Introduction
Breast cancer is the most common malignant tumor in
females worldwide and is the second leading cause of cancer-related
mortality in the USA (1,2). There are multiple risk factors for
breast cancer, including genetic, reproductive/hormonal,
environmental and other lifestyle factors (3). Epidemiological studies indicate that
alcohol consumption increases the risk of breast cancer in a
dose-dependent manner (4–9). In addition, alcohol enhances the
growth of existing breast tumors and promotes metastasis (10–12).
However, the mechanisms underlying these effects remain
unclear.
Tumor growth and metastasis are dependent on
angiogenesis. Vascular endothelial growth factor (VEGF) is one of
the most important known factors which stimulates vasculogenesis
and angiogenesis. VEGF has an important role in tumor angiogenesis
via promoting proliferation, migration, stabilization and survival
of endothelial cells as well as tumor cells (13,14).
It has been demonstrated that the VEGF expression in breast cancer
tissues is significantly higher than that in the adjacent normal
tissues (15). We previously
demonstrated that alcohol promoted angiogenesis and induced the
expression of monocyte chemotactic protein-1 (MCP-1). However,
MCP-1 only partially mediated the effect of alcohol, that is,
blocking MCP-1 signaling only partially reversed the effect of
alcohol on angiogenesis and mammary tumor growth (16). Alcohol-induced tumor promotion may
be mediated by multiple factors and signaling pathways. Considering
the important role of VEGF in tumor angiogenesis and progression,
we hypothesized that VEGF signaling is involved in alcohol
promotion of tumor angiogenesis and mammary tumor growth. In the
present study, we utilized in vitro and in vivo model
systems to address this hypothesis.
Materials and methods
Materials
Ethanol, fibrinogen, aprotinin and thrombin were
purchased from Sigma-Aldrich (St. Louis, MO, USA), and SU5416 was
purchased from Calbiochem (San Diego, CA, USA). Rat anti-mouse CD31
monoclonal antibody was obtained from BD Biosciences (San Diego,
CA, US), while anti-VEGF antibody was obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Cytodex 3 beads were
obtained from Amersham Pharmacia Biotech Inc. (Piscataway, NJ,
USA).
Cell culture
Mouse mammary adenocarcinoma cell line (E0771) was
provided by Dr. Enrico Mihich (Roswell Park Cancer Institute,
Buffalo, NY, USA) and maintained in DMEM (Gibco-BRL, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan,
UT, USA), penicillin (100 U/ml;), streptomycin (100 U/ml) and
amphotericin B (0.25 mg/ml) at 37°C with 5% CO2 (GBCBIO™
Technologies, Guangzhou, China). MDA-MB231 breast cancer cells and
SVEC4-10EE2 murine endothelial cells (both American Type Culture
Collection, Manassas, VA, USA) were grown in DMEM medium containing
10% FBS and 100 U/ml penicillin and streptomycin at 37°C with 5%
CO2. Human umbilical vein endothelial cells (HUVECs;
American Type Culture Collection) were isolated from fresh human
placentas with type I collagenase (1 mg/ml) and grown in Clonetics
Endothelial Cell Growth Medium-2 (EGM-2; Lonza, Walkersville, MD,
USA). HUVECs were used between passages 3 and 10.
Animals and alcohol exposure
Female C57BL/6 mice (5–6 weeks old) were purchased
from the Experimental Animal Center of Anhui Province (Hefei,
China). All procedures were conducted according to the Guidelines
of the Animal Welfare Act approved by the Institutional Animal Care
and Use Committee of the Anhui Medical University (Hefei, China).
The mice were placed on a standard chow diet and were allowed to
acclimatize for one week prior to starting the study. The paradigm
of alcohol exposure has been previously described (16). Briefly, mice were divided into two
groups and fed with standard chow ad libitum. In the control
group, the mice were provided with regular drinking water (n=20).
In the alcohol-exposed group (n=18), the mice received repeated
cycles of chronic intermittent ethanol (2% v/v) exposure (12 h/day,
8:00pm–8:00am) for three weeks. The consumption of regular water or
alcohol-containing water in the two groups was monitored daily and
no significant difference in the liquid intake between the control
and ethanol group was identified. The average consumption of water
or ethanol-containing water for each mouse was ~4 ml/day. The study
was approved by the ethics committee of Anhui Medical
University.
Mouse tumor xenograft model
E0771 mouse breast cancer cells are syngeneic to
C57BL/6 mice. E0771 cells, implanted subcutaneously in C57BL/6
mice, are immunosuppressive and highly aggressive, invading locally
into dermal layers and the peritoneum as well as distantly to the
lung, and have characteristics which reflect the human disease
(16,17). These cells have been extensively
used for mouse tumor xenograft models. Briefly, three days
following alcohol exposure, E0771 cells [2.5×105 in 100
μl phosphate-buffered saline (PBS)] were injected into the
secondary mammary fat pad of mice using a 23-gauge needle.
Following implantation, the mice were continually provided normal
drinking water or water containing 2% ethanol. The tumor size was
monitored every three days. Two perpendicular dimensions of tumors
were measured using a dial caliper and the tumor volume was
calculated based on the formula: V=0.24a × b2 (a, the
longest dimension and b, the shortest dimension). Following
implantation, 24 days later the mice were sacrificed and the tumors
were harvested for further detection (16).
To investigate the role of VEGF in alcohol-induced
tumor promotion, an inhibitor of the VEGF receptor, Z-3-[(2,
4-dimethylpyrrol-5-yl) methylidenyl]-2-indolinone (SU5416;
Sigma-Aldrich) was injected into the mice. One day following
alcohol exposure, animals received intraperitoneal injection of
SU5416 [10 mg/kg in 100 μl, Su5416 was dissolved in ethanol and
diluted in 5% Tween-80 (Amresco LLC, Solon, OH, USA) and 5% PEG-400
(Shandong Lunan Chemical Technology Co., Ltd, Tengzhou City, China)
prior to injection] every three days. The dosage has been
previously demonstrated to effectively inhibit VEGF signaling in
mice (18). The tumor size was
monitored every three days.
Immunohistochemistry (IHC) and evaluation
of average microvessel density (AMVD)
The procedure for immunohistochemical analysis was
performed as described previously (19). Tumor tissues were sectioned at the
thickness of 4 μm. The sections were incubated in methanol (0.3%
H2O2) for 30 min and treated with 0.1% Triton
X-100 (Amresco LLC) for 10 min. The sections were washed with PBS
three times and blocked with 1% bovine serum albumin (BSA; Amresco
LLC) and 0.01% Triton X-100 for 1 h at room temperature. The
sections were incubated with anti-VEGF antibody (1:100) or
anti-CD31 (1:50) antibody overnight at 4°C. Negative controls were
performed by staining with isotype-matched IgG or PBS. Following
rinsing in PBS, the sections were incubated with goat anti-rat
biotinylated monoclonal secondary antibodies (Vector Laboratories
Inc., Burlingame, CA, USA) for 1 h at room temperature. The
sections were washed three times with PBS, then incubated in
avidin-biotin-peroxidase complex (1:100 in PBS; Vector Laboratories
Inc., Burlingame, CA, USA) for 1 h and developed in 0.05%
3,3′-diaminobenzidine (Sigma-Aldrich) containing 0.003%
H2O2 in PBS. The tumor microvessels that were
visualized by CD31 IHC were examined under a microscope (Olympus
CX31; Olympus Corporation, Tokyo, Japan). Ten random fields were
quantified and the AMVD was expressed as the number of
microvessels/mm2 area.
Three-dimensional (3D) endothelial tumor
cell co-culture system
To investigate the effect of alcohol on tumor
angiogenesis, a 3D tumor/endothelial cell co-culture was performed
as described previously (16,20).
In this model, endothelial cells were cultured on a fibrin gel bead
system alone or with tumor cells to form a 3D capillary tube-like
network. Briefly, HUVECs or SVEC4 cells were trypsinized and the
cells (1×106) were mixed with cytodex beads
(3×103) in a 4 ml medium (EGM-2 for HUVECs and DMEM for
SVECs). The mixtures were incubated at 5% CO2 at 37°C
and gently shaken every 20 min for 4 h. Following this, 4 ml of
fresh medium was added to the tubes and the incubation continued
for another 4 h. The mixtures of cells/cytodex beads were
transferred to 25 ml tissue culture flasks and incubated overnight
to allow the bead-non-attached cells to adhere to the flask.
Following incubation, the mixtures of cells/cytodex beads were
transferred to a 50 ml centrifuge tube and washed three times with
20 ml of Ca2+- and Mg2+-free PBS. Then, the
beads with adherent cells were resuspended in medium [2.5 mg/ml
fibrinogen, 0.15 U/ml aprotinin (Amresco LLC), pH 7.4]. Following
this, 0.5 ml of the fibrinogen/bead suspension was added to 24-well
cell culture plates which were pre-coated with 0.625 U of thrombin
(Sigma-Aldrich). The fibrinogen/bead solution was allowed to
coagulate for 5 min at room temperature and was then cultured in 5%
CO2 at 37°C for 20 min. The resulting fibrin gels
contained endothelial cells (HUVECs or SVECs) adhering to the
beads. Then, 1 ml of medium (0.15 U/ml aprotinin) was added to each
well to equilibrate with the fibrin clot for 30 min at 37°C and 5%
CO2. The medium was removed and replaced with 1 ml of
fresh medium (0.15 U/ml aprotinin). For co-culture of endothelial
cells/breast tumor cells, E0771 or MDA-MB231 breast cancer cells
(2×104 or 4×104, respectively) were layered
on top of the fibrin gels. The medium was changed every day.
In vitro alcohol exposure
A method utilizing sealed containers was used to
maintain alcohol concentrations in the cell culture system
(21). Briefly, the appropriate
amount of ethanol (using 95% ethanol stock) was added to the
culture medium to reach the desired concentration (0.2%). The cell
culture plates were placed in a sealed plastic container. In each
container, a water bath with 200 ml 0.2% ethanol was deposited in
order to maintain the ethanol concentration. Prior to sealing each
container, CO2 (60 ml) was injected. The containers were
placed in a humidified environment and maintained at 37°C with 5%
CO2. With this method, ethanol concentrations were
maintained constantly over time in a cell culture medium (21).
Immunoblotting
The immunoblotting was performed as previously
described (22). Briefly, aliquots
of the protein samples (30 μg) were separated on an
SDS-polyacrylamide gel (Sigma-Aldrich) and were transferred to
nitrocellulose membranes. The membranes were blocked with 5% BSA
solution [pH 7.4, 0.05% Tween-20 (Amresco LLC) in PBS] for 1 h at
room temperature. Anti-VEGF antibody (1:500) was added to the
membranes (Millipore, Billerica, MA, USA) for 1 h at room
temperature. Membranes were probed with goat anti-rat monoclonal
horseradish peroxidase-conjugated secondary antibody (Amersham Life
Science, Arlington Heights, IL, USA). The signals were detected by
using the enhanced chemiluminescence method (Amersham Life
Sciences) and were exposed to X-ray film for autoradiography. The
membranes were stripped with a stripping buffer for 15 min at room
temperature and immunoblotted with a rabbit anti-mouse actin
monoclonal antibody (Santa Cruz Biotechnology, Inc.). The images
were scanned and the signal intensity was quantified with Image J
software (NIH, Bethesda, MD, USA).
VEGF enzyme-linked immunosorbent assay
(ELISA)
ELISA was performed in 100-μl volumes in triplicate
using commercial kits for VEGF, according to the manufacturer’s
instructions (R&D Systems, Minneapolis, MN, USA). The plates
were read at 450 nm on an ELx800 absorbance microplate reader
(Bio-Tek Instruments Inc., Winooski, VT, USA).
Statistical analysis
The data were analyzed using SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA). All data are represented as the
mean ± SEM. Differences among the treatment groups were examined
using analysis of variance. In the cases where significant
differences were detected, specific post-hoc comparisons between
the treatment groups were examined with Student-Newman-Keuls tests.
In a number of the experiments, the results were analyzed by an
unpaired Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Ethanol induces VEGF expression and tumor
angiogenesis
The effect of alcohol on VEGF expression and tumor
angiogenesis was investigated in a mouse xenograft model of mammary
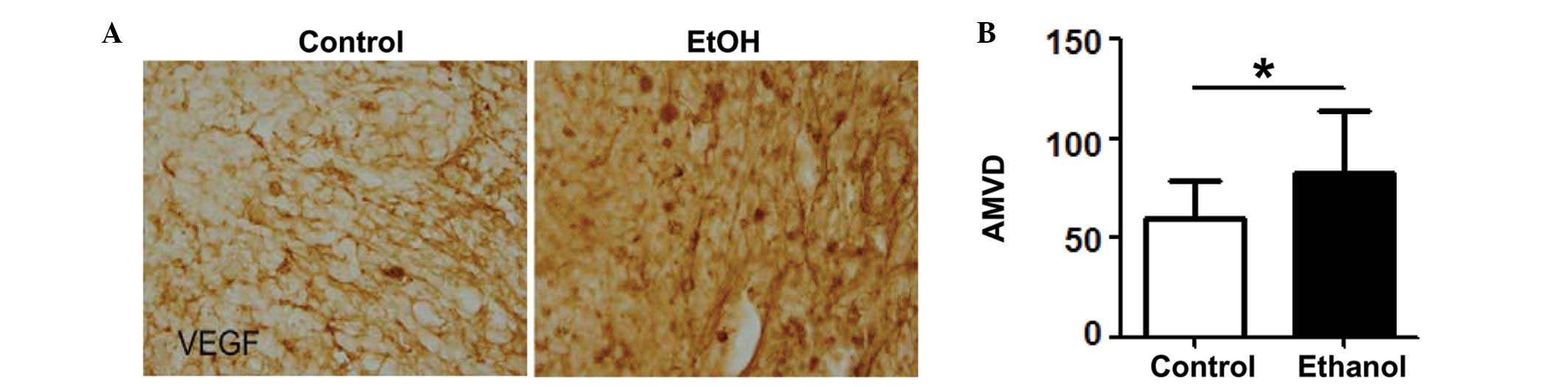
tumors. As demonstrated in Fig. 1A,
the VEGF immunoreactivity in the mammary tumor tissues of mice
exposed to alcohol was higher than that of the control mice. The
AMVD was also examined using CD31 (a marker of endothelial cells)
IHC. The quantification data are presented in Fig. 1B. Alcohol consumption significantly
increased the AMVD in mammary tissues. To confirm alcohol
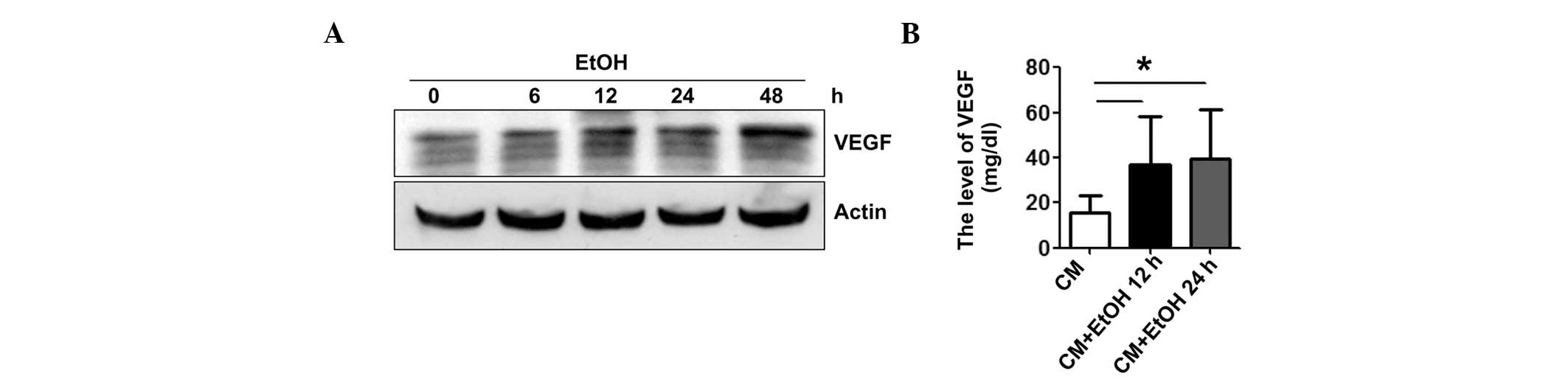
stimulation of VEGF expression, we examined the effect of alcohol
on E0771 mouse breast cancer cells in culture. Alcohol exposure
upregulated VEGF expression in E0771 cells (Fig. 2A) and increased the secretion of
VEGF to the culture medium (Fig.
2B).
Ethanol promotes tumor angiogenesis
We hypothesized that alcohol increased VEGF
production in breast cancer cells and stimulated angiogenesis of
endothelial cells. A previously described 3D angiogenic model was
utilized to test this hypothesis. With this system, we previously
demonstrated that breast cancer cells (E0771 cells or MDA-MB231
cells) significantly increased the vascular sprout formation of
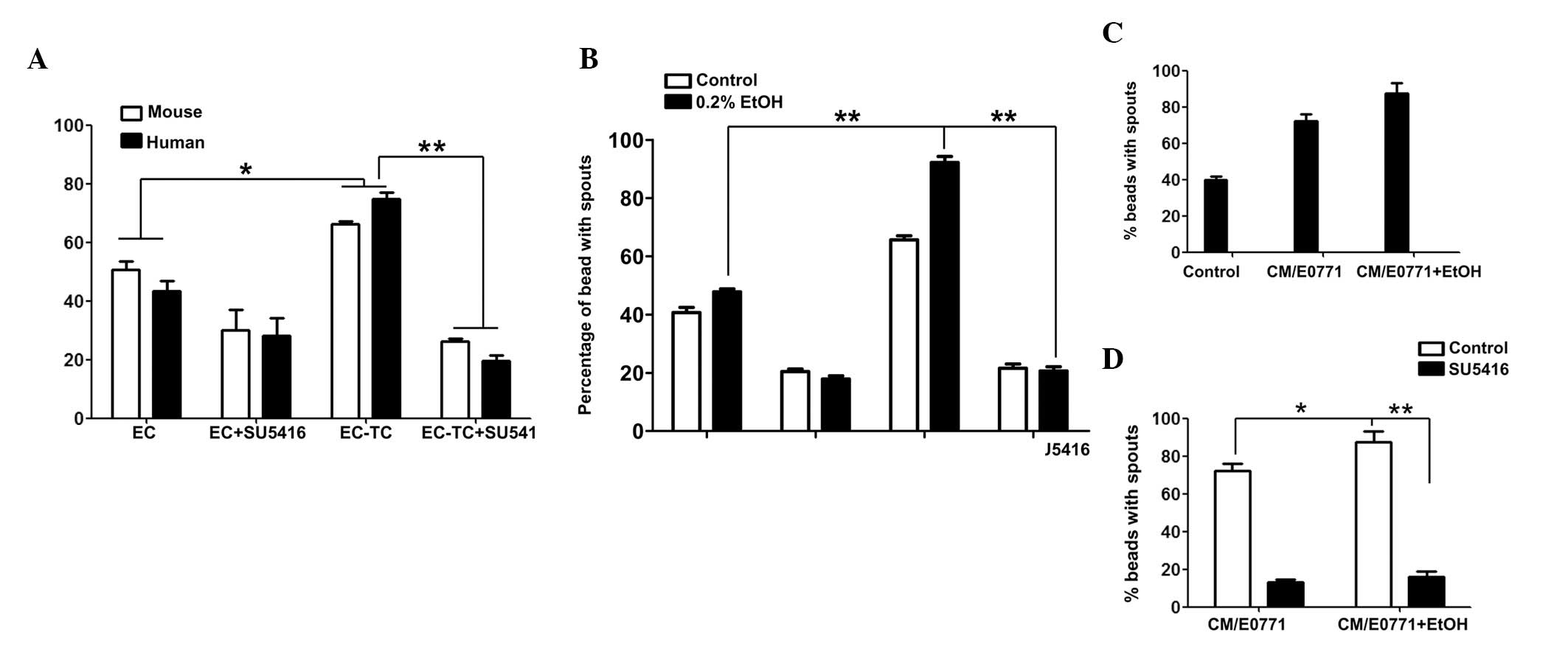
endothelial cells (SVECs or HUVECs) (16). In the present study, VEGF signaling
in tumor/endothelial cell co-culture was blocked using SU5416, an
inhibitor of VEGF receptor 2 (VEGFR2). As demonstrated in Fig. 3A, inclusion of breast cancer cells
(E0771 or MDA-MB231) in this 3D co-culture system increased
endothelial cell sprouting, which was indicative of enhanced
angiogenesis. Treatment with SU5416 completely blocked the
promotion of angiogenesis mediated by breast cancer cells. Alcohol
exposure did not increase endothelial cell sprouting when the
endothelial cells were cultured alone; however, it significantly
increased endothelial cell sprouting in SVEC/E0771 co-culture
(Fig. 3B). More importantly, SU5416
blocked alcohol-stimulated angiogenesis (Fig. 3B). To confirm the role of VEGF in
alcohol-stimulated angiogenesis, the effect of conditioned medium
collected from alcohol-treated E0771 cells on angiogenesis was
examined. As demonstrated in Fig.
3C, E0771 cell-conditioned medium significantly increased
endothelial cell sprouting and alcohol exposure further enhanced
this effect. SU5416 completely blocked alcohol-stimulated
angiogenesis. These results suggested that alcohol-enhanced tumor
angiogenesis was mediated by VEGF signaling.
SU5416 inhibits ethanol-promoted tumor
growth in mouse tumor xenograft model
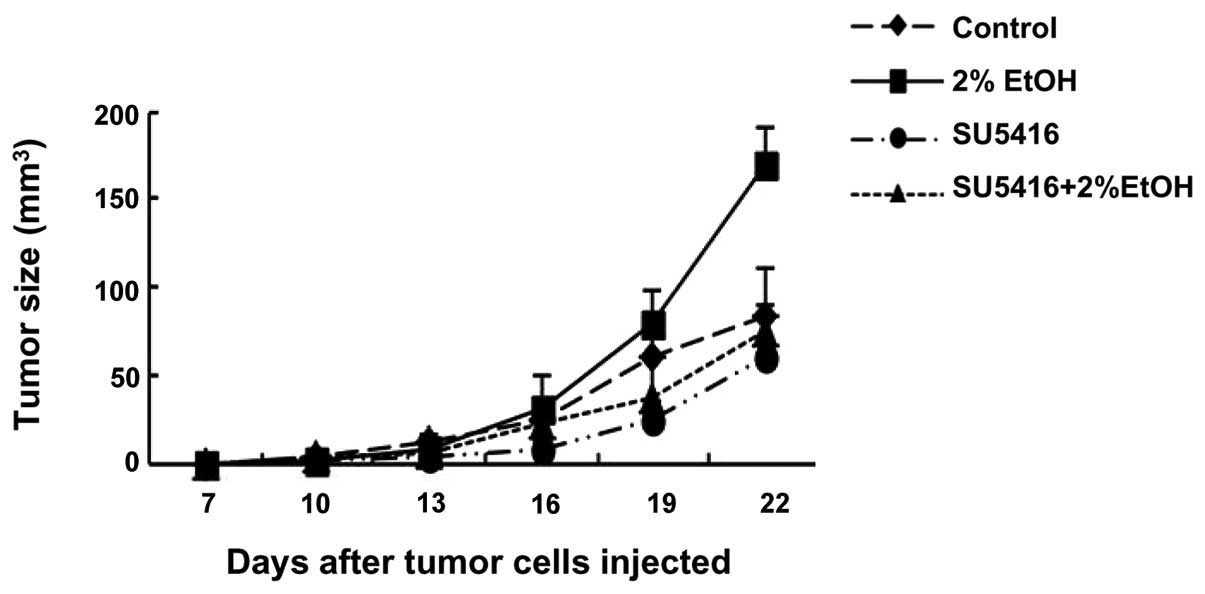
A well-established mouse xenograft model of mammary
tumors was utilized to validate the role of VEGF in alcohol-induced
tumor promotion in vivo. As demonstrated in Fig. 4, alcohol consumption significantly
promoted the growth of mammary tumors in C57BL6 mice. Injection of
SU5416 inhibited alcohol-stimulated tumor growth. These results
suggested that alcohol promotion of tumor growth may be mediated by
VEGF-dependent angiogenesis.
Discussion
Alcohol consumption promotes the growth and
metastasis of breast cancer (10–12).
However, the mechanisms underlying this effect remain unclear. VEGF
has been implicated in tumor angiogenesis and progression. In the
present study, it was demonstrated that alcohol increases tumor
angiogenesis and accelerates tumor growth. Alcohol upregulates VEGF
expression in breast cancer cells in vitro and in
vivo. Blocking VEGF signaling inhibits alcohol-stimulated tumor
angiogenesis in a 3D tumor/endothelial cell co-culture system.
Furthermore, blocking VEGF signaling inhibited alcohol-accelerated
mammary tumor growth in mice. These results suggest that
VEGF-dependent angiogenesis has an important role in
alcohol-mediated tumor promotion.
Consistent with epidemiological evidence, alcohol
exposure promotes tumor progression and malignancy in animals
(16,23,24,25).
In the present study, it was demonstrated that alcohol at a
moderate concentration (2% in drinking water during the dark cycle)
enhances mammary tumor growth in C57BL6 mice. Our previous results
and studies by others suggested that alcohol exposure may enhance
angiogenesis (16,23,26,27).
The mechanisms underlying alcohol-stimulated angiogenesis, however,
are complex and unclear. Alcohol may directly target endothelial
cells, or regulate the interaction between endothelial and tumor
cells. To the best of our knowledge, our previous study was the
first to demonstrate that alcohol promotes tumor/endothelial cell
interaction and enhances tumor angiogenesis in a 3D co-culture of
tumor/endothelial cells (16). In
this earlier study, we demonstrated that alcohol stimulated MCP-1
secretion from mammary tumor cells, which enhanced endothelial
angiogenesis. However, blocking MCP-1 signaling only partially
reversed the effect of alcohol on tumor angiogenesis. Therefore, we
hypothesized that other factors are responsible for mediating
alcohol-stimulated angiogenesis.
VEGF is one of the most potent effectors of
physiological and pathological angiogenesis, which has been proved
to be an important factor for tumor occurrence, progression and
metastasis of breast cancer (13).
In the tumor microenvironment, VEGF is derived from various
sources, including tumor cells, inflammatory and stromal cells,
platelets and vascular cells (28).
VEGF regulates multiple aspects of tumor angiogenesis through two
high-affinity receptor tyrosine kinases, VEGFR1 (Flt-1) and
VEGFR2/KDR (Flk-1), on endothelial cells. The binding of VEGF and
VEGFR results in endothelial cell proliferation, migration,
differentiation, tube formation and upregulation of vascular
permeability (29). Furthermore,
VEGF has been reported to be an indirect leukocyte migrating factor
through inducing the expression of MCP-1 and IL-8 (30,31).
In the present study, it was demonstrated that SU5416, a tyrosine
kinase inhibitor of VEGFR2, not only blocks tumor cell-stimulated
angiogenesis but also eliminates alcohol-mediated promotion of
tumor angiogenesis (Fig. 3).
Furthermore, SU5416 significantly inhibits alcohol-stimulated
mammary tumor growth in mice. Therefore, this study confirms that
VEGF has an important role in alcohol promotion of mammary tumor
progression. Furthermore, it appears that the role of VEGF in
alcohol-mediated tumor promotion is not tumor type-specific. It has
been reported that moderate alcohol consumption increases the
expression of VEGF and angiogenesis in a mouse xenograft model of
melanoma (16). Multiple factors
and signaling pathways may be responsible for alcohol promotion of
mammary tumor progression and VEGF signaling appears to be an
important one. Future studies are required to elucidate the
underlying mechanisms whereby alcohol regulates the expression of
VEGF.
Acknowledgements
This study was supported by the Scientific Research
Foundation for the Returned Overseas Chinese Scholars (Professor
Siying Wang) and Academic Leaders Fund of Anhui Province (Professor
Siying Wang).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108.
2005.
|
|
2
|
Breast Cancer Facts & Figures
2009–2010. American Cancer Society; Atlanta: pp. 1–36. 2009
|
|
3
|
Salehi F, Turner MC, Phillips KP, Wigle
DT, Krewskia D and Aronson KJ: Review of the etiology of breast
cancer with special attention to organochlorines as potential
endocrine disruptors. J Toxicol Environ Health B Crit Review.
11:276–300. 2008.
|
|
4
|
Singletary KW and Gapstur SM: Alcohol and
breast cancer: review of epidemiologic and experimental evidence
and potential mechanisms. JAMA. 286:2143–2151. 2001.
|
|
5
|
Seitz HK and Becker P: Alcohol metabolism
and cancer risk. Alcohol Res Health. 30:38–41. 44–47. 2007.
|
|
6
|
Seitz HK and Maurer B: The relationship
between alcohol metabolism, estrogen levels, and breast cancer
risk. Alcohol Res Health. 30:42–43. 2007.
|
|
7
|
Key J, Hodgson S, Omar RZ, et al:
Meta-analysis of studies of alcohol and breast cancer with
consideration of the methodological issues. Cancer Causes Control.
17:759–770. 2006.
|
|
8
|
Tjønneland A, Christensen J, Olsen A, et
al: Alcohol intake and breast cancer risk: the European Prospective
Investigation into Cancer and Nutrition (EPIC). Cancer Causes
Control. 18:361–373. 2007.
|
|
9
|
Visvanathan K, Crum RM, Strickland PT, et
al: Alcohol dehydrogenase genetic polymorphisms, low-to-moderate
alcohol consumption, and risk of breast cancer. Alcohol Clin Exp
Res. 31:467–476. 2007.
|
|
10
|
Weiss HA, Brinton LA, Brogan D, et al:
Epidemiology of in situ and invasive breast cancer in women aged
under 45. Br J Cancer. 73:1298–1305. 1996.
|
|
11
|
Vaeth PA and Satariano WA: Alcohol
consumption and breast cancer stage at diagnosis. Alcohol Clin Exp
Res. 22:928–934. 1998.
|
|
12
|
Stoll BA: Alcohol intake and late-stage
promotion of breast cancer. Eur J Cancer. 35:1653–1658. 1999.
|
|
13
|
Delli Carpini J, Karam AK and Montgomery
L: Vascular endothelial growth factor and its relationship to the
prognosis and treatment of breast, ovarian, and cervical cancer.
Angiogenesis. 13:43–58. 2010.
|
|
14
|
Amini A, Masoumi Moghaddam S, Morris DL
and Pourgholami MH: Review: Utility of vascular endothelial growth
factor inhibitors in the treatment of ovarian cancer: from concept
to application. J Oncol. 2012:5407912011.
|
|
15
|
Yoshiji H, Gomez D, Shibuya M and
Thorgeirsson UP: Expression of vascular endothelial growth factor,
its receptor, and other angiogenic factors in human breast cancer.
Cancer Res. 56:2013–2016. 1996.
|
|
16
|
Wang S, Xu M, Li F, et al: Ethanol
promotes mammary tumor growth and angiogenesis: the involvement of
chemoattractant factor MCP-1. Breast Cancer Res Treat.
133:1037–1048. 2012.
|
|
17
|
Ewens A, Luo L, Berleth E, et al:
Doxorubicin plus interleukin-2 chemoimmunotherapy against breast
cancer in mice. Cancer Res. 66:5419–5426. 2006.
|
|
18
|
Li WL, Fraser JL, Yu SP, Zhu J, Jiang YJ
and Wei L: The role of VEGF/VEGFR2 signaling in peripheral
stimulation-induced cerebral neurovascular regeneration after
ischemic stroke in mice. Exp Brain Res. 214:503–513. 2011.
|
|
19
|
Liu Y, Chen G, Ma C, Bower KA, Xu M, Fan
Z, Shi X, Ke ZJ and Luo J: Overexpression of glycogen synthase
kinase 3beta sensitizes neuronal cells to ethanol toxicity. J
Neurosci Res. 87:2793–2802. 2009.
|
|
20
|
Chen Z, Htay A, Dos Santos W, Gillies GT,
Fillmore HL, Sholley MM and Broaddus WC: In vitro angiogenesis by
human umbilical vein endothelial cells (HUVEC) induced by
three-dimensional co-culture with glioblastoma cells. J Neurooncol.
92:121–128. 2009.
|
|
21
|
Luo J and Miller MW: Differential
sensitivity of human neuroblastoma cell lines to ethanol:
correlations with their proliferative responses to mitogenic growth
factors and expression of growth factor receptors. Alcohol Clin Exp
Res. 21:1186–1194. 1997.
|
|
22
|
Xu M, Bower KA, Wang S, Frank JA, Chen G,
Ding M, Wang S, Shi X, Ke Z and Luo J: Cyanidin-3-glucoside
inhibits ethanol-induced invasion of breast cancer cells
overexpressing ErbB2. Mol Cancer. 9:2852010.
|
|
23
|
Tan W, Bailey AP, Shparago M, Busby B,
Covington J, Johnson JW, Young E and Gu JW: Chronic alcohol
consumption stimulates VEGF expression, tumor angiogenesis and
progression of melanoma in mice. Cancer Biol Ther. 6:1211–1217.
2007.
|
|
24
|
Hong J, Holcomb VB, Tekle SA, Fan B and
Núñez NP: Alcohol consumption promotes mammary tumor growth and
insulin sensitivity. Cancer Lett. 294:229–235. 2010.
|
|
25
|
Yirmiya R, Ben-Eliyahu S, Gale RP, Shavit
Y, Liebeskind JC and Taylor AN: Ethanol increases tumor progression
in rats: possible involvement of natural killer cells. Brain Behav
Immun. 6:74–86. 1992.
|
|
26
|
Gu JW, Elam J, Sartin A, Li W, Roach R and
Adair TH: Moderate levels of ethanol induce expression of vascular
endothelial growth factor and stimulate angiogenesis. Am J Physiol
Regul Integr Comp Physiol. 281:R365–R372. 2001.
|
|
27
|
Qian Y, Luo J, Leonard SS, Harris GK,
Millecchia L, Flynn DC and Shi X: Hydrogen peroxide formation and
actin filament reorganization by Cdc42 are essential for
ethanol-induced in vitro angiogenesis. J Biol Chem.
278:16189–16197. 2003.
|
|
28
|
Greenberg JI and Cheresh DA: VEGF as an
inhibitor of tumor vessel maturation: implications for cancer
therapy. Exp Opin Biol Ther. 9:1347–1356. 2009.
|
|
29
|
Rahimi N, Dayanir V and Lashkari K:
Receptor chimeras indicate that the vascular endothelial growth
factor receptor-1 (VEGFR-1) modulates mitogenic activity of VEGFR-2
in endothelial cells. J Biol Chem. 275:16986–16992. 2000.
|
|
30
|
Ziche M, Morbidelli L, Choudhuri R, Zhang
HT, Donnini S, Granger HJ, et al: Nitric oxide synthase lies
downstream from vascular endothelial growth factor-induced but not
basic fibroblast growth factor-induced angiogenesis. J Clin Invest.
99:2625–2634. 1997.
|
|
31
|
Marumo T, Schini-Kerth VB and Busse R:
Vascular endothelial growth factor activates nuclear factor-kappaB
and induces monocyte chemoattractant protein-1 in bovine retinal
endothelial cells. Diabetes. 48:1131–1137. 1999.
|