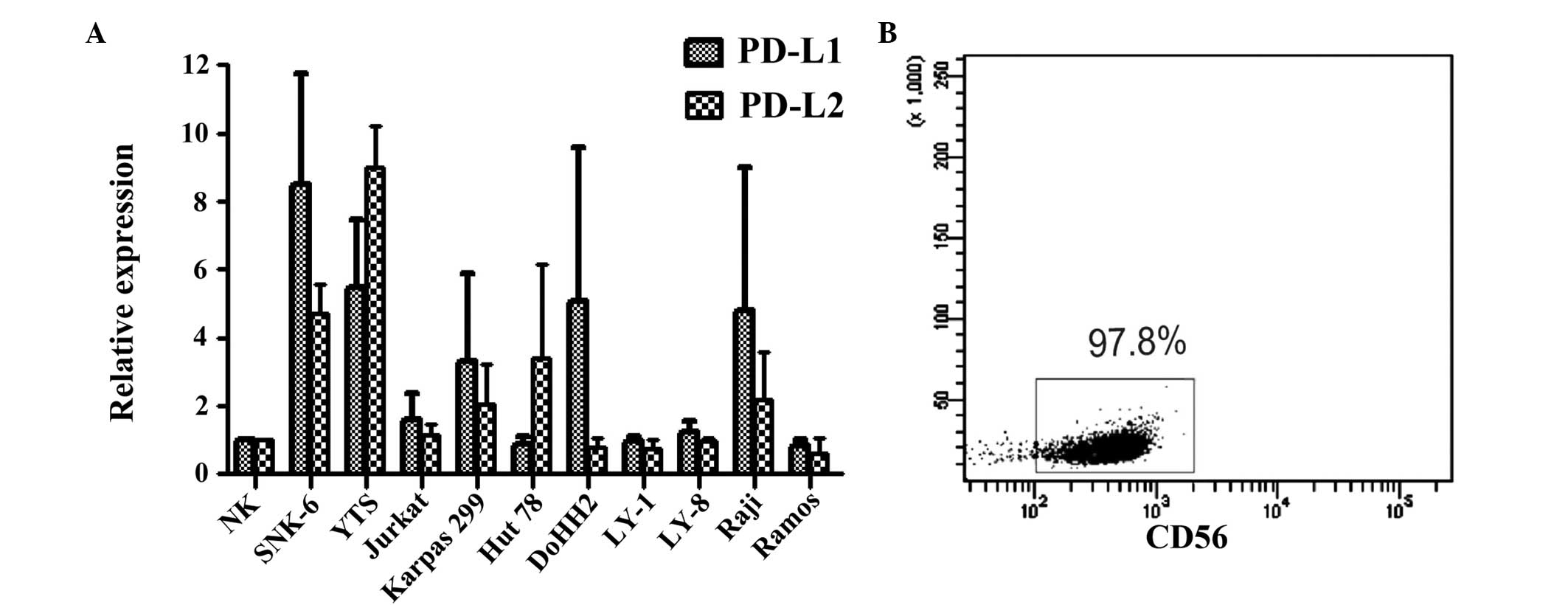
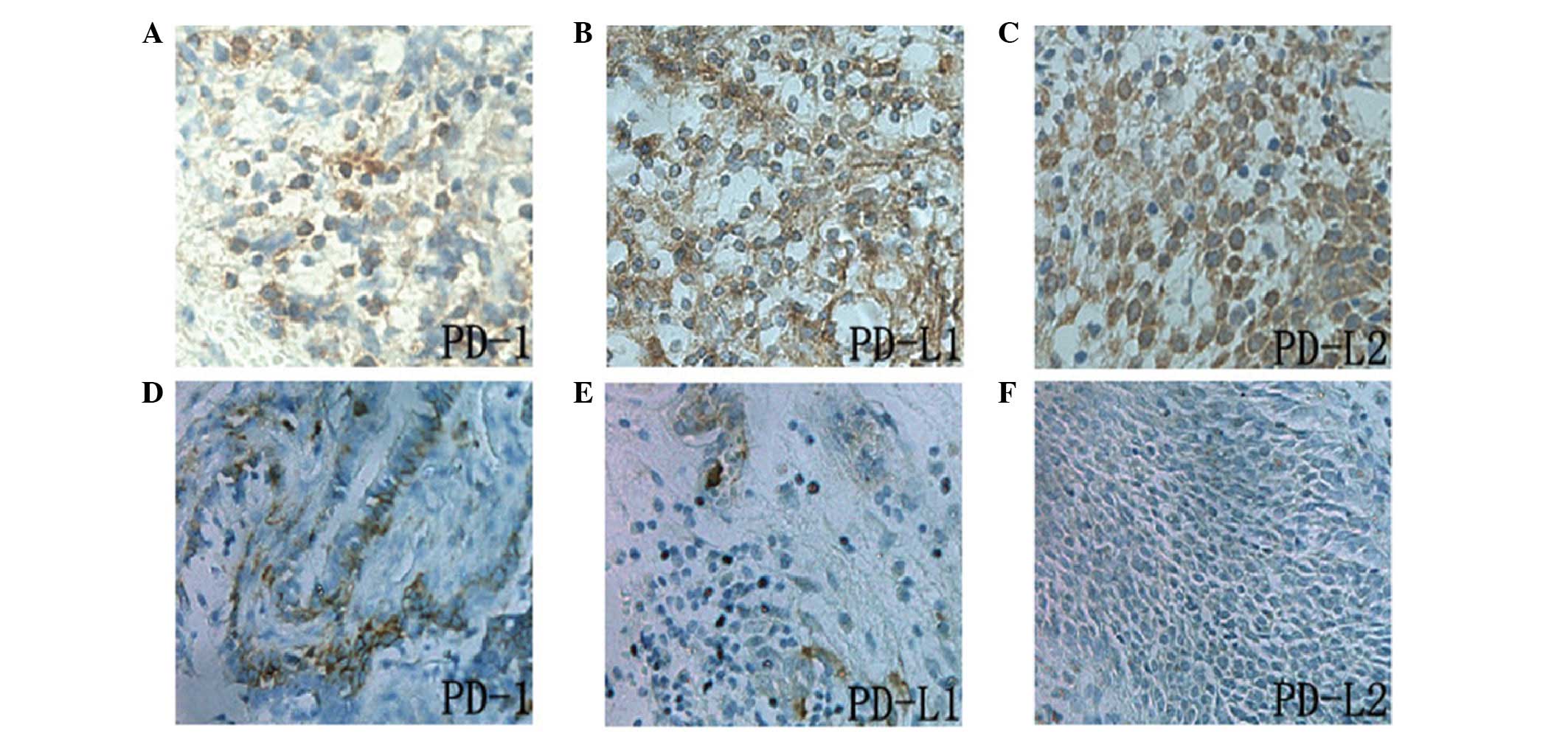
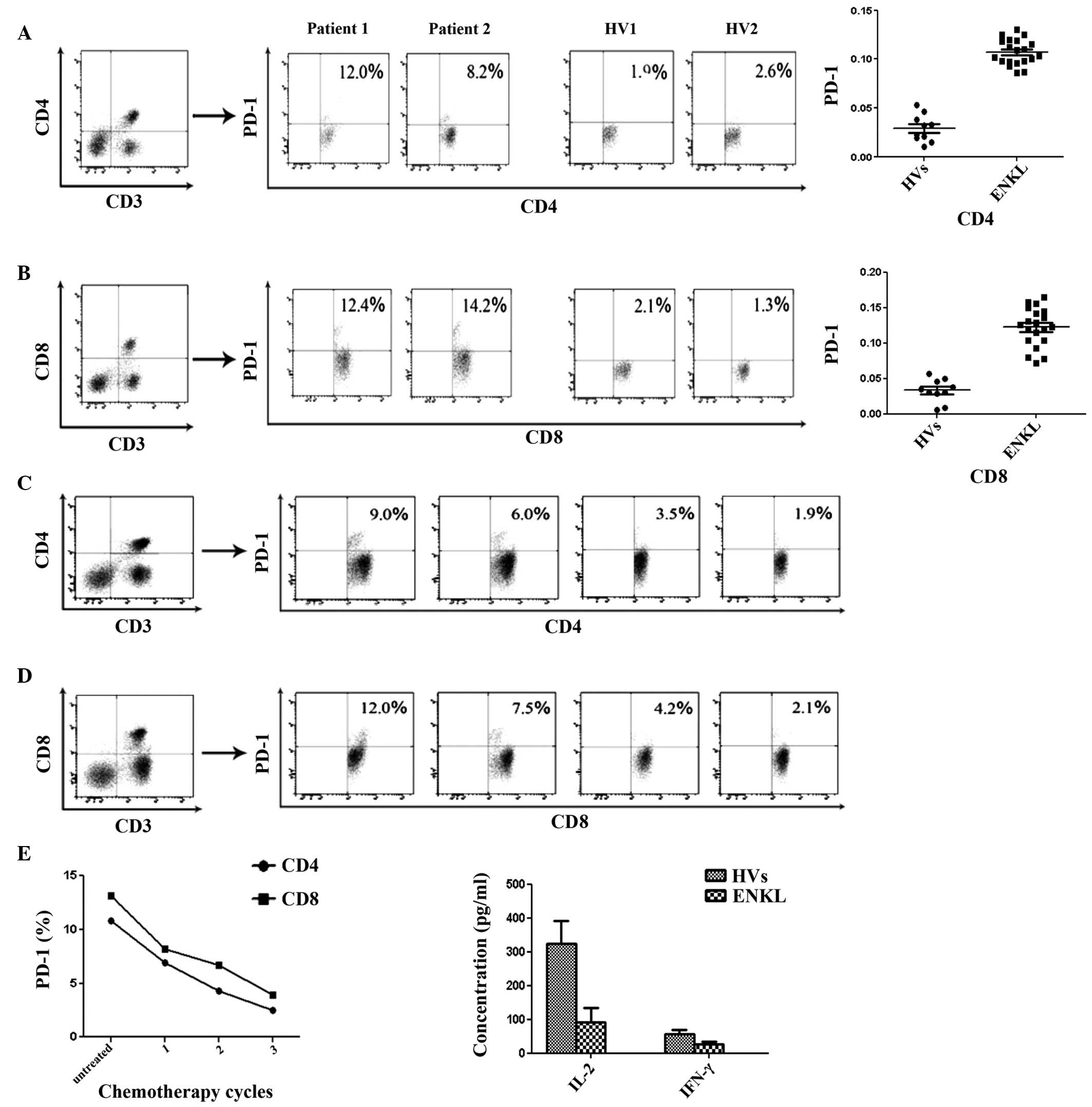
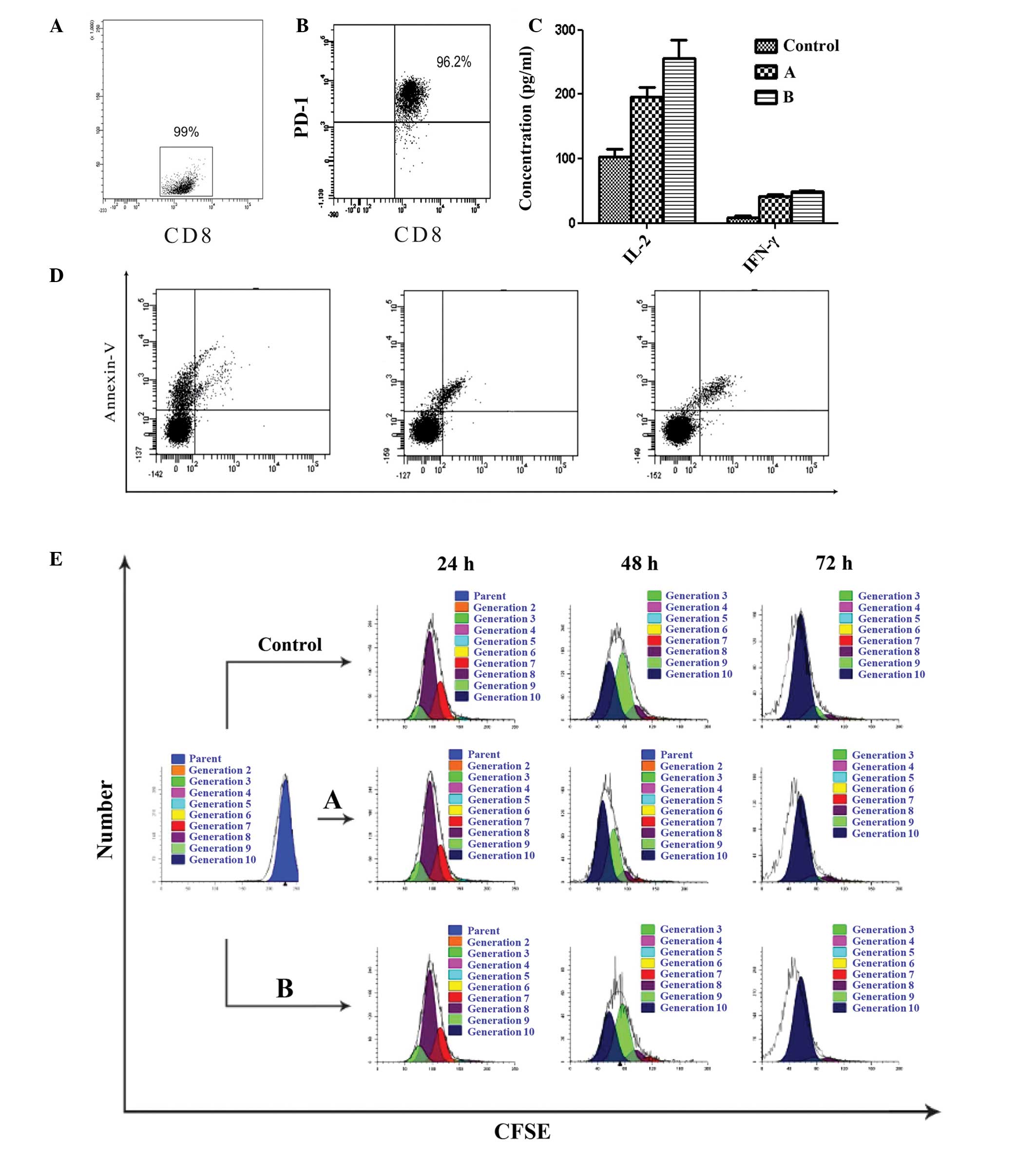
|
1
|
Harabuchi Y, Takahara M, Kishibe K, et al:
Nasal natural killer (NK)/T-cell lymphoma: clinical, histological,
virological, and genetic features. Int J Clin Oncol. 14:181–190.
2009.
|
|
2
|
Jaffe ES: The 2008 WHO classification of
lymphomas: implications for clinical practice and translational
research. Hematology Am Soc Hematol Educ Program. 2009:523–531.
2009.
|
|
3
|
Mao Y, Zhang DW, Zhu H, et al: LMP1 and
LMP2A are potential prognostic markers of extranodal NK/T-cell
lymphoma, nasal type (ENKTL). Diag Pathol. 7:1782012.
|
|
4
|
Roma E and Smith AG: Epidemiology of
lymphoma. Histopathology. 58:4–14. 2011.
|
|
5
|
Suzuki R: NK/T-Cell Lymphomas:
pathobiology, prognosis and treatment paradigm. Curr Oncol Rep.
14:395–402. 2012.
|
|
6
|
Chen L: Co-inhibitory molecules of the
B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol.
4:336–347. 2004.
|
|
7
|
Fife BT, Panken KE, Eagar TN, et al:
Interractions between PD-1 and PD-L1 promote tolerance by blocking
the TCR-induced stop signal. Nat Immunol. 10:1185–1192. 2009.
|
|
8
|
Atanackovic D, Luetkens T and Kröger N:
Coinhibitory molecule PD-1 as a potential target for the
immunotherapy of multiple myeloma. Leukemia. Oct 23–2013.(Epub
ahead of print).
|
|
9
|
Zitvogel L and Kroemer G: Targeting
PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology.
1:1223–1225. 2012.
|
|
10
|
Iwamura K, Kato T, Miyahara Y, et al:
siRNA-mediated silencing of PD-1 ligands enhances tumor-specific
human T-cell effector functions. Gene Ther. 19:959–966. 2012.
|
|
11
|
Brahmer JR, Tykodi SS, Chow LQ, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012.
|
|
12
|
Topalian SL, Hodi FS, Brahmer JR, et al:
Safety, activity, and immune correlates of anti-PD-1 antibody in
cancer. N Engl J Med. 366:2443–2454. 2012.
|
|
13
|
Chen BJ, Chapuy B, Ouyang J, et al: PD-L1
expression is characteristic of a subset of aggressive B-cell
lymphomas and virus-associated malignancies. Clin Cancer Res.
19:3462–3473. 2013.
|
|
14
|
Beahrs OH, Henson DE and Hutter RVP:
Handbook for staging of cancer, from the manual for staging of
cancer. 4th ed. American joint committee on cancer (AJCC). JB
Lippincott Co; 257. pp. 1992
|
|
15
|
Taube JM, Anders RA, Young GD, et al:
Colocalization of inflammatory response with B7-h1 expression in
human melanocytic lesions supports an adaptive resistance mechanism
of immune escape. Sci Transl Med. 4:127ra372012.
|
|
16
|
Li Y, Wang J, Li C and Ke XY: Contribution
of PD-L1 to oncogenesis of lymphoma and its RNAi-based targeting
therapy. Leuk Lymph. 53:2015–2023. 2012.
|
|
17
|
Greaves P and Gribben JG: The role of B7
family molecules in hematological malignancy. Blood. 121:734–744.
2013.
|
|
18
|
Rozali EN, Hato SV, Robinson BW, et al:
Programmed death ligand 2 in cancer-induced immune suppression.
Clin Dev Immunol. 2012:6563402012.
|
|
19
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of PD-1, a novel member of the immunoglobulin
gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895.
1992.
|
|
20
|
Loke P and Allison JP: PD-L1 and PD-L2 are
differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci
USA. 100:5336–5341. 2003.
|
|
21
|
Andorsky DJ, Yamada RE, Said J, et al:
Programmed death ligand 1 is expressed by non-hodgkin lymphomas and
inhibits the activity of tumor-associated T cells. Clin Cancer Res.
17:4232–4244. 2011.
|
|
22
|
Yamamoto R, Nishikori M, Kitawaki T, et
al: PD-1-PD-1 ligand interaction contributes to immunosuppressive
microenvironment of Hodgkin lymphoma. Blood. 111:3220–3224.
2008.
|
|
23
|
Brusa D, Serra S, Coscia M, et al: The
PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic
lymphocytic leukemia. Haematologica. 98:953–963. 2013.
|
|
24
|
Nomi T, Sho M, Akahori T, et al: Clinical
significance and therapeutic potential of the programmed death-1
ligand/programmed death-1 pathway in human pancreatic cancer. Clin
Cancer Res. 13:2151–2157. 2007.
|
|
25
|
Ohigashi Y, Sho M, Yamada Y, et al:
Clinical significance of programmed death-1 ligand-1 and programmed
death-1 ligand-2 expression in human esophageal cancer. Clin Cancer
Res. 11:2947–2953. 2005.
|
|
26
|
Grzywnowicz M, Zaleska J, Mertens D, et
al: Programmed death-1 and its ligand are novel immunotolerant
molecules expressed on leukemic B cells in chronic lymphocytic
leukemia. PLoS One. 7:e351782012.
|
|
27
|
Green MR, Rodig S, Juszczynski P, et al:
Constitutive AP-1 activity and EBV infection induce PD-L1 in
Hodgkin lymphomas and posttransplant lymphoproliferative disorders:
implications for targeted therapy. Clin Cancer Res. 18:1611–1618.
2012.
|
|
28
|
Takahashi H, Tomita N, Sakata S, et al:
Prognostic significance of programmed cell death-1-positive cells
in follicular lymphoma patients may alter in the rituximab era. Eur
J Haematol. 90:286–290. 2013.
|
|
29
|
Ohmatsu H, Suqaya M, Fujita H, et al:
Primary cutaneous follicular helper T-cell lymphoma treated with
allogeneic bone marrow transplantation: immunohistochemical
comparison with angioimmunoblastic T-cell lymphoma. Acta Derm
Venereol. Jan;2014.(Epub ahead of print).
|
|
30
|
Chen L: B7-H1 connection of innate and
adaptive immunity against tumor dormancy. Blood. 105:2242–2243.
2005.
|
|
31
|
Abiko A, Mandai M, Hamanishi J, et al:
PD-L1 on tumor cells is induced in ascites and promotes peritoneal
dissemination of ovarian cancer through CTL dysfunction. Clin
Cancer Res. 19:1363–1374. 2013.
|
|
32
|
Yamamoto R, Nishikori M, Tashima M, et al:
B7-H1 expression is regulated by MEK/ERK signaling pathway in
anaplastic large cell lymphoma and Hodgkin lymphoma. Cancer Sci.
100:2093–2100. 2009.
|
|
33
|
Küppers R: The biology of Hodgkin’s
lymphoma. Nat Rev Cancer. 9:15–27. 2009.
|