Introduction
Chronic myeloid leukemia (CML) is a clonal
myeloproliferative hematopoietic stem cell disease, characterized
by the presence of the Philadelphia chromosome, which is generated
by the reciprocal translocation of the ABL1 oncogene localized on
chromosome 9 with the breakpoint cluster region (BCR) on chromosome
22 [t (9; 22)] (1,2). The BCL-ABL fusion gene, which has been
identified as a crucial step in the pathogenesis of CML, encodes
the fusion BCR-ABL1 protein, which possesses constitutive tyrosine
kinase activity (3,4). The BCR-ABL tyrosine kinase
significantly influences several cellular signaling cascades,
including the Ras, mitogen-activated protein kinase,
phosphatidylinositide 3-kinase (PI3K)/protein kinase B (Akt),
signal transducer and activator of transcription 5, and Src
(5–9). Abnormal overactivation of these
signaling cascades leads to increased cellular proliferation,
resistance to apoptosis and genetic instability.
The selective tyrosine kinase inhibitors (TKI),
which target the ATP binding site of BCR/ABL, block BCR/ABL kinase
activity and exhibit a positive therapeutic effect for CML
(10,11). TKIs, such as imatinib, are
considered as a conventional treatment option for CML. However, CML
patients may become relatively resistant to TKI therapy, including
the second generation TKIs (12,13)
and, thus, the development of a therapeutic strategy that targets
abnormal signaling cascades other than BCR/ABL is urgently required
for CML treatment.
Resveratrol is a naturally occurring polyphenolic
compound that is abundant in various plants, including grapes and
nuts, as well as red wine and other plant-based food sources
(14). Resveratrol has been
reported to exert multiple beneficial effects, including
anti-inflammatory, -oxidant and -viral effects, as well as
neuroprotection (15,16). Resveratrol inhibits the initiation,
promotion and progression of tumors and, therefore, based on the
above report, resveratrol may present a potential preventive and
therapeutic agent (17). Numerous
studies have demonstrated that resveratrol is involved in the
regulation of cell cycle arrest, differentiation and apoptosis of
different tumor cell lines and experimental cancer models (18–22).
Resveratrol causes cell cycle arrest at the G1 phase
by inducing the expression of the cyclin-dependent kinase
inhibitors, p21WAF1/CIP1 and p27KIP1 (23). Additionally, resveratrol induces
cell apoptosis by upregulating the expression of Bax, Bak, Bim,
p53, tumor necrosis factor-related apoptosis-inducing ligand
(TRAIL), TRAIL-receptor (R)1/R4 and TRAIL-R2/R5, whilst
simultaneously downregulating the expression of B-cell lymphoma
(Bcl)-2 and Bcl-XL (24,25). Numerous studies have shown that
resveratrol modulates several cell signaling pathways, which are
involved in the proliferation of tumor cells. Furthermore,
resveratrol functions through different mechanisms in different
types of tumor cells (26).
The aim of the present study was to investigate
whether resveratrol has potential antitumor effects in human CML
and to determine whether the modulation of the PI3K/Akt/mammalian
target of rapamycin (mTOR) signaling pathway by resveratrol is
crucial for its anticancer effects in the human CML K562 cell
line.
Materials and methods
Reagents and antibodies
Resveratrol was purchased from Sigma-Aldrich (St.
Louis, MO, USA) and dissolved in dimethylsulfoxide (Sigma-Aldrich)
as a stock solution of 100 mM. Resveratrol was further diluted in
RPMI-1640 medium (Gibco, Big Cabin, OK, USA) plus 10% fetal bovine
serum (FBS; Gibco) to the appropriate final concentrations. The
primary polyclonal rabbit anti-human antibodies, anti-PI3K,
-phosphorylated (p)-PI3K (Tyr458), -Akt, -p-Akt (Ser473), -mTOR,
-p-mTOR (Ser2448), -p70S6K, -p-p70S6K (Thr389), -4EBP1, -p-4EBP1
(Ser65), -cyclin D1, -procaspase-3, -cleaved caspase-3 and-β-actin,
were obtained from Cell Signaling Technology, Inc. (Beverly, MA,
USA), and the secondary horseradish peroxidase (HRP)-labeled mouse
anti-rabbit IgG polyclonal antibodies for western blot analysis
were provided by Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd. (Beijing, China). Annexin V-fluorescein isothiocyanate (FITC)
and propidium iodide (PI) were purchased from BD Biosciences (Palo
Alto, CA, USA), LY294002 and SH-6 were provided by Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA), and rapamycin (RAPA) was
purchased from the North China Pharmaceutical Group Corporation
(Shijiazhuang, China).
Cell culture
The human CML K562 cell line was purchased from the
Peking Union Medical College Cell Library (Beijing, China). The
cells were cultured in RPMI-1640 medium supplemented with 100 U/ml
of penicillin, 100 μg streptomycin (both Gibco) and 10% FBS at 37°C
in a humidified atmosphere containing 5% CO2.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was determined using
water-soluble tetrazolium salt-8 dye (Sigma-Aldrich) according to
the manufacturer’s instructions. Briefly, the cells were suspended
in RPMI-1640 medium containing 10% FBS and seeded at a density of
5×103 cells/well in 96-well plates. Resveratrol was then
added to the medium at various concentrations of up to 60 μM for
different time durations. Next, the CCK-8 solution (10 μl;
Sigma-Aldrich) was added to each well and further incubated at 37°C
for 3 h. The absorbance was determined at a wavelength of 450 nm
using a microplate reader (Bio-Rad, Hercules, CA, USA). In total,
three duplicate wells were set up for each experimental
condition.
Apoptosis analysis
A total of 1×106 cells/well were treated
with resveratrol for 24 h and double-staining with Annexin V-FITC
(1 μl) and PI (1 μg) was performed. The cells were then washed with
phosphate-buffered saline (PBS; Beyotime, Haikou, China) and
analyzed by flow cytometry (FACS sort; BD Biosciences).
Western blot analysis
Western blot analysis was performed on whole cell
extracts obtained by the direct dissolution of cells using a whole
cell protein extract reagent (M-PER; Pierce Biotechnology, Inc.,
Rockford, IL, USA) according to the manufacturer’s instructions.
Protein concentrations were then determined using a bicinchoninic
acid protein assay kit and bovine serum albumin [both Sangon
Biotech (Shanghai) Co,. Ltd., Shanghai, China] was used as a
control. Next, the proteins (40 μg/lane) were separated on 12%
SDS-PAGE gels (Beyotime) and transferred onto polyvinylidene
difluoride membranes (Bio-Rad). The membranes were then blocked
with 5% non-fat milk in phospshate-buffered saline with Tween
[PBST; 0.2% Tween-20 in PBS (pH 7.6); Beyotime] and incubated with
the primary antibodies (1:1,000) for 18–24 h at 4°C. The membranes
were subsequently incubated with the secondary antibodies
conjugated to HRP (1:5,000) for 1 h at 37°C. Finally, the protein
bands were visualized using an enhanced chemiluminescence western
blot detection kit (Pierce Biotechnology, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using SPSS 16.0 software (SPSS, Inc., Chicago,
IL, USA). Significant differences were determined using one-way
analysis of variance or a two-tailed Student’s t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
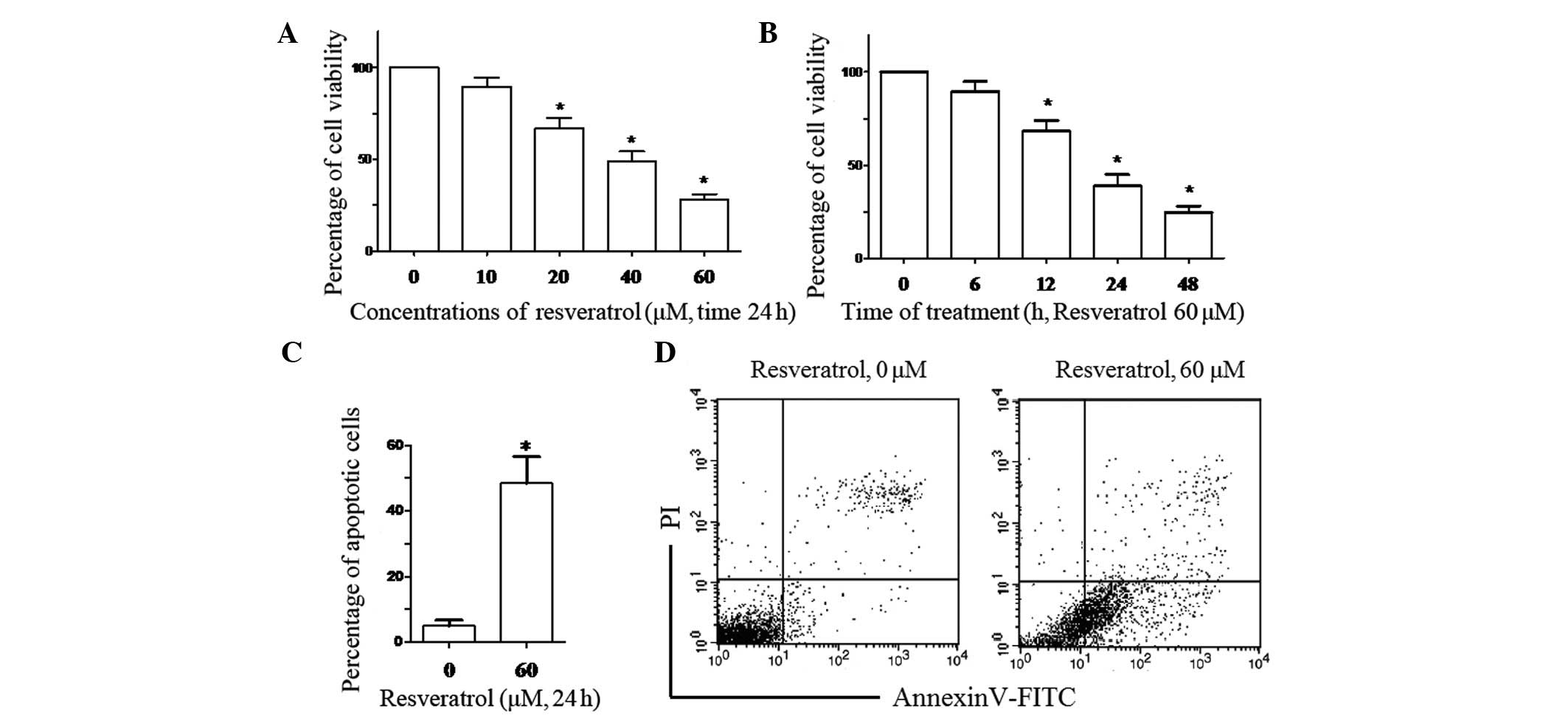
Resveratrol inhibits the proliferation
and induces the apoptosis of K562 cells
To investigate the effect of resveratrol on the
proliferation of K562 cells, the cells were treated with
resveratrol at serial concentrations and its ability to inhibit
proliferation was measured using a CCK-8 assay. The results showed
that resveratrol may inhibit the proliferation of K562 cells in a
dose- and time-dependent manner (Fig.
1A and B). To further investigate whether resveratrol can
induce the apoptosis of K562 cells, the cells were treated with 60
μM of resveratrol for 24 h and the apoptotic rate of the K562 cells
was detected using Annexin V-FITC/PI staining (Fig. 1C and D). The results suggested that
resveratrol inhibits cell proliferation and induces apoptosis in
K562 human CML cells.
Resveratrol blocks PI3K/Akt
phosphorylation in K562 cells
The PI3K/Akt signaling pathway is important in cell
proliferation, differentiation and survival and resveratrol has
been demonstrated to inhibit proliferation and induce apoptosis in
various types of cancer cells (27). In the present study, to investigate
whether PI3K/Akt phosphorylation is responsible for
resveratrol-induced inhibition of proliferation, the K562 cells
were treated with various concentrations of resveratrol for 24 h.
The levels of p-PI3K and p-Akt were then detected and the results
demonstrated that resveratrol significantly blocks the constitutive
phosphorylation of PI3K at Tyr458 and Akt at Ser473 in a
dose-dependent manner (Fig. 2A).
The K562 cells were then treated with 60 μM of resveratrol for 0,
6, 12 and 24 h, and the levels of p-PI3K and p-Akt were observed to
decrease following 24 h of treatment (Fig. 2B). The K562 cells were also treated
with the selective PI3K inhibitor, LY294002, or the selective Akt
inhibitor, SH-6. The selective inhibitors were observed to further
inhibit the phosphorylation of PI3K and Akt in K562 cells induced
by resveratrol, respectively (Fig.
2C). These results indicated that the antiproliferative effects
of resveratrol in K562 cells are associated with blocking the
activation of the PI3K/Akt signaling cascade.
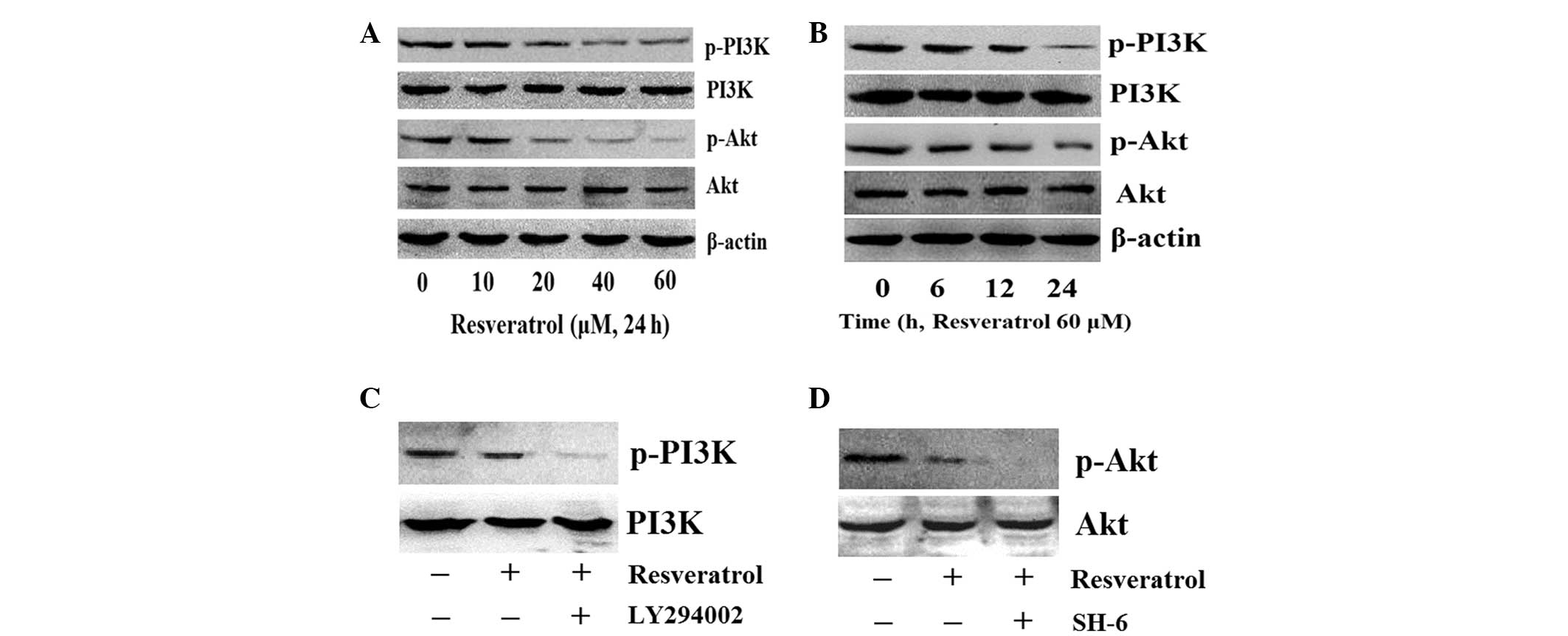 | Figure 2Resveratrol reduces the
phosphorylation of PI3K and Akt in K562 cells. Levels of p-PI3K and
p-Akt in K562 cells treated with (A) various concentrations of
resveratrol for 24 h and (B) 60 μM of resveratrol for different
time periods, as determined by western blot analysis. (C) The
selective inhibitor, LY294002, enhanced the resveratrol effect on
reducing the phosphorylation of PI3K and (D) the selective
inhibitor, SH-6, enhanced the resveratrol effect on reducing the
phosphorylation of Akt. β-actin was used as a loading control for
PI3K and Akt, while PI3K and Akt were used as loading controls for
p-PI3K and Akt, respectively. PI3K, phosphatidylinositide 3-kinase;
p-PI3K, phosphorylated-PI3K; Akt, protein kinase B; p-Akt,
phosphorylated-Akt. |
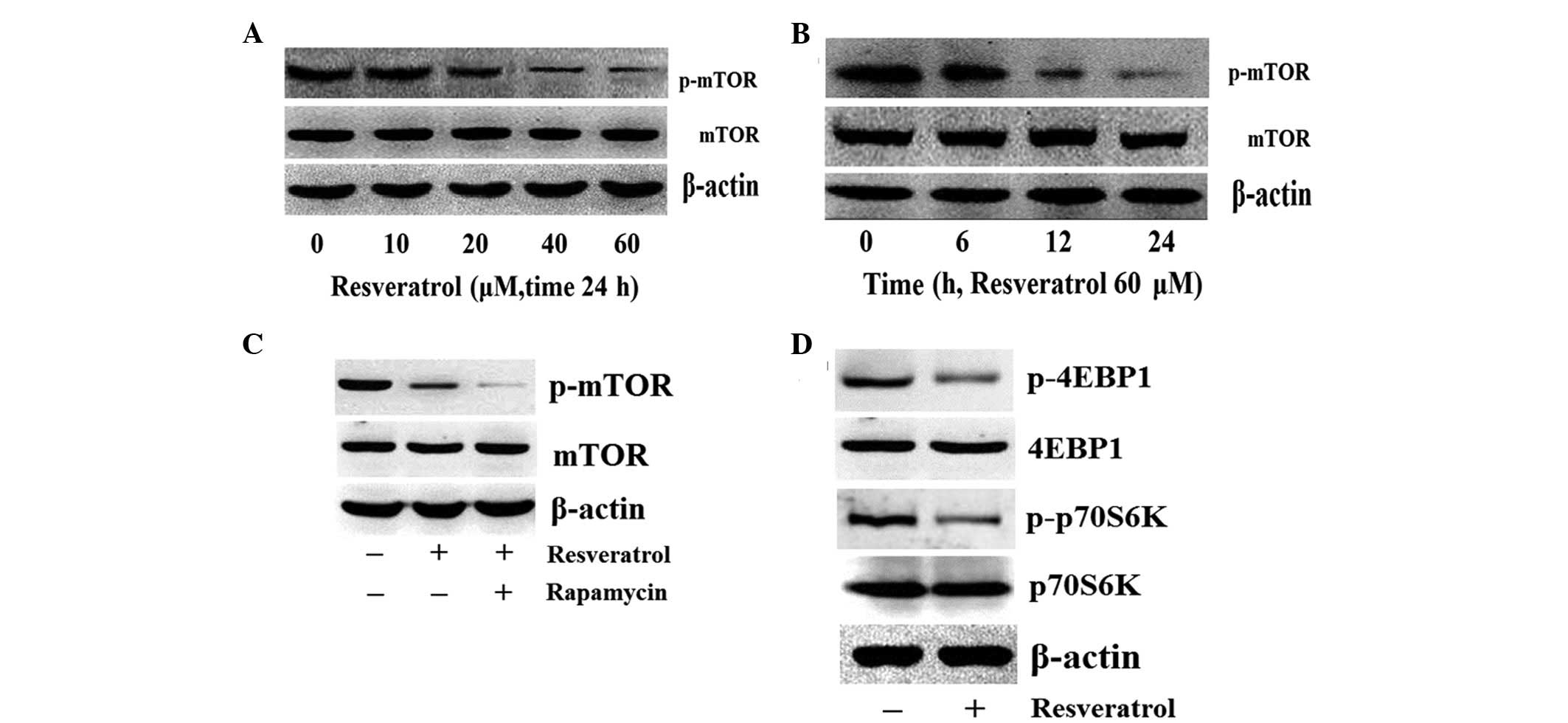
Resveratrol suppresses the
phosphorylation of mTOR in K562 cells
The mTOR protein is one of the downstream targets of
PI3K/Akt. To investigate the effect of resveratrol on mTOR
phosphorylation, the K562 cells were treated with a serial
concentration of resveratrol for 24 h and resveratrol was found to
reduce the levels of p-mTOR (Ser2448) in a dose-dependent manner
(Fig. 3A). The K562 cells were then
treated with 60 μM of resveratrol for 0, 6, 12 and 24 h and the
level of p-mTOR was observed to decrease following resveratrol
treatment for 12 h (Fig. 3B), and
was further reduced following rapamycin treatment (Fig. 3C). These results indicated that
resveratrol inhibits the PI3K/Akt/mTOR signaling pathway and that
the specific inhibitor of mTOR enhances the effect induced by
resveratrol.
Next, the effect of resveratrol on the downstream
targets of mTOR was investigated. The K562 cells were treated with
0 or 60 μM of resveratrol for 24 h and the downstream targets were
detected using western blot analysis. The results revealed that
treatment with resveratrol reduced the phosphorylation of the
downstream targets, p70S6K and 4EBP1; however, total p706SK and
4EBP1 levels were not affected by resveratrol treatment (Fig. 3D), which indicated that resveratrol
may downregulate the Akt/mTOR signaling pathway.
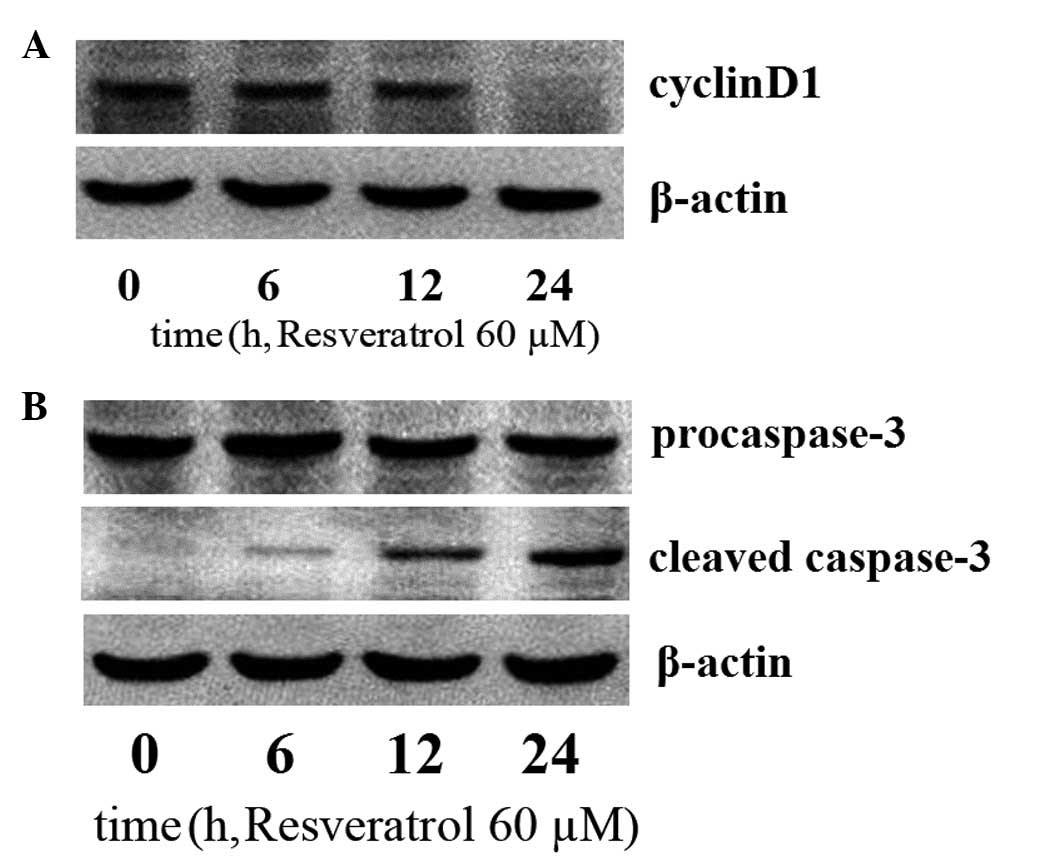
Resveratrol suppresses cyclin D1 and
enhances caspase-3 expression
Constitutive activation of the PI3K/Akt/mTOR
signaling pathway has been demonstrated to exhibit a critical
function in the cell cycle and antiapoptosis by affecting several
regulatory molecules, including the upregulation of cyclin D1 and
downregulation of caspase-3 expression (28). In the current study, to investigate
whether resveratrol downregulates cyclin D1 expression and
upregulates caspase-3 expression, the K562 cells were treated with
60 μM of resveratrol. A marked decline in cyclin D1 levels and an
increase in caspase-3 levels in the resveratrol-treated cells was
observed (Fig. 4).
Discussion
Although resveratrol has been reported to have a
wide range of potential targets during the inhibition of
proliferation and induction of apoptosis in a variety tumor cell
types (28), the underlying
molecular mechanisms of its anticancer effects are not well
understood, particularly in human leukemia which often proves
difficult to treat as multiple signaling pathways may be involved
(17). Contradictory results have
previously been reported with regard to the inhibition of
proliferation and induction of apoptosis by resveratrol. Certain
studies have reported that resveratrol treatment induces apoptosis
in various tumor cells (29–33).
However, other studies have reported that resveratrol induces
differentiation, but not apoptosis, in certain types of cancer
cells (34–36). In the current study, one of the
possible mechanisms of resveratrol-induced apoptosis of human CML
K562 cells, was investigated.
There is much evidence to support the critical
function of the PI3K/Akt/mTOR signaling pathway in cancer
proliferation, tumor genesis and metastasis (37,38),
and numerous studies have demonstrated that PI3K/Akt/mTOR activity
is increased in a variety of tumor cell lines (39–42),
including leukemia (43). In
addition, it has been demonstrated that the inhibition of PI3K/Akt
activation or expression may inhibit cancer cell proliferation and
invasion. This signaling pathway involves three key driving
proteins, PI3K, Akt and mTOR. The PI3K proteins are a family of
lipid kinases, which include PI3K1, PI3K2 and PI3K3. Akt belongs to
a family of serine/threonine protein kinases, which can be
activated in a PI3K-dependent manner following stimulation by
growth factors, stress or protein phosphatases. Furthermore, mTOR
is one of the main downstream target molecules of PI3K/Akt, which
performs a crucial function in the regulation of proliferation,
differentiation and survival of cells. It has been demonstrated
that the activation of the PI3K/Akt/mTOR signaling pathway
increases the phosphorylation of p70S6K and 4EBP1, and that
p-p70S6K subsequently induces the phosphorylation of the ribosomal
protein S6.
The effect of resveratrol on the PI3K/Akt/mTOR
pathway in CML cells has not been widely investigated. The results
of the current study revealed that resveratrol inhibits the
proliferation and induces the apoptosis of K562 cells in a
dose-dependent manner. In addition, resveratrol downregulates the
PI3K/Akt/mTOR signaling pathway and the inhibitors of the proteins
involved in this signaling pathway enhance the inhibitory effects
induced by resveratrol in K562 cells. Furthermore, resveratrol
inhibits proliferation and induces apoptosis in human leukemia K562
cells, which is considered to occur via the deregulation of the
cell cycle machinery and activation of mitochondria-mediated
caspase-3 dependent apoptotic signaling cascades. These results
indicated that resveratrol is an attractive candidate for use in
leukemia therapy. Therefore, further understanding of the
underlying molecular signaling mechanisms of the inhibition of
proliferation and induction of CML cell apoptosis induced by
resveratrol may aid the development of additional therapeutic
targets for the treatment of CML.
Resveratrol inhibits the proliferation and induces
the apoptosis of cells mediated by the regulation of cell cycle,
proapoptotic and antiapoptotic proteins, such as cyclin D1 and
caspase-3. Gao et al (44)
demonstrated that the PI3K/Akt/mTOR signaling pathway exhibits a
key function in the cell cycle progression in human prostate cancer
cells. In addition, resveratrol has been demonstrated to inhibit
the proliferation and induce the apoptosis of cells by
downregulation of the nuclear factor-κβ signaling pathway (45).
In conclusion, the present study demonstrated that
resveratrol downregulates and inactivates the PI3K/Akt/mTOR
signaling pathway, which may exhibit a critical function in
resveratrol-induced apoptosis in K562 cells and, therefore, the
PI3K/Akt/mTOR signaling pathway may present a potential therapeutic
target for the treatment of human CML.
Acknowledgements
The present study was supported by grants from the
Tianjin Science and Technology Committee (grant no. 11JCZDJC18600)
and the Tianjin Municipal Health Bureau (grant no. 13KG106).
References
|
1
|
Chandra HS, Heisterkamp NC, Hungerford A,
Morrissette JJ, Nowell PC, Rowley JD and Testa JR: Philadelphia
Chromosome Symposium: commemoration of the 50th anniversary of the
discovery of the Ph chromosome. Cancer Genet. 204:171–179. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deininger MW, Goldman JM and Melo JV: The
molecular biology of chronic myeloid leukemia. Blood. 96:3343–3356.
2000.PubMed/NCBI
|
|
3
|
Hantschel O and Superti-Furga G:
Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat Rev Mol
Cell Biol. 5:33–44. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sattler M and Griffin JD: Molecular
mechanisms of transformation by the BCR-ABL oncogene. Semin
Hematol. 40(Suppl 2): S4–S10. 2003. View Article : Google Scholar
|
|
5
|
Barrett D, Brown VI, Grupp SA and Teachey
DT: Targeting the PI3K/AKT/mTOR signaling axis in children with
hematologic malignancies. Paediatr Drugs. 14:299–316.
2012.PubMed/NCBI
|
|
6
|
Horita M, Andreu EJ, Benito A, Arbona C,
Sanz C, Benet I, et al: Blockade of the Bcr-Abl kinase activity
induces apoptosis of chronic myelogenous leukemia cells by
suppressing signal transducer and activator of transcription
5-dependent expression of Bcl-xL. J Exp Med. 191:977–984. 2000.
View Article : Google Scholar
|
|
7
|
Pene-Dumitrescu T and Smithgall TE:
Expression of a Src family kinase in chronic myelogenous leukemia
cells induces resistance to imatinib in a kinase-dependent manner.
J Biol Chem. 285:21446–21457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baum KJ and Ren R: Effect of Ras
inhibition in hematopoiesis and BCR/ABL leukemogenesis. J Hematol
Oncol. 1:52008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Notari M, Neviani P, Santhanam R, Blaser
BW, Chang JS, Galietta A, et al: A MAPK/HNRPK pathway controls
BCR/ABL oncogenic potential by regulating MYC mRNA translation.
Blood. 107:2507–2516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baran Y and Saydam G: Cumulative clinical
experience from a decade of use: imatinib as first-line treatment
of chronic myeloid leukemia. J Blood Med. 3:139–150. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mauro MJ and Deininger MW: Chronic myeloid
leukemia in 2006: a perspective. Haematologica.
91:1522006.PubMed/NCBI
|
|
12
|
Shah NP and Sawyers CL: Mechanisms of
resistance to STI571 in Philadelphia chromosome-associated
leukemias. Oncogene. 22:7389–7395. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sawyers CL, Hochhaus A, Feldman E, Goldman
JM, Miller CB, Ottmann OG, et al: Imatinib induces hematologic and
cytogenetic responses in patients with chronic myelogenous leukemia
in myeloid blast crisis: results of a phase II study. Blood.
99:3530–3539. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soleas GJ, Diamandis EP and Goldberg DM:
Resveratrol: a molecule whose time has come? And gone? Clin
Biochem. 30:91–113. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jang DS, Kang BS, Ryu SY, Chang IM, Min KR
and Kim Y: Inhibitory effects of resveratrol analogs on unopsonized
zymosan-induced oxygen radical production. Biochem Pharmacol.
57:705–712. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inoue H, Jiang XF, Katayama T, Osada S,
Umesono K and Namura S: Brain protection by resveratrol and
fenofibrate against stroke requires peroxisome
proliferator-activated receptor alpha in mice. Neurosci Lett.
352:203–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu W, Fu YC and Wang W: Cellular and
molecular effects of resveratrol in health and disease. J Cell
Biochem. 113:752–759. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Delmas D, Solary E and Latruffe N:
Resveratrol, a phytochemical inducer of multiple cell death
pathways: apoptosis, autophagy and mitotic catastrophe. Curr Med
Chem. 18:1100–1121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tinhofer I, Bernhard D, Senfter M, Anether
G, Loeffler M, Kroemer G, et al: Resveratrol, a tumor-suppressive
compound from grapes, induces apoptosis via a novel mitochondrial
pathway controlled by Bcl-2. FASEB J. 15:1613–1615. 2001.PubMed/NCBI
|
|
20
|
Mgbonyebi OP, Russo J and Russo IH:
Antiproliferative effect of synthetic resveratrol on human breast
epithelial cells. Int J Oncol. 12:865–869. 1998.PubMed/NCBI
|
|
21
|
Ahmad N, Adhami VM, Afaq F, Feyes DK and
Mukhtar H: Resveratrol causes WAF-1/p21-mediated G(1)-phase arrest
of cell cycle and induction of apoptosis in human epidermoid
carcinoma A431 cells. Clin Cancer Res. 7:1466–1473. 2001.PubMed/NCBI
|
|
22
|
Dörrie J, Gerauer H, Wachter Y and Zunino
SJ: Resveratrol induces extensive apoptosis by depolarizing
mitochondrial membranes and activating caspase-9 in acute
lymphoblastic leukemia cells. Cancer Res. 61:4731–4739. 2001.
|
|
23
|
Benitez DA, Pozo-Guisado E,
Alvarez-Barrientos A, Fernandez-Salguero PM and Castellón EA:
Mechanisms involved in resveratrol-induced apoptosis and cell cycle
arrest in prostate cancer-derived cell lines. J Androl. 28:282–293.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fulda S and Debatin KM:
Resveratrol-mediated sensitisation to TRAIL-induced apoptosis
depends on death receptor and mitochondrial signalling. Eur J
Cancer. 41:786–798. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shankar S, Siddiqui I and Srivastava RK:
Molecular mechanisms of resveratrol
(3,4,5-trihydroxy-trans-stilbene) and its interaction with
TNF-related apoptosis inducing ligand (TRAIL) in
androgen-insensitive prostate cancer cells. Mol Cell Biochem.
304:273–285. 2007. View Article : Google Scholar
|
|
26
|
Shankar S, Singh G and Srivastava RK:
Chemoprevention by resveratrol: molecular mechanisms and
therapeutic potential. Front Biosci. 12:4839–4854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang H, Zhang L, Kuo J, Kuo K, Gautam SC,
Croc L, et al: Resveratrol-induced apoptotic death in human U251
glioma cells. Mol Cancer Ther. 4:554–561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang H, Shang X, Wu H, Gautam SC,
Al-Holou S, Li C, et al: Resveratrol downregulates PI3K/Akt/mTOR
signaling pathways in human U251 glioma cells. J Exp Ther Oncol.
8:25–33. 2009.PubMed/NCBI
|
|
29
|
Yang CS, Landau JM, Huang MT and Newmark
HL: Inhibition of carcinogenesis by dietary polyphenolic compounds.
Annu Rev Nutr. 21:381–406. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Quoc Trung L, Espinoza JL, Takami A and
Nakao S: Resveratrol induces cell cycle arrest and apoptosis in
malignant NK cells via JAK2/STAT3 pathway inhibition. PLoS One.
8:e551832013.PubMed/NCBI
|
|
31
|
Miki H, Uehara N, Kimura A, Sasaki T, Yuri
T, Yoshizawa K and Tsubura A: Resveratrol induces apoptosis via
ROS-triggered autophagy in human colon cancer cells. Int J Oncol.
40:1020–1028. 2012.PubMed/NCBI
|
|
32
|
Hussain AR, Uddin S, Bu R, Khan OS, Ahmed
SO, Ahmed M and Al-Kuraya KS: Resveratrol suppresses constitutive
activation of AKT via generation of ROS and induces apoptosis in
diffuse large B cell lymphoma cell lines. PLoS One. 6:e247032011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Truong M, Cook MR, Pinchot SN,
Kunnimalaiyaan M and Chen H: Resveratrol induces Notch2-mediated
apoptosis and suppression of neuroendocrine markers in medullary
thyroid cancer. Ann Surg Oncol. 18:1506–1511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen Q, Ganapathy S, Singh KP, Shankar S
and Srivastava RK: Resveratrol induces growth arrest and apoptosis
through activation of FOXO transcription factors in prostate cancer
cells. PLoS One. 5:e152882010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leong CW, Wong CH, Lao SC, Leong EC, Lao
IF, Law PT, et al: Effect of resveratrol on proliferation and
differentiation of embryonic cardiomyoblasts. Biochem Biophys Res
Commun. 360:173–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kaminski J, Lançon A, Aires V, Limagne E,
Tili E, Michaille JJ and Latruffe N: Resveratrol initiates
differentiation of mouse skeletal muscle-derived C2C12 myoblasts.
Biochem Pharmacol. 84:1251–1259. 2012. View Article : Google Scholar
|
|
37
|
Yu XM, Jaskula-Sztul R, Ahmed K, Harrison
AD, Kunnimalaiyaan M and Chen H: Resveratrol induces
differentiation markers expression in anaplastic thyroid carcinoma
via activation of Notch1 signaling and suppresses cell growth. Mol
Cancer Ther. 12:1276–1287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yap TA, Garrett MD, Walton MI, Raynaud F,
de Bono JS and Workman P: Targeting the PI3K-AKT-mTOR pathway:
progress, pitfalls, and promises. Curr Opin Pharmacol. 8:393–412.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wetzker R and Rommel C: Phosphoinositide
3-kinases as targets for therapeutic intervention. Curr Pharm Des.
10:1915–1922. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004.PubMed/NCBI
|
|
42
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: size matters. Oncogene.
22:8983–8998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Morgensztern D and McLeod HL:
PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer
Drugs. 16:797–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Martelli AM, Evangelisti C, Chiarini F,
Grimaldi C, Manzoli L and McCubrey JA: Targeting the PI3K/AKT/mTOR
signaling network in acute myelogenous leukemia. Expert Opin
Investig Drugs. 18:1333–1349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gao N, Zhang Z, Jiang BH and Shi X: Role
of PI3K/AKT/mTOR signaling in the cell cycle progression of human
prostate cancer. Biochem Biophys Res Commun. 310:1124–1132. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun C, Hu Y, Liu X, Wu T, Wang Y, He W and
Wei W: Resveratrol downregulates the constitutional activation of
nuclear factor-kappaB in multiple myeloma cells, leading to
suppression of proliferation and invasion, arrest of cell cycle,
and induction of apoptosis. Cancer Genet Cytogenet. 165:9–19. 2006.
View Article : Google Scholar
|