Introduction
Activated fibroblasts or myofibroblasts with
α-smooth muscle actin (α-SMA) expression are considered to be the
main cellular constituents of reactive stroma in a number of solid
tumors (1). These activated
fibroblasts have been proposed to be important in promoting tumor
progression and metastasis by releasing growth factors,
extracellular matrix proteins and angiogenic factors (2,3). The
hepatic stellate cell (HSC), an important hepatic stromal cell, is
known to be activated or transdifferentiated into a
myofibroblast-like cell through intercellular communication between
HSCs and damaged hepatocytes in various liver tumors, including
hepatocellular carcinoma (HCC) (4).
These activated HSCs demonstrate a high expression of α-SMA and are
considered to be a vital stromal component in HCC (5). Increasing evidence has indicated that
activated HSCs play a critical role in promoting HCC cell
proliferation and invasiveness (6,7). In
addition, HSCs are now being considered as a new potential
therapeutic target in patients with HCC (3). To the best of our knowledge, no direct
evidence has been identified documenting a correlation between
fibroblast activation and survival in HCC patients. The aim of the
present study was to assess the value of activated fibroblasts with
high α-SMA expression as an indicator for survival in patients with
HCC.
Patients and methods
A case-control study was performed at the Veterans
Affairs Medical Center (Memphis, TN, USA) following appropriate
Institutional Review Board approval. A review of the computerized
hospital records was conducted to identify the cases of patients
diagnosed with HCC who had undergone a liver biopsy between January
2000 and December 2009. A total of 10 age- and gender-matched cases
with no histopathological abnormality on liver biopsy were also
identified and used as controls. The clinical information with
regard to age and survival period were obtained for all study
cases. The immunohistochemical staining for α-SMA was performed on
the formalin-fixed, paraffin-embedded tissue sections of all study
and control cases. The α-SMA staining in the HSCs within the tumor
stroma and perisinusoidal spaces was qualitatively classified into
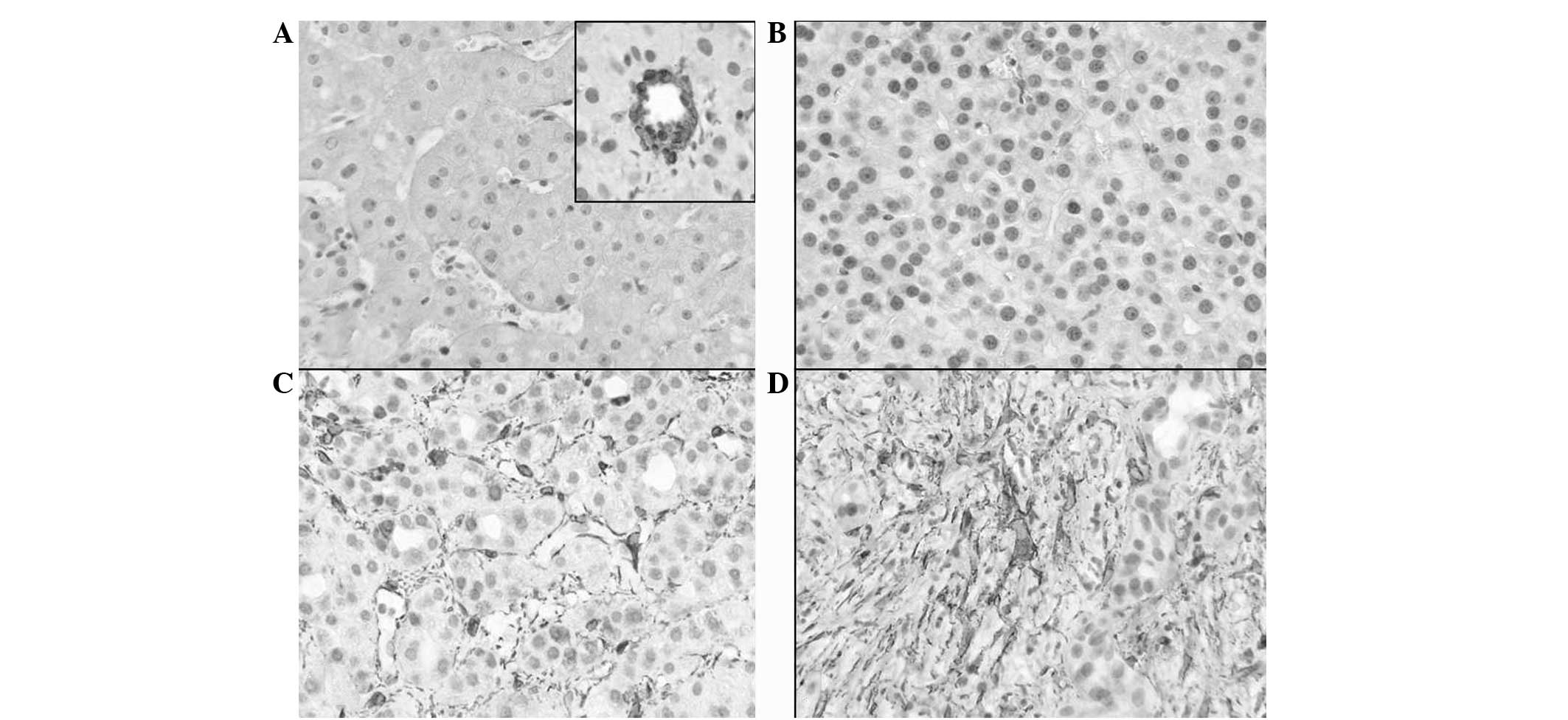
the following 4 groups compared with vascular smooth muscle cells
(VSMCs): 0, no staining; +1, staining intensity considerably lower
than VSMCs (Fig. 1B); +2, staining
intensity lower than VSMCs; and +3, staining intensity similar to
that of VSMCs (Fig. 1C and D). In
addition, the α-SMA staining was quantitatively classified into the
following 4 groups: 0, ≤1 positive cell/high-power field (hpf); +1,
<10 positive cells/hpf (Fig.
1B); +2, 10–20 positive cells/hpf; and +3, >20
positive cells/hpf (Fig. 1C and
1D). Qualitative and quantitative classification was performed
by a fellowship-trained gastrointestinal pathologist without
matching knowledge of the clinical data. For statistical analysis,
grades 0 and +1 were categorized as low expression and grades +2
and +3 as high expression.
Results
A total of 47 patients (age range, 45–85 years; mean
age, 64.45 years) were diagnosed with HCC between January 2000 and
December 2009. All the study subjects subsequently succumbed to
their condition. The survival period following diagnosis ranged
between 1 and 94 months (mean survival, 19 months). All the study
and control subjects were male. Positive staining for α-SMA in
control tissue was mainly observed in VSMCs (Fig 1A; inset) and in extremely few cells
in the perisinusoidal spaces (Fig.
1A). High qualitative (strong) and quantitative (extensive)
expression of α-SMA was revealed in 41 and 42 of the 47 patients,
respectively. Low qualitative (weak) and quantitative (sparse)
expression of α-SMA was revealed in 6 and 5 of the 47 patients,
respectively. Strong and extensive expression of α-SMA revealed a
statistically significant negative correlation with the 3-year [OR,
0.021 and 0.111; 95% confidence interval (CI), 0.002–0.234 and
0.015–0.810; P=0.001 and 0.0302, respectively] and 1.5-year (OR,
0.040 and 0.051; 95% CI, 0.002–0.771 and 0.002–0.990; P=0.0330 and
0.0492, respectively) survival rates in comparison with weak and
sparse expression (Table I). No
significant correlation was identified between age and α-SMA
expression.
 | Table IQualitative and quantitative
expression of α-SMA in HCC. |
Table I
Qualitative and quantitative
expression of α-SMA in HCC.
| | α-SMA staining intensity | | | α-SMA-positive HSC | | |
|---|
| |
| | |
| | |
|---|
| Characteristics | n, (%) | High, n (%) | Low, n (%) | OR (95% CI) | P-value | High, n (%) | Low, n (%) | OR (95% CI) | P-value |
|---|
| Age, years 1 |
| ≤62 | 23 (49) | 18 (38) | 5 (11) | 0.156 | 0.103 | 19 (40) | 4 (9) | 0.206 | 0.174 |
| >62 | 24 (51) | 23 (49) | 1 (2) | | | 23 (49) | 1 (2) | | |
| 3-year survival |
| >3 | 9 (19) | 4 (8) | 5 (11) | 0.021
(0.002–0.234) | 0.0016 | 6 (13) | 3 (6) | 0.111
(0.015–0.810) | 0.0302 |
| ≤3 | 38 (81) | 37 (79) | 1 (2) | | | 36 (77) | 2 (4) | | |
| 1.5-year
survival |
| >1.5 | 20 (43) | 14 (30) | 6 (13) | 0.040
(0.002–0.771) | 0.0330 | 15 (32) | 5 (11) | 0.051
(0.002–0.990) | 0.0492 |
| ≤1.5 | 27 (57) | 27 (57) | 0 (0) | | | 27 (57) | 0 (0) | | |
Discussion
The interaction of tumor cells with surrounding
stromal cells has been recognized to promote tumor development by
affecting cell proliferation, survival and invasiveness (7). As important hepatic stromal cells,
HSCs have been considered to play a key role in liver fibrosis when
excessively activated in various liver diseases, including HCC
(8). HCC is one of the most common
types of malignant cancer in humans, with an overall 5-year
survival rate of only 3% in the United States (9). Surgical resection is the choice of
treatment for HCC, however, this is not feasible when patients
present in late stages of the disease (10). In addition, radiotherapy and
chemotherapy are not effective in advanced disease (10). Activated HSCs that express extremely
high levels of α-SMA have emerged as potent suppressors of hepatic
immunity by affecting T-cell responses and thus, play a vital role
in the progression of HCC (6,7,11,12).
Previous studies have also indicated that activated HSCs are
potential targets for HCC treatment (4).
The current study revealed that the detection of
α-SMA-expressing fibroblasts in HCC provides valuable prognostic
information. As α-SMA is a routinely used and relatively
inexpensive immunohistochemical stain, the majority of pathology
laboratories efficiently employ α-SMA to predict future survival in
HCC patients. The present study also supports the role of α-SMA
expressing activated HSCs in promoting carcinogenesis, which is
considered to be a possible target for future antitumor therapy in
HCC.
In the present study, due to lack of follow-up
staging information for all the patients, the results were not
corrected for stage, metastasis and other factors. Due to this same
reason, it was not possible to determine whether α-SMA is
predictive of metastasis-related survival or is an independent
predictive factor. Multicenter-based large studies with detailed
and frequent staging evaluation data, which enables multifactorial
analysis, are recommended to improve the present understanding of
the role of α-SMA-expressing fibroblasts for predicting poor
survival in HCC.
In conclusion, the current study demonstrated a
predictive role of strong and extensive α-SMA expression in
activated fibroblasts for poor survival in HCC patients. Strong
expression of α-SMA is an excellent marker to anticipate poor
3-year survival (OR, 0.021; 95% CI, 0.002–0.234; P=0.001).
Qualitative α-SMA expression levels in activated fibroblasts appear
to present an improved marker for predicting survival compared with
quantitative expression.
References
|
1
|
Micke P and Ostman A: Exploring the tumor
environment: cancer-associated fibroblasts as targets in cancer
therapy. Expert Opin Ther Targets. 9:1217–1233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Olumi AF, Grossfeld GD, Hayward SW, et al:
Carcinoma-associated fibroblasts direct tumor progression of
initiated human prostatic epithelium. Cancer Res. 59:5002–5011.
1999.
|
|
3
|
Chuaysri C, Thuwajit P, Paupairoj A, et
al: Alpha-smooth muscle actin-positive fibroblasts promote biliary
cell proliferation and correlate with poor survival in
cholangiocarcinoma. Oncol Rep. 21:957–969. 2009.
|
|
4
|
Kuang P, Zhao W, Su W, et al:
18β-glycyrrhetinic acid inhibits hepatocellular carcinoma
development by reversing hepatic stellate cell-mediated
immunosuppression in mice. Int J Cancer. 132:1831–1841. 2013.
|
|
5
|
Hautekeete ML and Geerts A: The hepatic
stellate (Ito) cell: its role in human liver disease. Virchows
Arch. 430:195–207. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carpino G, Morini S, Ginanni CS, et al:
Alpha-SMA expression in hepatic stellate cells and quantitative
analysis of hepatic fibrosis in cirrhosis and in recurrent chronic
hepatitis after liver transplantation. Dig Liver Dis. 37:349–356.
2005. View Article : Google Scholar
|
|
7
|
Desmoulière A, Guyot C and Gabbiani G: The
stroma reaction myofibroblast: a key player in the control of tumor
cell behavior. Int J Dev Biol. 48:509–517. 2004.PubMed/NCBI
|
|
8
|
Friedman SL: Mechanism of hepatic
fibrogenesis. Gastroenterology. 134:1655–1669. 2008. View Article : Google Scholar
|
|
9
|
Desmet VJ and Rosai J: Liver: tumors and
tumorlike conditions. Rosai and Akerman’s Surgical Pathology. Rosai
J: 10th Edition. Mosby Elsevier; New York: pp. 944–953. 2011
|
|
10
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases. Management of
hepatocellular carcinoma: an update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu MC, Chen CH, Liang X, et al: Inhibition
of T-cell responses by hepatic stellate cells via B7-H1-mediated
T-cell apoptosis in mice. Hepatology. 40:1312–1321. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen CH, Kuo LM, Chang Y, et al: In vivo
immune modulatory activity of hepatic stellate cells in mice.
Hepatology. 44:1171–1181. 2006. View Article : Google Scholar : PubMed/NCBI
|