Introduction
The cancer stem cell theory proposes that cancer is
a stem cell disease, where tumor recurrence is caused by cancer
stem cells. Cancer stem cells are tumorigenic cells that possess
self-renewal ability, have unlimited proliferative potential, and
can differentiate into multiple cell types. These cells are
hypothesized to be the cause of tumor initiation, abnormal
proliferation, invasion, metastasis, drug resistance and recurrence
(1–3). Previously, our group cultured HO8910
cells (a cell line from a poorly-differentiated human ovarian
serous cystadenocarcinoma) in suspension with serum-free medium
supplemented with paclitaxel, and isolated CD133+, CD117+ cells,
which were determined to be cancer stem cells by a series of in
vitro and in vivo experiments (4). These cells provide a valuable tool
with which to study the biological characteristics of ovarian
cancer stem cells.
The tumor suppressor gene WW domain-containing
oxidoreductase (WWOX) was isolated by Bednarek et al
(5) using shot-gun sequencing. WWOX
is a 414-amino acid protein, with two WW structural domains at its
amino-terminus. The WW domain mediates protein-protein
interactions, which is essential for the signaling pathways used by
tumor suppressors to inhibit tumor growth. The WWOX protein
localizes to the mitochondria under normal conditions but, in
response to stress stimuli, the synthesis of this protein
increases, mitochondrial permeability is altered, and WWOX
translocates to the nucleus where it regulates gene expression.
WWOX may inhibit tumor initiation and progression through multiple
signaling pathways, including the following: Tumor necrosis factor
receptor type 1-associated DEATH domain protein and tumor necrosis
factor receptor-associated factor 2-mediated apoptosis pathways;
c-Jun N-terminal kinase 1-mediated stress response pathways; and
p53-initiated apoptotic pathways (6,7). In
addition, our previous studies have demonstrated that WWOX alters
the biological phenotype of ovarian cancer stem cells, and is
important in the formation and progression of ovarian cancer
(8–10).
In the current study, a WWOX gene-containing
eukaryotic expression vector was introduced into ovarian cancer
stem cells by transfection, in order to evaluate the effects of
WWOX on the stem cell properties of these cells.
Materials and methods
Materials
Human ovarian cancer stem cells were isolated and
stored in the Laboratory of Obstetrics and Gynecology at The
Affiliated Hospital of Xuzhou Medical College (Xuzhou, China).
These cells have previously been confirmed to possess stem cell
properties, including self-renewal ability, differentiation
potential, in vivo tumorigenic capability, high-level
expression of stem cell genes and multidrug resistance (4). The pcDNA3.1-WWOX eukaryotic
expression vector was constructed by and stored in the same
laboratory (11). The pcDNA3.1
empty plasmid was provided by Professor Shuqun Hu at the Molecular
Biology Research Center of Xuzhou Medical College. Serum-free
medium, supplemented with epidermal growth factor (EGF), basic
fibroblast growth factor (bFGF), Noggin and leukemia inhibitory
factor (LIF), was purchased from Sigma-Aldrich (St. Louis, MO,
USA). Primary antibodies to CD133 (cat. no. MAB4310), CD117 (cat.
no. MA1-12192), ATP-binding cassette sub-family G member 2 (ABCG2;
cat. no. AM1125a), Nanog (cat. no. AP1486c), octamer-binding
transcription factor 4 (OCT4; cat. no. NRG1.1), breast cancer
resistance protein (BCRP; cat. no. 254515) and E-cadherin (cat. no.
MA5-12547) were purchased from Chemicon (Billerica, MA, USA).
Cisplatin, doxorubicin and mitoxantrone were purchased from XinYu
Biotechnology (Shanghai, China). Female non-obese diabetic
(NOD)/severe combined immunodeficiency (SCID) mice (4–6 weeks of
age) were purchased from the Chinese Academy of Sciences Shanghai
Laboratory Animal Center (Shanghai, China). All animal studies were
approved by the Ethics Committee of The Affiliated Hospital of
Xuzhou Medical College. Written informed consent was obtained from
the patient.
Cell culture
Human ovarian cancer stem cells were cultured in
serum-free medium supplemented with EGF, bFGF, Noggin, and LIF at
37°C in a humidified incubator with 5% CO2 in compressed
air.
Gene transfection
Plasmids were transfected into ovarian cancer stem
cells using the Lipofector Liposomal Transfection Kit (Beyotime
Institute of Biotechnology, Shanghai, China), following the
manufacturer’s instructions (transfection efficiency, 68%), and
stably transfected cells were subsequently isolated and expanded in
culture. A eukaryotic expression vector containing the WWOX
gene (pcDNA3.1-WWOX) was used to produce WWOX expression.
Cells transfected with the empty vector (pcDNA3.1) and
untransfected cells were used as controls.
Western blot assay
The three types of cells (WWOX-transfected,
empty vector-transfected and untransfected) were harvested during
the growth phase and lysed on ice in 200 μl lysis buffer. Protein
concentrations were determined using a bicinchoninic acid assay.
Proteins were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, and transferred onto
nitrocellulose membranes. Membranes were blocked with 5% non-fat
dry milk for 60 min, incubated with rabbit-anti-human WWOX
antibodies (dilution, 1:1,000; cat. no. 15800667461; Hangzhou
Sijiqing Biology Engineering Materials Co., Ltd., Hangzhou, China)
at 4°C overnight, and subsequently incubated with horseradish
peroxidase-conjugated goat-anti-rabbit antibodies (dilution,
1:10,000; cat. no. 13764022678; Sigma-Aldrich) at room temperature
for 2 h. Membranes were developed with enhanced chemiluminescence
reagents (Hangzhou Sijiqing Biology Engineering Materials Co.,
Ltd.), and exposed to high sensitivity X-ray films in the dark.
Analysis of self-renewal abilities of
ovarian cancer stem cells
Cells were dissociated into single cells suspensions
using 0.25% trypsin, and the number of viable cells was counted
following trypan blue staining. Cells were subsequently diluted to
a density of 1×103 cells/ml in serum-free medium, and
plated into 96-well plates at 100 μl/well; thus each well contained
100 cells. Each cell type was plated into 20 wells, and 100 μl
medium was added to each well, prior to incubation in a humidified
incubator at 37°C in an atmosphere of 5% CO2 for 48 h.
An additional 25 μl of medium was added to each well every
subsequent day, and the cells were observed each day for sphere
formation. Following an incubation period of eight days, the number
of cell spheres in each well was counted under a microscope.
Analysis of cell differentiation
Stem cells were resuspended in RPMI1640 medium
containing 10% fetal bovine serum, and allowed to adhere to the
plate and differentiate. Cell differentiation was observed under a
microscope. Expression of CD133+ and CD117+ on the cells prior to
and following differentiation was analyzed by flow cytometry, while
the expression of ABCG2, Nanog, OCT4, BCRP, and E-cadherin was
analyzed by Western blot analysis.
Drug-sensitivity test
Cell suspensions were stained with trypan blue to
count the number of viable cells, plated into 96-well plates at
6,000 cells/well, and cultured in serum-free medium in the presence
of one of the three drugs to be tested (cisplatin, doxorubicin and
mitoxantrone). Each drug was tested at two concentrations near its
half maximal inhibitory concentration (IC50): Cisplatin,
0.25 and 0.5 μg/ml; doxorubicin, 0.5 and 1.5 μmol/l; and
mitoxantrone, 0.05 and 0.25 μg/ml. Five replicate wells were
prepared for each condition. Following a 48 h incubation, the
medium was removed, and 10% water-soluble tetrazolium salt-8
(Pfizer Pharmaceutical Co., Ltd., Billerica, MA, USA) was added to
the wells for measurement. The CCK-8 reagent was also added to
wells without cells as blanks. The plates were placed in a
humidified incubator, with an atmosphere of 5% CO2, for
2 h at 37°C, and the absorbance (A) at 450 nm was measured with an
ultraviolet spectrophotometer. The cellular viability following
drug treatment was compared with the same type of cells without
drug treatment, and relative viability was calculated as
(Adrug treated − Ablank) /
(Auntreated − Ablank).
In vivo tumorigenesis assay
Cell suspensions were stained with trypan blue to
count the number of viable cells. Various quantities of cells
(2×105, 2×104, 2×103 or
1×103) were subsequently injected subcutaneously into
the right axilla of female NOD/SCID mice. The 15 mice at 4–6 weeks
of age were randomly divided into three groups, with five mice per
group. Mice were housed in a pathogen-free room with controlled
temperature (25–27°C), controlled humidity (45–50%) and filtered
fresh air, and received sterile food and water ad libitum.
Tumor growth was recorded twice per week. Tumorigenicity was
represented as tumorigenesis rate (number of mice that developed
tumors / total number of mice that received cells) and
tumor-forming time (the time from cell inoculation until the tumors
became palpable). When tumors reached 1 cm3 in size, or
at three months following cell transplantation if no tumor was
formed, mice were sacrificed. All procedures were conducted in
accordance with animal research ethical guidelines. This study was
approved by the ethics committee of The Affiliated Hospital of
Xuzhou Medical College.
Statistical Analysis
All data are presented as mean ± standard deviation
(SD). Statistical analysis was performed using SPSS version 13.0
software (SPSS, Inc., Chicago, IL, USA). Analysis of variance was
used for intergroup comparisons, while Tukey’s test was used for
pairwise comparisons between multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Ectopic expression of WWOX protein in
ovarian cancer stem cells by gene transfection
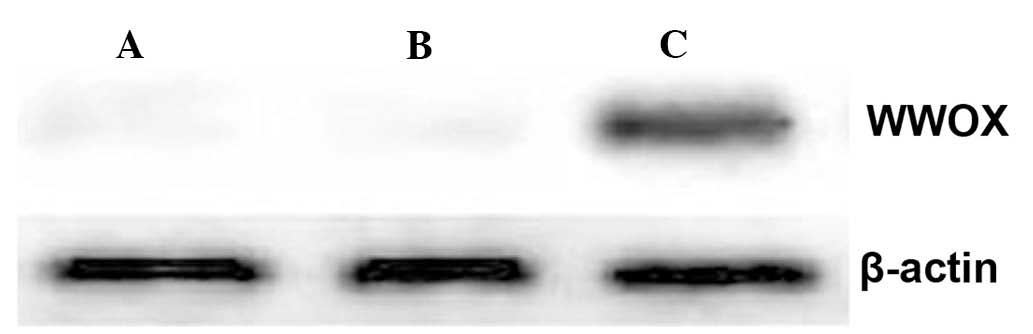
Western blot analysis demonstrated that cells
transfected with the WWOX-expression plasmid produced high levels
of WWOX protein, while it was undetectable in untransfected cells,
or cells transfected with the empty vector (Fig. 1).
WWOX inhibits the self-renewal ability of
ovarian cancer stem cells
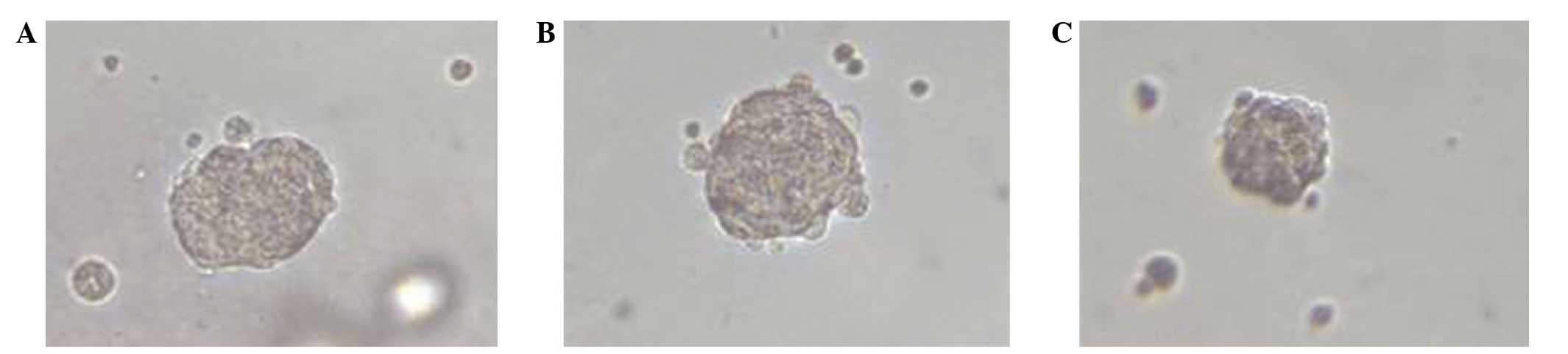
A sphere-forming assay was utilized to determine the
effect of WWOX expression on the self-renewal ability of ovarian
cancer stem cells. Untransfected stem cells and cells transfected
with the empty vector began to form spheres following three days in
culture, and reached a plateau at seven days. By contrast,
WWOX-expressing cells began to form spheres at six days, and
reached a plateau at 10 days. Furthermore, WWOX-expressing cells
formed significantly fewer cell spheres/well (mean ± SD, 7.61±2.02)
(P=0.019) in serum-free medium compared with the cells transfected
with the empty vector (mean ± SD, 32.82±3.29) or untransfected
cells (mean ± SD, 35.74±2.98). No significant difference was
observed between empty vector-transfected cells and untransfected
cells. In addition, the largest spheres formed by WWOX-expressing
cells were smaller in size compared with those formed by empty
vector-transfected cells or untransfected cells (Fig. 2).
WWOX reduces the differentiation
potential of ovarian cancer stem cells
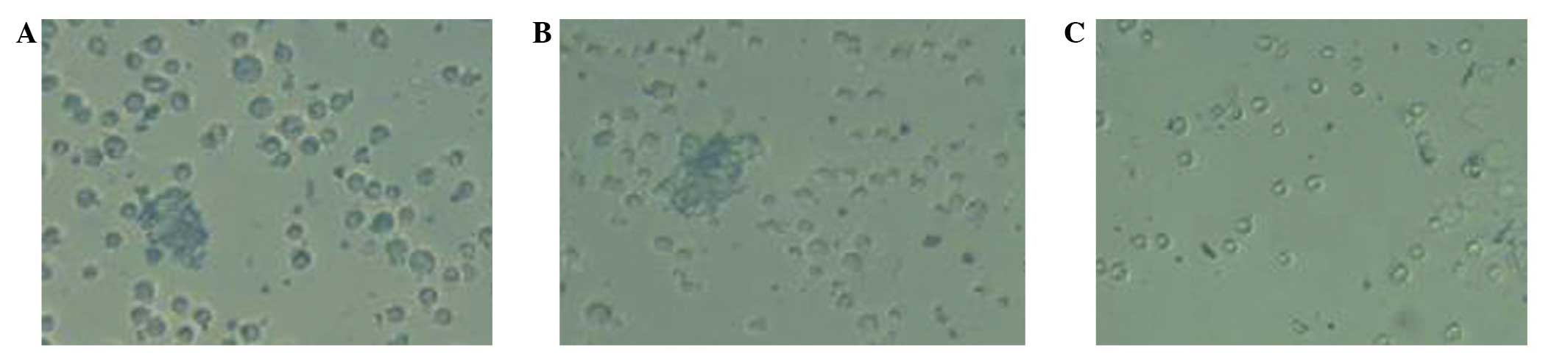
When plated in serum-containing medium to allow for
cellular differentiation, WWOX-expressing cells began to attach to
the culture plate surface after 2 h and almost all cells had
adhered to the culture plates at 12 h. Untransfected cells and
empty vector-transfected cells, by contrast, displayed a delay in
attachment to the culture plate surface: A number of cells adhered
to the culture plates after 6 h, whilst others maintained a
spherical morphology and did not become adherent until 24 h
(Fig. 3).
The expression of two stem cell markers, CD133 and
CD117, was analyzed in these cells using flow cytometry. The
percentages of CD133+ and CD117+ populations in the WWOX-expressing
cells were 21.3% and 23.4%, respectively, prior to cellular
differentiation, and 19.9% and 21.1%, respectively, following
differentiation; this difference was not significant. In cells
transfected with the empty vector, the percentages of CD133+ and
CD117+ cells prior to differentiation were 76.4% and 81.8%,
respectively, and decreased significantly following differentiation
(25.3% and 28.3%, respectively). Similarly, untransfected cells
comprised 79.7% CD133+ cells and 78.5% CD117+ cells prior to
differentiation, decreasing significantly to 23.1% and 21.5%,
respectively, following differentiation.
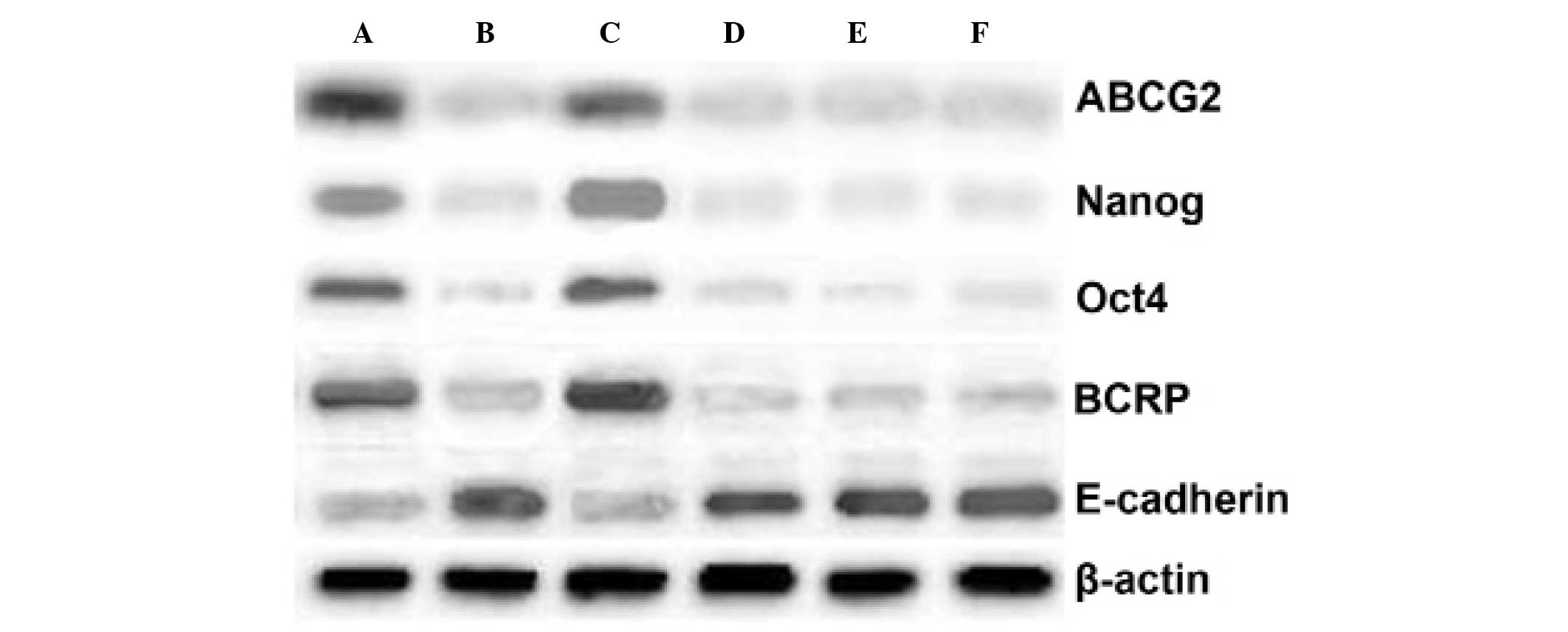
The protein levels of the stem cell markers ABCG2,
Nanog, OCT4, and BCRP in these cells were also analyzed by Western
blotting. Protein levels were quantified as follows: The target
belt and β-actin protein band gray values were detected and the
ratio of target belt/β-actin indicated the relative expression
level of the target proteins. In WWOX-expressing stem cells prior
to differentiation, the mean ± SD levels of these four proteins
were 0.38±0.11, 0.42±0.07, 0.31±0.03, and 0.37±0.13, respectively,
and did not differ significantly following differentiation
(0.34±0.79, 0.45±0.09, 0.29±0.03, and 0.39±0.12, respectively)
(P=0.062). In empty vector-transfected stem cells, the levels of
these proteins prior to differentiation were 0.72±0.17, 0.81±0.11,
0.79±0.09, and 0.75±0.15, respectively; the levels of all four
proteins decreased significantly following differentiation
(0.32±0.09, 0.41±0.07, 0.32±0.13, and 0.36±0.06, respectively).
Similar changes were observed in untransfected cells, which
expressed these proteins at levels of 0.78±0.03, 0.83±0.01,
0.81±0.04, and 0.77±0.05, respectively, prior to differentiation,
and at significantly lower levels following differentiation
(0.31±0.11, 0.36±0.12, 0.37±0.11, and 0.38±0.02, respectively). The
levels of E-cadherin (a marker of differentiation) in these cells
were also determined by Western blotting. In WWOX-expressing cells,
the E-cadherin level did not alter significantly (mean ± SD prior
to differentiation, 0.69±0.09; following differentiation,
0.71±0.02). In empty vector-transfected cells, the E-cadherin level
was 0.41±0.02 prior to differentiation, and increased significantly
to 0.72±0.01 following differentiation. Similarly, the E-cadherin
level was 0.46±0.06 in untransfected stem cells prior to
differentiation, increasing significantly to 0.71±0.04 when the
cells had differentiated (Fig.
4).
WWOX confers sensitivity to
chemotherapeutic drugs in ovarian cancer stem cells
Ovarian cancer stem cells were treated with
cisplatin, doxorubicin and mitoxantrone, to evaluate their
sensitivity to chemotherapeutic drugs (Table I). Under the same treatment
conditions for all drugs, the relative viability of WWOX-expressing
cells was significantly lower than that of empty vector-transfected
cells or untransfected cells, indicating that WWOX-expressing cells
had an attenuated resistance to the three drugs. When treated with
0.25 μg/ml cisplatin, the relative viability of WWOX-expressing
cells was 0.593±0.136 (P=0.009), significantly lower than empty
vector-transfected cells (0.823±0.103) or untransfected cells
(0.861±0.201). Similarly, in the presence of 0.5 μg/ml cisplatin,
the relative viability of WWOX-expressing cells (0.372±0.136) was
significantly lower compared with that of empty vector-transfected
cells (0.731±0.167) or untransfected cells (0.702±0.129). Treatment
with 0.5 μmol/l doxorubicin significantly lowered the relative
viability of WWOX-expressing cells (0.461±0.092) compared with
empty vector-transfected cells (0.782±0.133) or untransfected cells
(0.841±0.211). When doxorubicin was used at 1.5 μmol/l, the
relative viability of WWOX-expressing cells (0.266±0.004) was also
significantly lower compared with that of empty vector-transfected
cells (0.601±0.007) or untransfected cells (0.582±0.121). When
treated with 0.05 μg/ml mitoxantrone, the relative viability of
WWOX-expressing cells (0.396±0.101) was significantly lower than
either empty vector- transfected cells (0.702±0.141) or
untransfected cells (0.631±0.161); when doxorubicin was used at
0.25 μg/ml, the relative viability of WWOX-expressing cells
(0.103±0.136) was significantly lower compared with that of empty
vector-transfected cells (0.424±0.002) or untransfected cells
(0.371±0.003).
 | Table IWWOX increases chemosensitivity of
ovarian cancer stem cells to chemotherapeutic drugs following gene
transfection. |
Table I
WWOX increases chemosensitivity of
ovarian cancer stem cells to chemotherapeutic drugs following gene
transfection.
| Relative cell
viability, mean ± standard deviation |
|---|
|
|
|---|
| Cisplatin | Doxorubicin | Mitoxantrone |
|---|
|
|
|
|
|---|
| Cell group | 0.25 μg/ml | 0.5 μg/ml | 0.5 μmol/l | 1.5 μmol/l | 0.05 μg/ml | 0.25 μg/ml |
|---|
| WWOX-expressing
cells | 0.593±0.136 | 0.372±0.136 | 0.461±0.092 | 0.266±0.004 | 0.396±0.101 | 0.103±0.136 |
| Empty-vector
cells | 0.823±0.103 | 0.731±0.167 | 0.782±0.133 | 0.601±0.007 | 0.702±0.141 | 0.424±0.002 |
| Untransfected
cells | 0.861±0.201 | 0.702±0.129 | 0.841±0.211 | 0.582±0.121 | 0.631±0.161 | 0.371±0.003 |
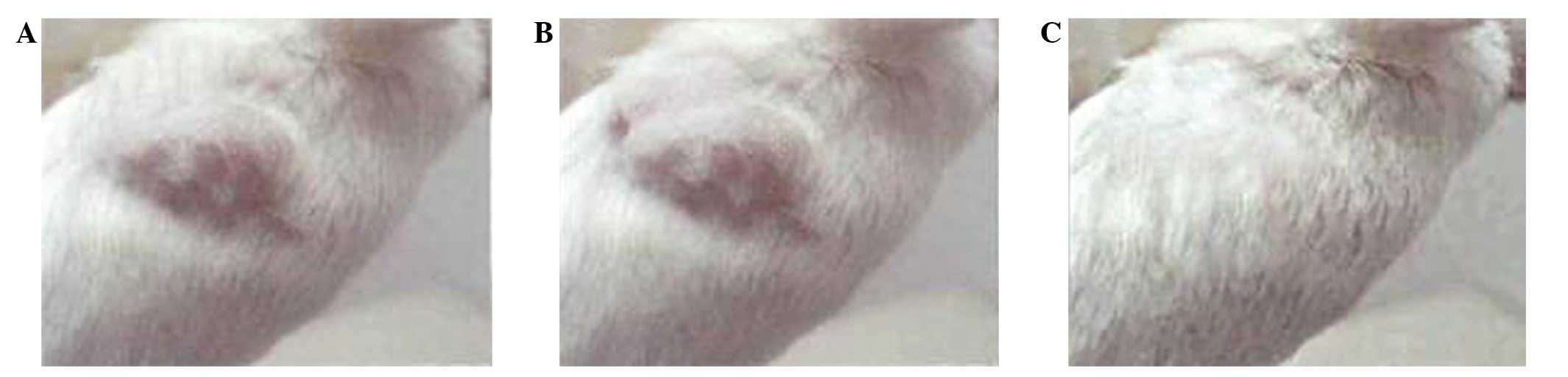
WWOX inhibits the in vivo tumorigenicity
of ovarian cancer stem cells
When ovarian cancer stem cells were transplanted
into NOD/SCID mice, empty vector-transfected cells and
untransfected cells formed tumors with a minimum of
1×103 cells, exhibiting a tumorigenesis rate of 2/5 and
a tumor-forming time of 43–55 days for the two groups.
Transplanting a greater number of cells led to an increase in
tumorigenesis rate, and reduction in tumor-forming time.
WWOX-expressing cells only formed tumors in vivo when a
minimum of 2×104 cells were transplanted. Compared with
empty vector-transfected cells and untransfected cells at the same
dose, WWOX-expressing cells had a lower rate of tumorigenesis (1/5,
compared with 5/5 for empty vector-transfected and untransfected
cells) and a longer tumor-forming time (76.9 days, compared with
27.3 and 28.1 days for untransfected and empty vector-transfected
cells, respectively). Thus the tumorigenicity of WWOX-expressing
cells was 20-fold lower than that of untransfected or empty
vector-transfected cells (Table II
and Fig. 5).
 | Table IIWWOX reduces in vivo
tumorigenic capability of ovarian cancer stem cells. |
Table II
WWOX reduces in vivo
tumorigenic capability of ovarian cancer stem cells.
| Tumorigenesis rate
(tumor forming time, days) |
|---|
|
|
|---|
| Cells | 103
cells | 2×103
cells | 2×104
cells | 2×105
cells |
|---|
| Untransfected | 2/5 (43–52) | 5/5 (30–44) | 5/5 (19–33) | 5/5 (14–26) |
| Empty vector | 2/5 (48–55) | 5/5 (29–45) | 5/5 (20–31) | 5/5 (15–27) |
| WWOX-expressing | 0/5 (N/A) | 0/5 (N/A) | 1/5 (68) | 5/5 (73–87) |
Discussion
The cancer stem cell theory provides a novel
perspective on the processes of tumor-initiation and progression
(12). Cancer stem cells have the
following properties: i) Self-renewal capability; ii)
differentiation potential; iii) expression of stem cell markers;
iv) resistance to chemotherapeutic drugs; and v) tumorigenicity in
immune-deficient mice (13–16). In the current study, the plasmid
pcDNA3.1-WWOX was transfected into human ovarian cancer stem
cells to determine the effects of WWOX overexpression on stem cell
properties.
In the sphere-forming assay, WWOX-expressing cells
exhibited a significantly diminished sphere-forming ability
compared with the control cells (empty vector-transfected cells or
untransfected cells), indicating that WWOX expression inhibits the
self-renewal capability of ovarian cancer stem cells. Conversely,
when grown in serum-containing medium to induce cellular
differentiation, WWOX-expressing stem cells adhered and grew more
readily compared with control cells. Flow cytometry analysis
revealed that, in control cells, the CD133+ and CD117+ cell
populations decreased following differentiation, while fewer
WWOX-expressing cells were positive for CD133 and CD117 compared
with control cells prior to differentiation, and this did not alter
significantly following differentiation. In addition, Western blot
analysis revealed that the expression of the stem cell markers
ABCG2, Nanog, OCT4, and BCRP in control cells reduced following
differentiation; WWOX-expressing cells, by contrast, exhibited
lower levels of these proteins prior to differentiation, and these
levels did not decrease significantly following differentiation.
Conversely, control cells produced more E-cadherin following
differentiation, while WWOX-expressing cells produced more
E-cadherin compared with control cells prior to differentiation,
but exhibited no significant increase in E-cadherin production
following differentiation. Collectively, the results suggest that
WWOX-expressing cells lose their potential for differentiation.
Cancer stem cells differ from mature, differentiated
cells in their resistance to chemotherapeutic drugs (17). In the current study, the first-line
chemotherapy drug for ovarian cancer, cisplatin, and the two most
widely used drugs in cancer stem cell studies, doxorubicin and
mitoxantrone, were used to evaluate the alterations in drug
sensitivity in WWOX-expressing ovarian cancer stem cells.
Consistent with the results from other cancer types (18–22),
ovarian cancer stem cells exhibited multi-drug resistance. However,
when WWOX was expressed, these cells exhibited significantly
decreased resistance to these drugs, indicating that WWOX may
reverse the drug resistance of ovarian cancer stem cells.
Ovarian cancer stem cells were also transplanted
into female NOD/SCID mice in order to determine their in
vivo tumorigenicity. The results indicated that ovarian cancer
stem cells possessed high tumorigenicity, however, WWOX expression
led to a lowered tumorigenesis rate and a longer time for tumors to
form, suggesting that WWOX expression reduces tumorigenicity in
these cells.
In conclusion, cancer stem cells derived from the
human ovarian cancer cell line HO8910 possess stem cell properties,
including self-renewal ability, differentiation potential, in
vivo tumorigenic capability, expression of stem cell markers at
high levels, and multiple drug resistance. The results also
indicate that WWOX expression may suppress the stem cell properties
of these cells. The present study provides an experimental basis
for ovarian cancer gene therapy, as the WWOX gene was found to
exhibit a significant effect on the biological behavior of ovarian
cancer stem cells and thus, may present a novel therapeutic target
for ovarian cancer treatment. Future studies are required to
elucidate the mechanisms governing WWOX-mediated inhibition of stem
cell properties in ovarian cancer stem cells, and to develop WWOX
gene therapy as a novel biological therapeutic method to improve
survival of ovarian cancer patients.
References
|
1
|
Gangemi R, Paleari L, Orengo AM, et al:
Cancer stem cells: a new paradigm for understanding tumor growth
and progression and drug resistance. Curr Med Chem. 16:1688–1703.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bellayr IH and Li Y: Stem Cells: It’s Good
To Have Choices. J Am Col Certif Wound Spec. 23:92–94. 2009.
|
|
3
|
Ghadially R: The role of stem and
circulating cells in cancer metastasis. J Surg Oncol. 103:555–557.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan HC, Yu N and Tong JY: Isolation of
cancer stem cells from ovarian cancer cell line HO9810 and
identification of their biological characteristics. Jiang Su Yi
Yao. 10:1152–1155. 2012.(In Chinese).
|
|
5
|
Bednarek AK, Laflin KJ, Daniel RL, et al:
WWOX, a novel WW domain-containing protein mapping to human
chromosome 16q23.3–24.1, a region frequently affected in breast
cancer. Cancer Res. 60:2140–2145. 2000.PubMed/NCBI
|
|
6
|
Del Mare S, Salah Z and Aqeilan RI: WWOX:
its genomics, partners, and functions. J Cell Biochem. 108:737–745.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Liu J, Ren Y, Yang J and Liu P:
Common Chromosomal Fragile Site Gene WWOX in Metabolic Disorders
and Tumors. Int J Biol Sci. 10:142–148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan HC and Zhang J: Effects of sodium
valproate on the growth of human ovarian cancer cell line HO8910.
Asian Pac J Cancer Prev. 13:6429–6433. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan H and Sun J: Methylation status of
WWOX gene promoter CpG islands in epithelial ovarian cancer and its
clinical significance. Biomed Rep. 1:375–378. 2013.
|
|
10
|
Yan H, Yu N and Tong J: Effects of
5-Aza-2′-deoxycytidine on the methylation state and function of the
WWOX gene in the HO-8910 ovarian cancer cell line. Oncol Lett.
6:845–849. 2013.PubMed/NCBI
|
|
11
|
Yan HC, Lu XY, Han QY and Jin LS:
Construction and identification of WWOX gene eukaryotic expression
vector. Jiang Su Yi Yao. 3:287–288. 2008.(In Chinese).
|
|
12
|
Dick JE: Stem cell concepts renew cancer
research. Blood. 112:4793–4807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Suzuki Y, Ishii H, Sekimoto M, Doki Y and
Mori M: Cancer stem cell. Nihon Rinsho. 69:98–102. 2011.(In
Japanese).
|
|
14
|
Ghaffari S: Cancer, stem cells and cancer
stem cells: old ideas, new developments. F1000 Med Rep. 3:232011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Natarajan TG, Ganesan N and Fitzgerald KT:
Cancer stem cells and markers: new model of tumorigenesis with
therapeutic implications. Cancer Biomark. 9:65–99. 2010.
|
|
16
|
Maitland NJ and Collins AT: Cancer stem
cells - A therapeutic target? Curr Opin Mol Ther. 12:662–673.
2010.PubMed/NCBI
|
|
17
|
Stevenson K, McGlynn L and Shiels PG: Stem
cells: outstanding potential and outstanding questions. Scott Med
J. 54:35–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fukuda K, Saikawa Y, Ohashi M, et al:
Tumor initiating potential of side population cells in human
gastric cancer. Int J Oncol. 34:1201–1207. 2009.PubMed/NCBI
|
|
19
|
Ning ZF, Huang YJ, Lin TX, et al:
Subpopulations of stem-like cells in side population cells from the
human bladder transitional cell cancer cell line T24. J Int Med
Res. 37:621–630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hirose H, Yamamoto H, Miyoshi N, et al:
Cancer stem cells in solid tumors. Gan To Kagaku Ryoho.
37:2809–2812. 2010.(In Japanese). PubMed/NCBI
|
|
21
|
Roy S and Majumdar AP: Cancer Stem Cells
in Colorectal Cancer: Genetic and Epigenetic Changes. J Stem Cell
Res Ther. 7:103422012.
|
|
22
|
Cetin I and Topcul M: Cancer stem cells in
oncology. J BUON. 17:644–648. 2012.
|