Introduction
Lung cancer ranks among the most common malignant
diseases and is currently the leading cause of cancer-related
mortality worldwide (1). Lung
cancers are divided into two main classes, depending on their
histological appearance and presumed cellular origin. Small cell
lung cancer (SCLC) is of neuroendocrine origin, while non-small
cell lung cancer (NSCLC) is predominantly of epithelial origin.
NSCLC accounts for >85% of all lung cancer cases (2). The current treatment methods of NSCLC
most commonly include surgery, chemotherapy, radiation therapy and
targeted therapy. While early-stage lung cancer may be eminently
curable by surgery (3), the
majority of lung cancer cases are detected at advanced stages, so
combined therapies cannot stand to benefit all patients. The
overall 5-year survival rate of lung cancer patients stands at
<15%, so it remains a necessity that specific biomarkers and
therapeutic targets are explored for the development of novel
diagnostic and treatment strategies for the future.
Semaphorins, initially called collapsins, are a
large family of secreted, transmembrane and
glycosylphosphatidylinositol (GPI)-linked proteins, which were
first characterized for their role in axonal guidance in the
developing nervous system (4–6).
Semaphorins are expressed in numerous tissues where they regulate
cell survival, apoptosis, adhesion, directional cell migration, and
affect the cytoskeleton, actin filament organization and
microtubules (7,8). Based on structural features and amino
acid sequence similarity, semaphorins have been divided into eight
classes, with classes three to seven representing the vertebrate
proteins. Semaphorin-3A is a chemorepellent with multiple guidance
functions, including axon pathfinding, cardiac and peripheral
vascular patterning and branching morphogenesis (9). Semaphorin-3A signaling is mediated by
a complex of the binding receptor neuropilin 1 (NP1) and the
signaling receptors plexin A1 or A3 (10). The action of semaphorin-3A is not
limited to the nervous system, as NP1 is expressed on endothelial
cells, T cells, keratinocytes and tumor cells. It can inhibit
angiogenesis, proliferation of T cells, migration of keratinocytes
and tumor cells. In addition, it was recently revealed that
semaphorin-3A is involved in the entry of dendritic cells to the
lymphatic system. Several studies have demonstrated that
overexpression of semaphorin-3A attenuates invasion and matrigel
adhesion of tumor cells in certain types of cancer, including
prostate and breast (11).
Semaphorin-3A has been considered as a potent tumor suppressor in
certain malignant neoplasms (12).
Matrix metalloproteinases (MMPs) are the main
enzymes involved in the degradation of the extracellular matrix
(ECM)and are important in regulating tumor invasion and metastasis
(13). Among this family of
Zinc-dependent proteolytic enzymes, 28 species have been
identified. According to their differences in structure and
degradable substrate, MMPs are categorized into four types:
Collagenases, gelatinases, matrilysin and membrane-type MMP
(14). MMP-14, also known as
membrane type-1 MMP, was first discovered in the membrane-type MMP
family, at ~3.5 kb, encoding a protein containing 582 amino acids
and with a molecular weight of ~66 kda (15). Besides its expression in
fibroblasts, smooth muscle and endothelial cells, MMP-14 is also
found in the majority of tumor types, including skin, lung,
stomach, colon, liver, kidney, breast, bladder and brain, and is
one of the most closely linked enzymes, of the MMP family, to the
molecular mechanisms of tumor invasion and metastasis (16,17).
MMP-14 can degrade many macromolecular ECM components, including
type I–III collagen, laminin, fibronectin, vitronectin, cellulose
and proteoglycan, by enhancing the hydrolysis effects of the
pro-enzymes MMP-2 and MMP-13. MMP-2, in particular, is important in
the processes involved in tumor invasion. MMP-14 can affect cell
migration in the ECM either through the effects of different
intercellular adhesion molecules or by directly providing
non-invasive potential cells with the ability to penetrate type I
collagen, which is a process that does not depend on the activation
of proenzyme MMP-2. MMP-14 increases expression of vascular
endothelial growth factor (VEGF), promotes migration of endothelial
cells in the ECM and thereby contributes to the formation of new
blood vessels. Together, these effects establish an optimal
microenvironment to promote tumor invasion and metastasis (17,18).
The present study aimed to identify a correlation
between semaphorin-3A and MMP-14 protein expression in NSCLC.
Semaphorin-3A and MMP-14 protein expression were first examined in
NSCLC and normal lung tissues as the control. Their expression
levels were analyzed against clinical characteristics, including
pleural invasion, lymph node metastasis, the number of metastatic
lymph node, degree of differentiation, vascular invasion and
proliferating cell nuclear antigen (PCNA) expression. Finally, the
correlation of semaphorin-3A and MMP-14 expression with prognosis
was investigated using statistical methods.
Materials and methods
Tissue samples and patients
NSCLC tissues (46 cases of lung pulmonary squamous
cell carcinoma and 48 cases of pulmonary adenocarcinoma) and normal
lung tissues (80 cases) were collected from patients who underwent
surgery at the Department of Thoracic Surgery, Tangshan Worker
Hospital of Hebei Medical University (Tangshan, China) between
January and November 2007. All tumor tissues were
histopathologically diagnosed by at least two trained pathologists.
Written informed consent was obtained from all patients prior to
surgery and the study protocol was approved by the Institutional
Review Board for the use of Human Subjects at Tangshan Worker
Hospital. The NSCLC group was composed of 60 male and 34 female
patients aged 43–75 years (mean, 53.8 years). The control group was
composed of 60 male and 20 female patients aged 44–77 years (mean,
53.8 years). None of the patients received pre-operative
chemotherapy or radiation therapy. The tissues were immersed in 10%
formalin with a pH value of 7.4. Following dehydration in graded
ethanol and xylene, the specimens were paraffin-embedded and cut
into 4 μm-thick coronal sections.
HE staining
The sections were stained using hematoxylin/eosin
according to the standard procedures and observed under an Olympus
BX51 light microscope (Olympus Corporation, Tokyo, Japan).
Semaphorin-3A and MMP-14
immunohistochemistry
The procedures were processed according to the
protocols recommended for the mouse anti-human semaphorin-3A and
MMP-14 monoclonal antibodies (Boster Biological Technology Co.,
Ltd., Wuhan, China). After being deparaffinated and rehydrated,
sections were irradiated in 0.1 mol/l sodium citrate buffer (pH
6.0) in a microwave oven (medium/low temperature) for 12 min.
Subsequently, the sections were exposed to 3%
H2O2 for 10 min to bleach endogenous
peroxidases, followed by rinsing three times in phosphate-buffered
saline (PBS) for 10 min. Sections were respectively incubated with
a mouse anti-human semaphorin-3A monoclonal antibody (1:100) and a
mouse anti-human MMP-14 monoclonal antibody (1:50) for 1 h at 37°C,
washed three times in PBS and incubated in a biotinylated goat
secondary anti-mouse polyclonal antibody (Boster Biological
Technology Co., Ltd.) for 30 min at 37°C. The specificity of the
antibodies was examined by omission of the primary antibodies.
Following being washed in PBS, the tissues were visualized with
3,3′-diaminobenzidine tetrahydrochloride (DAB) and counterstained
with hematoxylin. Finally, the sections were dehydrated in graded
ethanol, immersed in xylene and coverslipped.
Semaphorin-3A and MMP-14 assay
The positive staining of semaphorin-3A and MMP-14
was located in the cytoplasm, and the cells with brown/yellow
particles were considered as positive ones. The zone with
concentrated positive cells was selected and the number of positive
cells in 10 randomly chosen high-power fields (original
magnification, ×400) was counted. If the average expression rate of
positive tumor cells per high-power field was ≥25%, it was judged
as positive, while that <25%, was judged as negative. Finally,
the positive rate was calculated.
Statistical analysis
Statistical analysis was conducted using SPSS for
Windows software, version 17.0 (SPSS, Inc., Chicago, IL, USA). The
χ2 test was used to compare the difference in
semaphorin-3A and MMP-14 expression between NSCLC and normal
tissues, and between different clinical characteristics of patients
in the NSCLC group. Bivariate correlation analysis (Pearson’s
product moment coefficient) was used to analyze the correlation
between semaphorin-3A and MMP-14 expression in the NSCLC group. A
life table was used to calculate survival function and a log-rank
test was used for survival analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of semaphorin-3A and MMP-14 in
NSCLC tissues and normal lung tissues
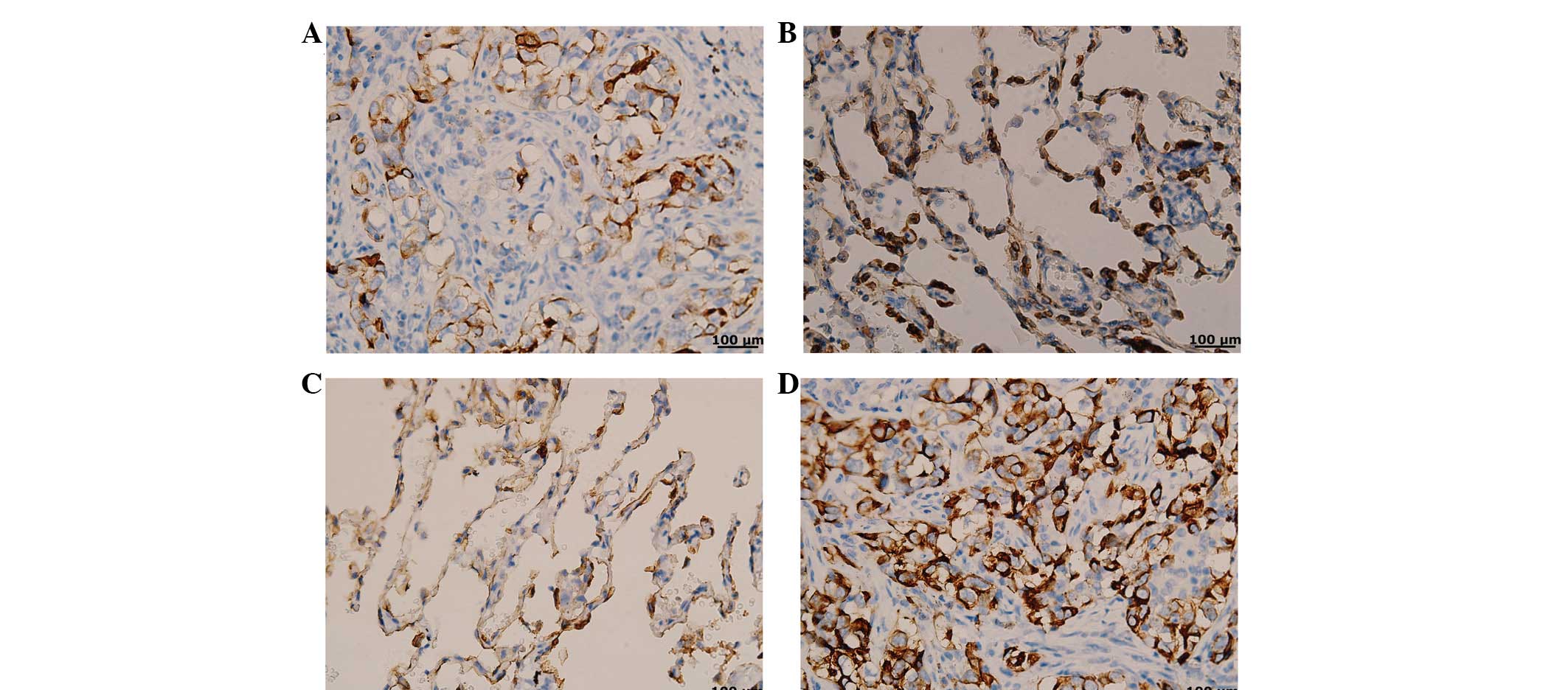
Semaphorin-3A expression in NSCLC tissues was lower
compared with that in the control tissues (Fig. 1A and B), while the MMP-14 expression
was higher than that in normal tissues (Fig. 1C and D). In Table I, the data demonstrates that
compared with the control group, the NSCLC group had a low positive
rate of semaphorin-3A expression (36.17 vs. 75.00%, respectively)
and a high positive rate of MMP-14 (75.53 vs. 25.00%,
respectively), and the difference was considered to be
statistically significant (P<0.05).
 | Table IComparison of semaphorin-3A and MMP-14
expression between the NSCLC group and control group. |
Table I
Comparison of semaphorin-3A and MMP-14
expression between the NSCLC group and control group.
| | Semaphorin-3A | | | MMP-14 | | |
|---|
| |
| | |
| | |
|---|
| Variable | n | + (%) | − (%) | χ2 | P-value | + (%) | − (%) | χ2 | P-value |
|---|
| NSCLC group | 94 | 34 (36.17) | 60 (63.83) | 26.2349 | <0.0001 | 71 (75.53) | 23 (24.47) | 44.2363 | <0.0001 |
| Control group | 80 | 60 (75.00) | 20 (25.00) | | | 20 (25.00) | 60 (75.00) | | |
Expression of semaphorin-3A and MMP-14 in
patients presenting with different clinical characteristics
In the NSCLC group, the positive rates of
semaphorin-3A and MMP-14 expression were relevant to pleural
invasion, lymph node metastasis, the number of metastatic lymph
nodes, the degree of differentiation, vascular invasion and PCNA
expression. In addition, the expression of semaphorin-3A correlated
with the maximum diameter of the tumor, while MMP-14 expression
revealed no such association (Table
II).
 | Table IIAnalysis of semaphorin-3A and MMP-14
expression in the NSCLC group. |
Table II
Analysis of semaphorin-3A and MMP-14
expression in the NSCLC group.
| | Semaphorin-3A | | | MMP-14 | | |
|---|
| |
| | |
| | |
|---|
| Variable | n | + (%) | − (%) | χ2 | P-value | + (%) | − (%) | χ2 | P-value |
|---|
| Pleural invasion | | | | 5.6360 | 0.0176 | | | 5.3965 | 0.0202 |
| No | 54 | 25 (46.30) | 29 (53.70) | | | 36 (66.67) | 18 (33.33) | | |
| Yes | 40 | 9 (22.50) | 31 (77.50) | | | 35 (87.50) | 5 (12.50) | | |
| Lymph node
metastasis | | | | 14.3348 | 0.0002 | | | 8.5288 | 0.0035 |
| No | 37 | 22 (59.46) | 15 (40.54) | | | 22 (59.46) | 15 (40.54) | | |
| Yes | 57 | 12 (21.05) | 45 (78.95) | | | 49 (85.96) | 8 (14.04) | | |
| Number of metastatic
lymph nodes | | | | 7.2598 | 0.0071 | | | 7.5552 | 0.0060 |
| <4 | 64 | 29 (45.31) | 35 (54.69) | | | 43 (67.19) | 21 (32.81) | | |
| ≥4 | 30 | 5 (16.67) | 25 (83.33) | | | 28 (93.33) | 2 (6.67) | | |
| Expression of PCNA,
% | | | | 23.4801 | <0.0001 | | | 5.7913 | 0.0161 |
| <25 | 49 | 29 (59.18) | 20 (40.82) | | | 32 (65.31) | 17 (34.69) | | |
| ≥25 | 45 | 5 (11.11) | 40 (88.89) | | | 39 (86.67) | 6 (13.33) | | |
| Degree of
differentiation | | | | 8.7037 | 0.0032 | | | 9.4801 | 0.0021 |
| Well and
moderately | 56 | 27 (48.21) | 29 (51.79) | | | 36 (64.29) | 20 (35.71) | | |
| Poorly | 38 | 7 (18.42) | 31 (81.58) | | | 35 (92.11) | 3 (7.89) | | |
| Vascular
invasion | | | | 5.7928 | 0.0161 | | | 6.4769 | 0.0109 |
| No | 66 | 29 (43.94) | 37 (56.06) | | | 45 (68.18) | 21 (31.82) | | |
| Yes | 28 | 5 (17.86) | 23 (82.14) | | | 26 (92.86) | 2 (8.70) | | |
| Maximum diameter of
tumor, cm | | | | 15.2753 | <0.0001 | | | 0.4966 | 0.4810 |
| <5 | 44 | 25 (56.82) | 19 (43.18) | | | 31 (73.81) | 11 (26.19) | | |
| ≥5 | 50 | 9 (18.00) | 41 (82.00) | | | 40 (80.00) | 10 (20.00) | | |
Correlation between the expression of
semaphorin-3A and MMP-14 in the NSCLC group
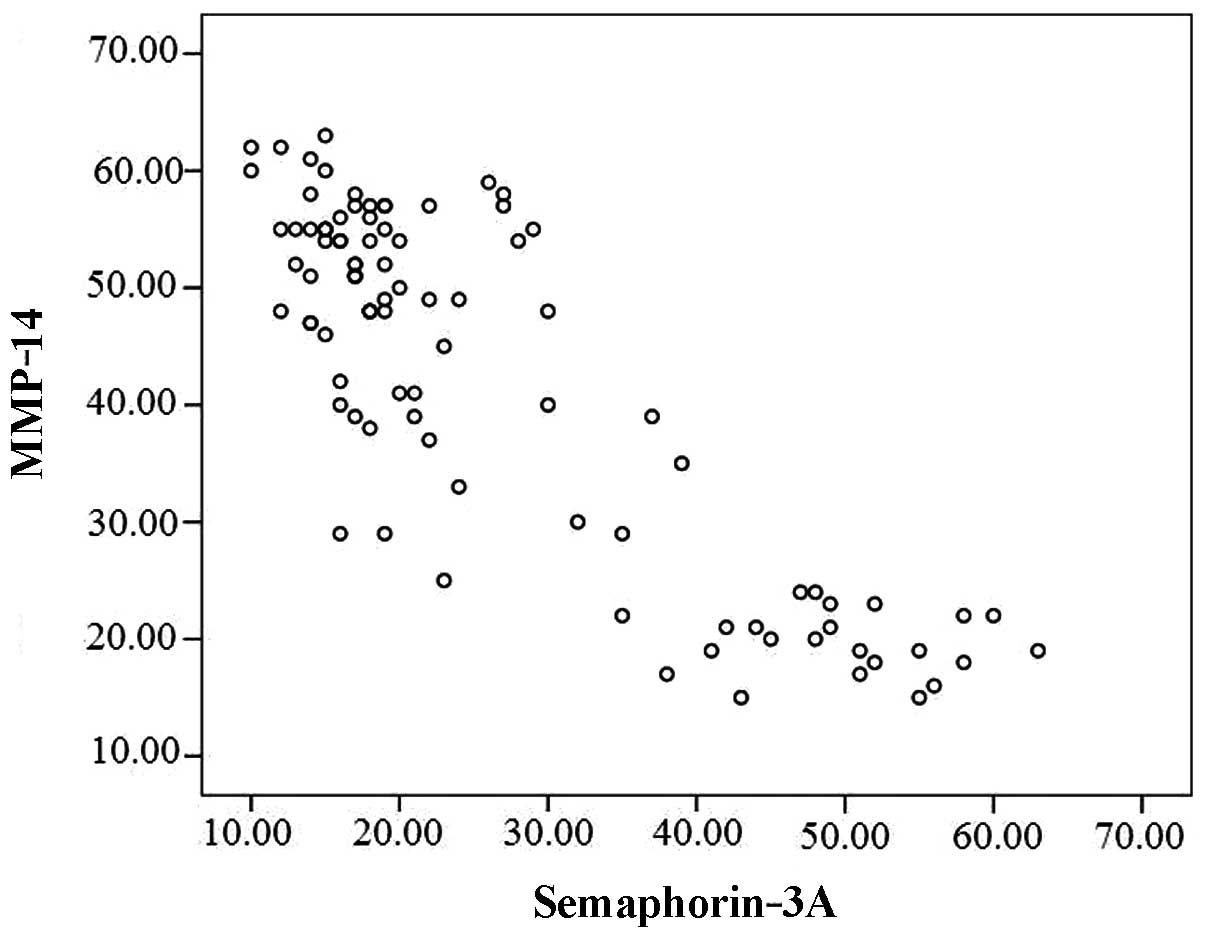
The linear correlation analysis revealed that
semaphorin-3A expression was negatively correlated with MMP-14
expression (r=−0.852, P<0.001; Fig.
2).
Survival analysis of semaphorin3-A and
MMP-14 expression in the NSCLC group
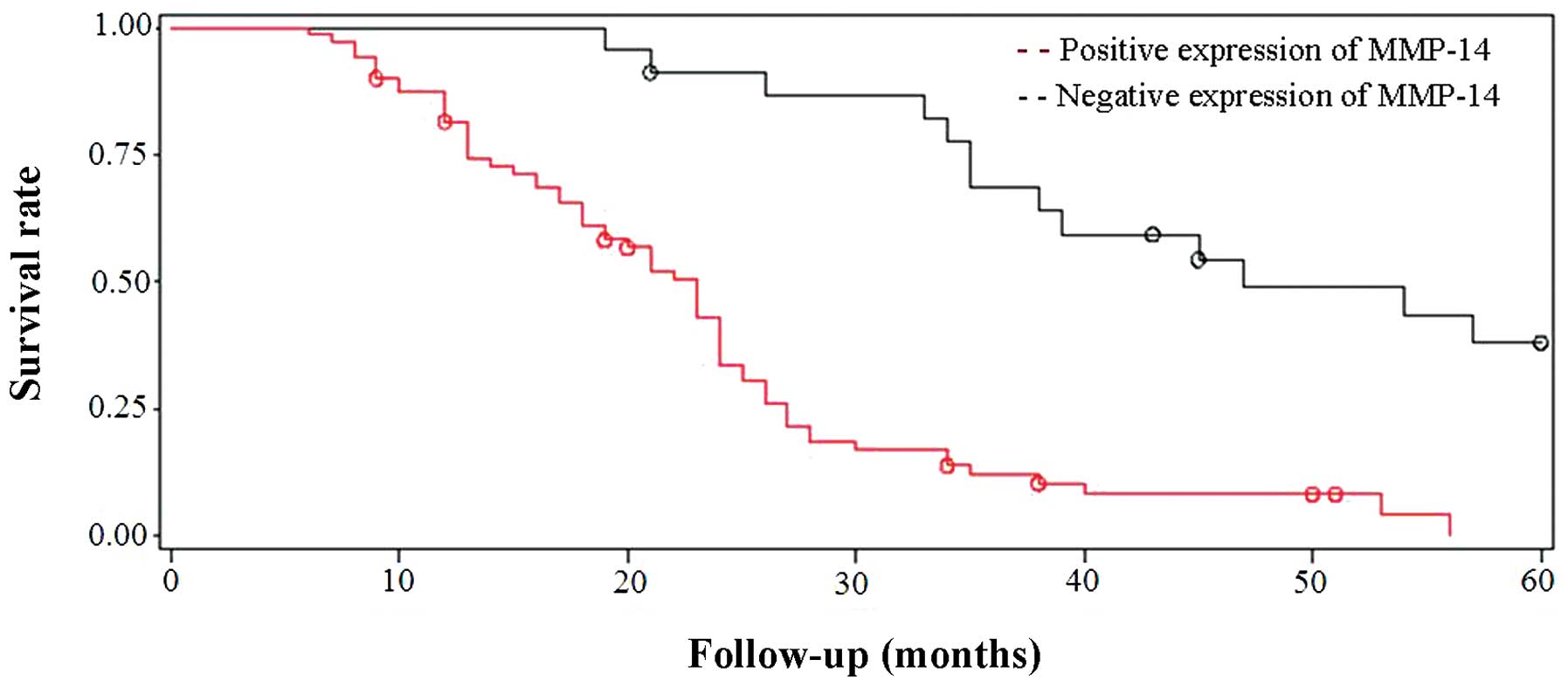
The clinical findings of NSCLC patients during
long-term follow-up were analyzed and compared with the expression
patterns of semaphorin-3A and MMP-14. The follow-up time was from 6
to 60 months (mean, 24.2 months). A life table was used to
calculate the survival function of patients with semaphorin-3A and
MMP-14 expression, and then the log-rank test was utilized for
survival analysis. The results indicated that the protein
expression levels of semaphorin-3A and MMP-14 were associated with
survival time. Patients with a lower expression of semaphorin-3A
and a higher expression of MMP-14 had a worse disease prognosis
(Figs. 3 and 4).
Discussion
Semaphorin-3A is an important protein that belongs
to a family of nerve axon guidance factors, which have a crucial
physiological role in nervous system development. As the product of
a tumor suppressor gene, its role in malignant tumors has attracted
much attention recently. Semaphorin-3A has numerous diverse
biological functions, including lymphocyte activation, vascular
endothelial cell migration, lung and bronchial morphogenesis and
promoting tumor cell migration (19). MMPs are a group of proteins with
high structural homology which, through a series of proteolytic
enzymatic reactions, play a key role in the degradation of the ECM.
MMP-14 is important within the MMP family, for its ability to
activate other members, including the cell-surface expressed MMP-2,
which may be critically involved in accelerating malignant
processes, such as tumor metastasis (20). Through the inhibitory control of MMP
activation, semaphorins may regulate the breakdown of the ECM
components and have thus been implicated as suppressors of tumor
metastasis (21).
In the present study, the expression profiles of
semaphorin-3A and MMP-14 in NSCLC tissues were examined. The
results revealed low level expression of semaphorin-3A and high
expression levels of MMP-14. These abnormal expression patterns may
have an important role in tumor development and metastasis, and
suggest that semaphorin-3A may be a suppressor gene and MMP-14 an
oncogene. The experimental results demonstrated that there is a
negative correlation between semaphorin-3A and MMP-14 expression in
NSCLC, suggesting that these protein levels have negative synergy
and promote tumor progression in a collaborative manner. The
mechanisms underlying this effect may be explained by the
hypothesis that the diverse biological effects of semaphorin-3A are
regulated by the NP receptor (22).
Since the NP receptor is also the receptor of VEGF isomer,
semaphorin-3A may act to limit the combined effects of the VEGF and
NP receptor complex, when it is interacting with the NP receptor.
As VEGF is a key regulator of blood vessel formation, semaphorin-3A
may prevent tumor progression by competitively inhibiting
VEGF-induced tumor angiogenesis. As mentioned above, semphorin-3A
can induce tumor cell migration by reducing MMP-14 secretion and
thus regulate ECM degradation. Recently, it has been hypothesized
that MMP-14 also has a role in promoting VEGF secretion and can
activate tumor angiogenesis (23).
So, it appears the regulatory effects of semaphorin-3A and MMP-14
on cancerous tumors may be associated with VEGF. That is, VEGF is
the molecule mediating the synergistic effects of semaphorin-3A and
MMP-14 on tumor progression and metastasis. However, its specific
mechanisms need to be confirmed by further investigations.
The present study demonstrated that both the
expression of semaphorin-3A and MMP-14 in the observation group
were closely associated with pleural invasion, lymph node
metastasis, the number of metastatic lymph nodes, the degree of
differentiation, vascular invasion and expression of PNCA. This
result indicated that semaphorin-3A and MMP-14 may be
synergistically involved in the processes of tumor invasion,
differentiation and vascular dissemination. In addition, the
expression of semaphorin-3A was correlated with the maximum
diameter of the tumors; the lower the expression, the larger the
maximum diameter of the tumor. MMP-14 had a weaker correlation with
tumor diameter. Therefore, lower expression of semaphorin-3A
exhibited a more evident promoting effect on tumor growth. PNCA, as
an important indicator of tumor proliferation index, is more
strongly associated with semaphorin-3A and MMP-14. The abnormal
expression levels of semaphorin-3A and MMP-14 appear to promote
tumor cell proliferation, which provides direct evidence for its
damaging impact on tumor progression and disease prognosis.
Survival analysis identified that the patients with low expression
of semaphorin-3A and high expression of MMP-14 had a worse
prognosis. Therefore, the combined detection of semaphorin-3A and
MMP-14 postoperatively is significantly valuable on the judgment of
prognosis of NSCLC.
The present study demonstrated that low expression
of semaphorin-3A and high expression of MMP-14 in NSCLC may promote
tumor progression, possibly due to negative synergy, and the
combined detection of semaphorin-3A and MMP-14 postoperatively was
valuable in the judgment of prognosis. The upregulation of
semaphorin-3A and downregulation of MMP-14 may provide a useful
strategy for future NSCLC inhibitory therapies.
Acknowledgements
This study was supported by a grant (no. 10276117D)
awarded by the Science and Technology Research Program of Hebei
Province, China.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar
|
|
3
|
Lee PC, Korst RJ, Port JL, Kerem Y,
Kansler AL and Altorki NK: Long-term survival and recurrence in
patients with resected non-small cell lung cancer 1 cm or less in
size. J Thorac Cardiovasc Surg. 132:1382–1389. 2006. View Article : Google Scholar
|
|
4
|
Shelly M, Cancedda L, Lim BK, Popescu AT,
Cheng PL, Gao H and Poo MM: Semaphorin3A regulates neuronal
polarization by suppressing axon formation and promoting dendrite
growth. Neuron. 71:433–446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Charoy C and Castellani V: The
neurotrophic factor GDNF, a novel modulator of the semaphorin
signaling pathway during axon guidance. Med Sci (Paris).
29:127–130. 2013.
|
|
6
|
Roney K, Holl E and Ting J: Immune plexins
and semaphorins: old proteins, new immune functions. Protein Cell.
4:17–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gu C and Giraudo E: The role of
semaphorins and their receptors in vascular development and cancer.
Exp Cell Res. 319:1306–1316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tamagnone L: Emerging role of semaphorins
as major regulatory signals and potential therapeutic targets in
cancer. Cancer Cell. 22:145–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goshima Y, Sasaki Y, Yamashita N and
Nakamura F: Class 3 semaphorins as a therapeutic target. Expert
Opin Ther Targets. 16:933–944. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bouvrée K, Brunet I, Del Toro R, et al:
Semaphorin3A, Neuropilin-1, and PlexinA1 are required for lymphatic
valve formation. Circ Res. 111:437–445. 2012.PubMed/NCBI
|
|
11
|
Staton CA, Shaw LA, Valluru M, et al:
Expression of class 3 semaphorins and their receptors in human
breast neoplasia. Histopathology. 59:274–282. 2011.PubMed/NCBI
|
|
12
|
Chakraborty G, Kumar S, Mishra R, Patil TV
and Kundu GC: Semaphorin 3A suppresses tumor growth and metastasis
in mice melanoma model. PLoS One. 7:e336332012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roy R, Yang J and Moses MA: Matrix
metalloproteinases as novel biomarkers and potential therapeutic
targets in human cancer. J Clin Oncol. 27:5287–5297. 2009.
View Article : Google Scholar
|
|
14
|
Murphy G and Nagase H: Progress in matrix
metalloproteinase research. Mol Aspects Med. 29:290–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagase H, Visse R and Murphy G: Structure
and function of matrix metalloproteinases and TIMPs. Cardiovasc
Res. 69:562–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cruz-Munoz W and Khokha R: The role of
tissue inhibitors of metalloproteinases in tumorigenesis and
metastasis. Crit Rev Clin Lab Sci. 45:291–338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong Q, Yu D, Yang CM, Jiang B and Zhang
H: Expression of the reversion-inducing cysteine-rich protein with
Kazal motifs and matrix metalloproteinase-14 in neuroblastoma and
the role in tumour metastasis. Int J Exp Pathol. 91:368–373. 2010.
View Article : Google Scholar
|
|
18
|
Perentes JY, Kirkpatrick ND, Nagano S, et
al: Cancer cell-associated MT1-MMP promotes blood vessel invasion
and distant metastasis in triple-negative mammary tumors. Cancer
Res. 71:4527–4538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rizzolio S and Tamagnone L: Multifaceted
role of neuropilins in cancer. Curr Med Chem. 18:3563–3575. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fullár A, Kovalszky I, Bitsche M, et al:
Tumor cell and carcinoma-associated fibroblast interaction
regulates matrix metalloproteinases and their inhibitors in oral
squamous cell carcinoma. Exp Cell Res. 318:1517–1527. 2012.
|
|
21
|
Zarrabi K, Dufour A, Li J, et al:
Inhibition of matrix metalloproteinase 14 (MMP-14)-mediated cancer
cell migration. J Biol Chem. 286:33167–33177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parker MW, Guo HF, Li X, Linkugel AD and
Vander Kooi CW: Function of members of the neuropilin family as
essential pleiotropic cell surface receptors. Biochemistry.
51:9437–9446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deng YP, Li W and Li YL, Xu H, Liang SS,
Zhang LH and Li YL: MT1-MMP up-regulates VEGF expression in human
breast carcinoma MCF-7 cells and induces tumor angiogenesis.
Zhonghua Zhong Liu Za Zhi. 31:727–731. 2009.(In Chinese).
|