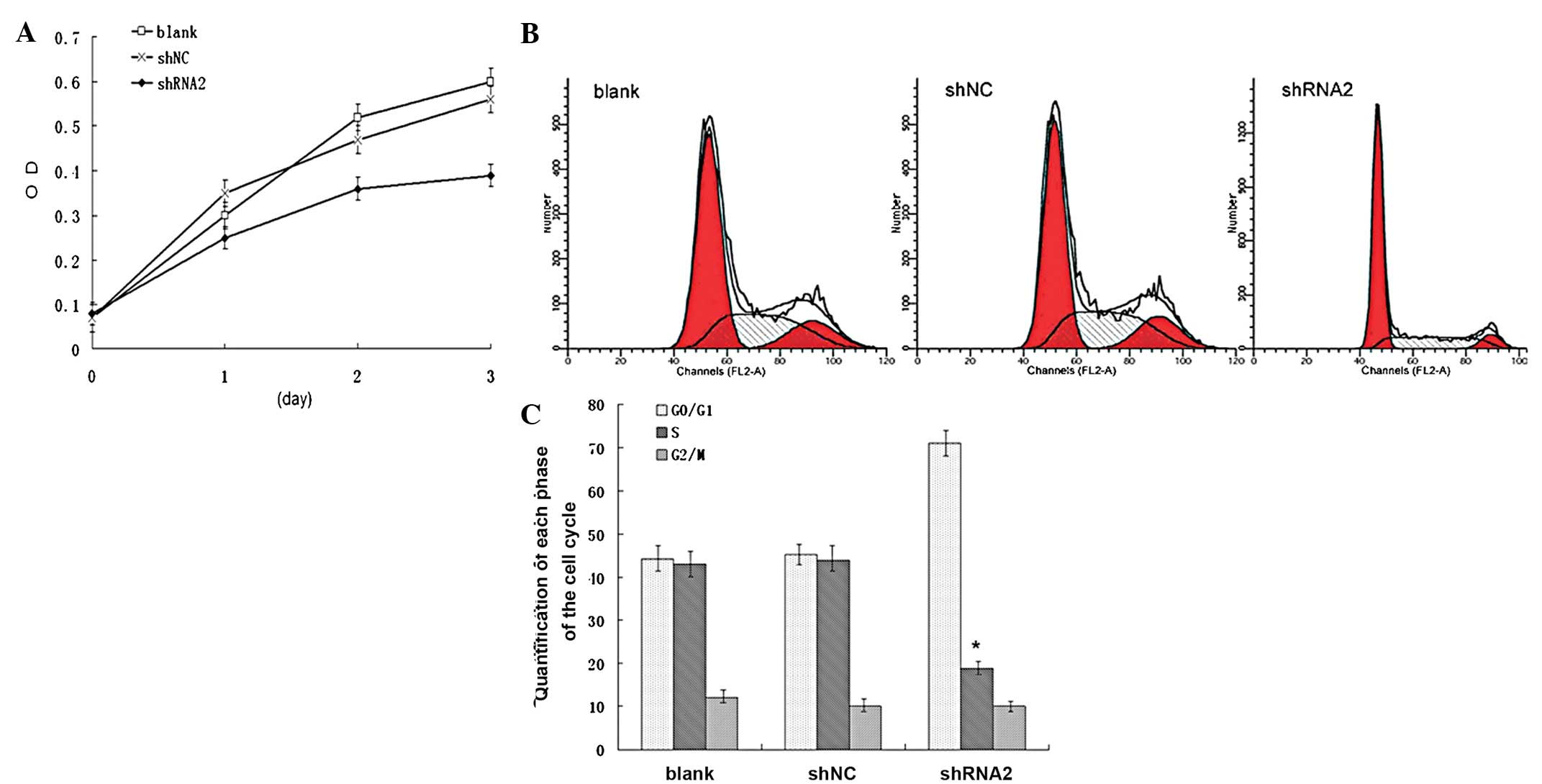
SGC7901, SGC7901_shNC and SGC7901_shRNA2 cells. Fig. 4A shows that the knockdown of LAT1
expression significantly inhibited the growth of the SGC7901_shRNA2
cells compared with the SGC7901_shNC and SGC7901 cells (P<0.05).
Next, the cell cycle of these cells was analyzed by a flow
cytometry assay. As shown in Fig. 4B
and C, the percentage of SGC7901_shRNA2 cells in
G0/G1 phase was significantly increased
(P<0.05), whereas the percentage in the S phase was
significantly decreased (P<0.05) compared with the SGC7901_shNC
cells. The difference in the percentage of cells in the
G0/G1 and S phases between the SGC7901_shNC
and SGC701 cells was not significant (P>0.05). These results
suggested that downregulation of LAT1 expression inhibited the
growth of SGC7901 cells and induced cell cycle arrest in the
G0/G1 phase.
Downregulation of LAT1 expression
inhibits the migration and invasion of gastric cancer cells
The role of LAT1 in cell migration was then
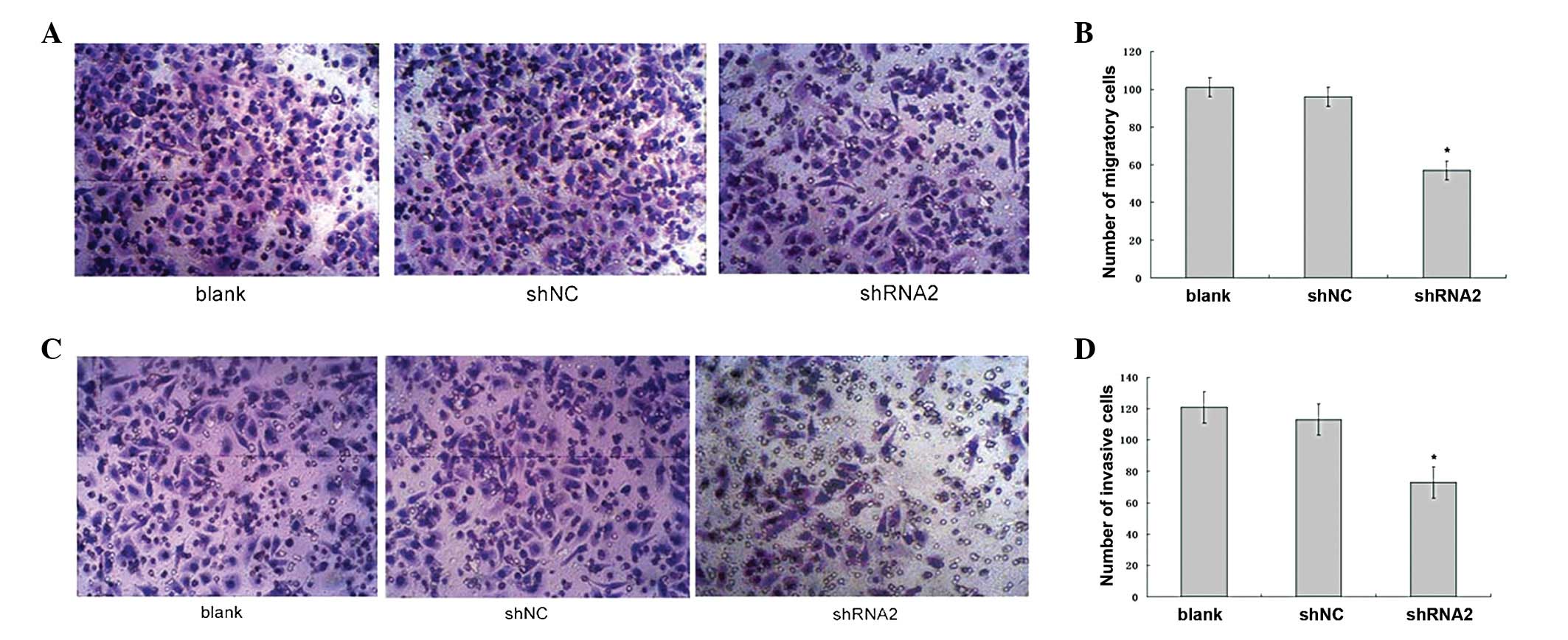
examined. Fig. 5A shows that the
number of SGC7901_shRNA2 cells that migrated to the lower side of
the membrane was decreased compared with that of the SGC7901_shNC
and SGC7901 cells. The quantitative analysis revealed that the
number of migratory SGC7901_shRNA2, SGC7901_shNC and SGC7901 cells
was 57±4.7, 96±6.5 and 101±3.4 per field, respectively (Fig. 5B). The difference in the number of
migratory cells between the SGC7901_shRNA2 and SGC7901_shNC cells
was significant (P<0.05), whereas the difference between the
SGC7901_shNC and SGC7901 cells was not significant (P>0.05).
The role of LAT1 in cell invasion was then examined.
Fig. 5C shows that the number of
SGC7901_shRNA2 cells that invaded to the lower side of the gel and
the membrane was decreased compared with that of the SGC7901_shNC
and SGC7901 cells. The quantitative analysis revealed that the
number of invasive SGC7901_shRNA2, SGC7901_shNC and SGC7901 cells
was 73±10.3, 121±11.8 and 113±9.3 per field, respectively (Fig. 5D). The difference in the number of
invasive cells between the SGC7901_shRNA2 and SGC7901_shNC cells
was significant (P<0.05), whereas the difference between the
SGC7901_shNC and SGC7901 cells was not significant (P>0.05).
These results suggested that the downregulation of
LAT1 expression inhibits the migration and invasion of gastric
cancer cells.
Discussion
In the present study, the role of LAT1 in gastric
cancer was identified by establishing stable cell lines with
successful LAT1 silencing and their relative control cell lines. To
the best of our knowledge, this study is the first to demonstrate
that the downregulation of LAT1 expression inhibits the
proliferation, migration and invasion of gastric cancer cells, as
well as inducing cell cycle arrest in the
G0/G1 phase.
LAT1, as one of the L-type amino acid transporters,
mainly transports large, neutral amino acids, including essential
amino acids, and is therefore important in cell metabolism
(2). Cell metabolism plays a
significant role in cancer progression and metastasis, as rapid
growing tumor tissues require a sufficient energy supply and stable
protein synthesis (3). Accumulating
studies in human tissues utilizing immunohistochemical staining
have suggested that LAT1 is upregulated in numerous types of
cancer, including gastric carcinoma (8). By detecting the Ki67 label index,
VEGF, HIF-1α and numerous other molecules that are significant in
cancer progression and metastasis, it has also been demonstrated
that the overexpression of LAT1 is correlated with cell
proliferation, angiogenesis and hypoxia (11). Moreover, the overexpression of LAT1
is a prognostic marker of lung cancer and gliomas (6,7). To
date, studies clarifying the mechanism and signaling pathway of
LAT1 in vitro, or in the transgenic mouse model, are
limited. In the present study, the role of LAT1 was investigated in
gastric cancer using a loss-of-function method. A total of two
plasmids with LAT1 knockdown, and a control plasmid, medicated by
shRNAs were constructed, and then the stable cell lines were
established using these constructs through transfection and
subsequent selection. Therefore, the results demonstrated the
biological features of gastric cancer cells with constitutive LAT1
silencing. These cell lines also provided an in vitro model
for further clarifying the LAT1 signaling pathway in gastric
cancer.
LAT1 links to CD98hc (also known as 4F2hc) by an
extracellular disulfide bridge for its cell membrane localization
and function. The heavy chain subunit, CD98hc, is a transmembrane
glycoprotein that heterodimerizes with one of the light chains,
such as LAT1 and LAT2, to form a functional complex for
transporting large, neutral amino acids (7). CD98hc is expressed in normal tissue,
particularly in the gastrointestinal tract, as well as in tumor
tissue. Due to its significance in transporting essential amino
acids, genetic disruption of CD98hc results in early embryonic
lethality (12). Additionally,
overexpression of CD98hc in the gastrointestinal epithelium induces
tumorigenesis (9). CD98hc has been
demonstrated to regulate cell proliferation, survival, migration
and transformation in numerous types of cell lines. Overexpression
of CD98hc has been observed in certain types of human cancer
tissue, including lung, breast and renal cell cancer (13–15).
It has been suggested that LAT1 requires CD98hc for its functional
expression, and numerous studies have revealed that the
overexpression of LAT1 is correlated with CD98hc expression in
human cancer tissue; however, CD98hc expression levels are not
always concordant with LAT1 expression levels, suggesting that
these two proteins may have separate signaling pathways and
functions (7). The present study
also found that stably silencing LAT1 did not affect the CD98hc
expression levels, but inhibited certain cell biological processes,
including growth, migration and invasion. Overall, the findings
suggested that LAT1 may have other functions in promoting
carcinogenesis, other than through transporting amino acids.
Therefore, further studies to clarify the signaling pathway of LAT1
in gastric cancer are required.
It has been documented that the upregulation of LAT1
expression in cancer cells not only induces an increase in the
transportation of amino acids, particularly essential amino acids
such as leucine, but also activates the mammalian target of
rapamycin (mTOR) signaling pathway (5,16).
mTOR is a serine/threonine kinase that functions as a complex
through interaction with other proteins, including rictor or raptor
(17). mTOR complexes respond to
growth factors, nutrients and energetic status, and regulate cell
growth, survival, autophagy and metabolism (18). mTOR complex 1 (mTORC1) predominantly
regulates protein translation through ribosomal p70 S6 kinase
(p70S6K) and eukaryotic translation initiation factor 4E-binding
protein 1 (4E-BP1). mTORC2 is a kinase that directly activates Akt
and other kinases, including protein kinase C (PKC) and serum- and
glucocorticoid-induced protein kinase 1 (SGK1) (18,19).
The increased transportation of amino acids by the LAT1/CD98hc
complex activates the mTORC1 signaling pathway by supplying it with
sufficient energy. However, it is possible that other mechanisms
exist that are regulated by LAT1 itself; further clarification of
the underlying mechanism whereby LAT1 regulates the mTOR signaling
pathway is required.
Overall, stable cell lines with successful silencing
of LAT1 expression, and relative control cell lines, were
established. The downregulation of LAT1 expression was identified
to inhibit the proliferation, cell cycle, migration and invasion of
gastric cancer cells. These findings suggested that LAT1 is
significant in gastric cancer and that it may be developed as a
therapeutic target in cancer therapy.
Acknowledgements
This study was supported by the
Natural Science Foundation of the Department of Education of Anhui
Province, China (grant no. KJ2011A264).
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2.
|
Kaira K, Oriuchi N, Imai H, et al: L-type
amino acid transporter 1 and CD98 expression in primary and
metastatic sites of human neoplasms. Cancer Sci. 99:2380–2386.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Shennan DB and Thomson J: Inhibition of
system L (LAT1/CD98hc) reduces the growth of cultured human breast
cancer cells. Oncol Rep. 20:885–889. 2008.PubMed/NCBI
|
|
4.
|
Sakata T, Ferdous G, Tsuruta T, et al:
L-type amino-acid transporter 1 as a novel biomarker for high-grade
malignancy in prostate cancer. Pathol Int. 59:7–18. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yamauchi K, Sakurai H, Kimura T, et al:
System L amino acid transporter inhibitor enhances anti-tumor
activity of cisplatin in a head and neck squamous cell carcinoma
cell line. Cancer Lett. 276:95–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kaira K, Oriuchi N, Imai H, et al:
Prognostic significance of L-type amino acid transporter 1
expression in resectable stage I-III nonsmall cell lung cancer. Br
J Cancer. 98:742–748. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Haining Z, Kawai N, Miyake K, et al:
Relation of LAT1/4F2hc expression with pathological grade,
proliferation and angiogenesis in human gliomas. BMC Clin Pathol.
12:42012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Ichinoe M, Mikami T, Yoshida T, et al:
High expression of L-type amino-acid transporter 1 (LAT1) in
gastric carcinomas: comparison with non-cancerous lesions. Pathol
Int. 61:281–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Nguyen HT, Dalmasso G, Torkvist L, et al:
CD98 expression modulates intestinal homeostasis, inf lammation,
and colitis-associated cancer in mice. J Clin Invest.
121:1733–1747. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kim CH, Park KJ, Park JR, et al: The RNA
interference of amino acid transporter LAT1 inhibits the growth of
KB human oral cancer cells. Anticancer Res. 26:2943–2948.
2006.PubMed/NCBI
|
|
11.
|
Kaira K, Oriuchi N, Takahashi T, et al:
LAT1 expression is closely associated with hypoxic markers and mTOR
in resected non-small cell lung cancer. Am J Transl Res. 3:468–478.
2011.PubMed/NCBI
|
|
12.
|
Tsumura H, Suzuki N, Saito H, et al: The
targeted disruption of the CD98 gene results in embryonic
lethality. Biochem Biophys Res Commun. 308:847–851. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Garber ME, Troyanskaya OG, Schluens K, et
al: Diversity of gene expression in adenocarcinoma of the lung.
Proc Natl Acad Sci USA. 98:13784–13789. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Esseghir S, Reis-Filho JS, Kennedy A, et
al: Identification of transmembrane proteins as potential
prognostic markers and therapeutic targets in breast cancer by a
screen for signal sequence encoding transcripts. J Pathol.
210:420–430. 2006. View Article : Google Scholar
|
|
15.
|
Prager GW, Poettler M, Schmidinger M, et
al: CD98hc (SLC3A2), a novel marker in renal cell cancer. Eur J
Clin Invest. 39:304–310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Imai H, Kaira K, Oriuchi N, et al:
Inhibition of L-type amino acid transporter 1 has antitumor
activity in non-small cell lung cancer. Anticancer Res.
30:4819–4828. 2010.PubMed/NCBI
|
|
17.
|
Kim DH, Sarbassov DD, Ali SM, et al: mTOR
interacts with raptor to form a nutrient-sensitive complex that
signals to the cell growth machinery. Cell. 110:163–175. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|