Introduction
Angiosarcomas are rare malignant sarcomas derived
from vascular endothelial cells, and accounting for 1–2% of all
soft-tissue sarcomas (1–5). In total, ~60% of angiosarcomas are
cutaneous and the majority of these occur in the head and neck
(1,3). Due to its high aggressiveness and
multifocality, the prognosis of angiosarcoma is poor, with a
reported five-year survival rate of ~35% in non-metastatic
angiosarcoma cases (1,4,6). The
majority of cases of recurrence (75%) occur within 24 months of
local treatment (1). For local
disease, radical resection and adjuvant radiotherapy are
recommended (5), while for
metastatic angiosarcoma, chemotherapy is the primary treatment
choice, although there is no standard regimen (5).
The present study describes a case of scalp
angiosarcoma, which was treated with surgery, followed by
recurrence and multiple metastases, which were treated with
post-operative combination chemotherapy and radiotherapy. The
patient obtained an overall survival time of 38 months following
initial diagnosis.
Case report
A 66-year-old male was admitted to the Department of
Dermatology of the Sir Run Run Shaw Hospital (Zhejiang University
School of Medicine, Hangzhou, Zhejiang, China) in October 2007 with
a four-month history of scalp masses. Upon physical examination,
two masses without ulcers or tenderness were noted in the right
temporoparietal area, measuring 3.0×3.0 cm and 1.5×1.5 cm,
respectively. No superficial lymph nodes were palpable, and the
rest of the physical exam was unremarkable. Brain magnetic
resonance imaging (MRI) showed a subcutaneous soft-tissue mass with
irregular margins in the right temporoparietal area, which was
moderately enhanced upon enhanced scanning. Serum tumor marker
levels were normal. Chest and abdominal computed tomography (CT)
scans did not indicate distant metastases.
Surgical excision was performed on October 31, 2007,
following a pre-operative evaluation in the Department of
Neurosurgery. The resection range was as large as 8.0×10.0 cm, so
skin grafting with a free flap taken from the outer side of the
right thigh was performed. The tumor did not invade the galea
aponeurosis, and complete resection was achieved. The
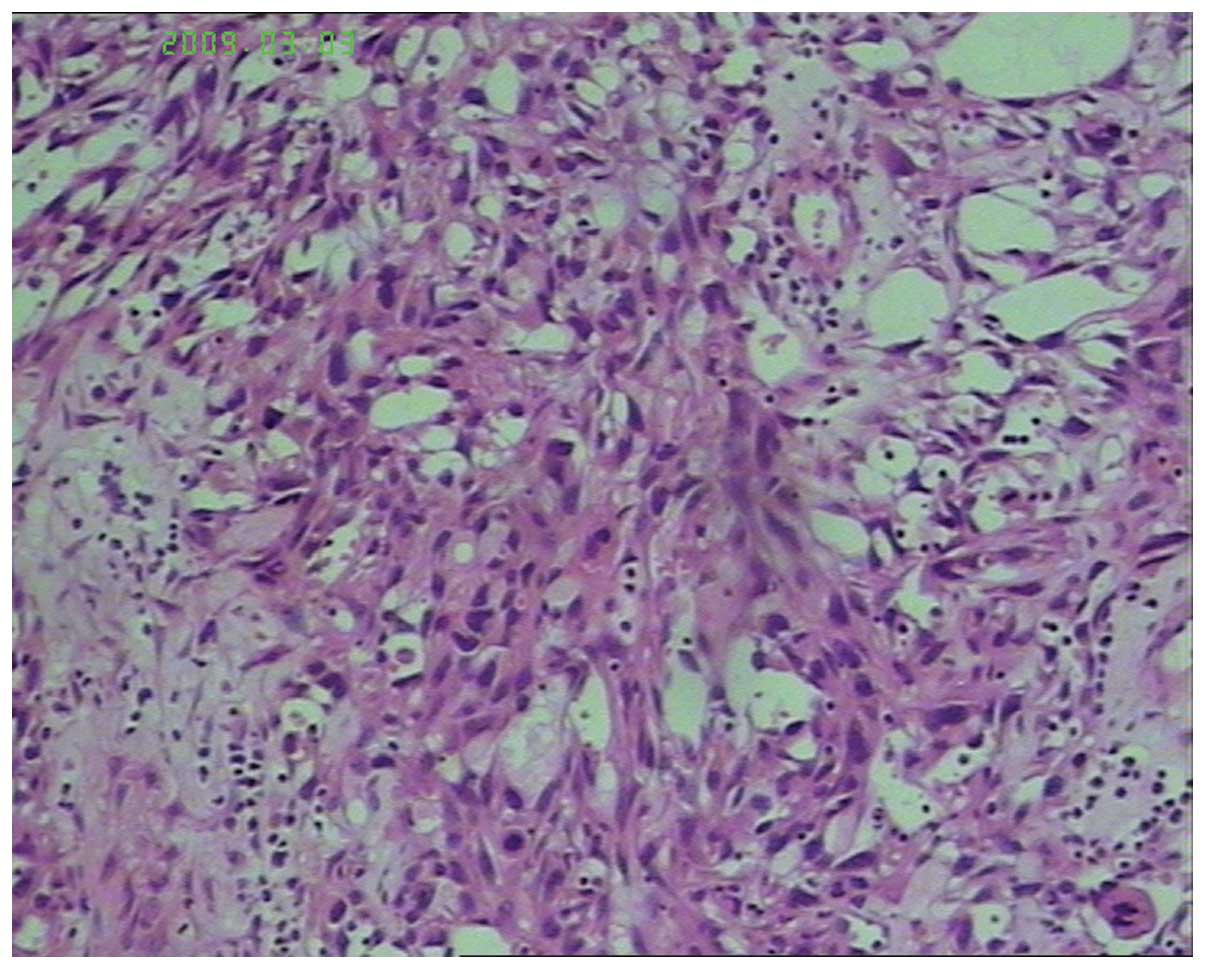
post-operative pathology revealed an infiltrative, irregularly
configured vascular channel tumor. The tumor formed a pattern of
vascular channels which were interlacing and anastamosing, which
were lined with hyperchromatic endothelial cells, which exhibited
mitotic activity. The pathological diagnosis was angiosarcoma of
the scalp, with negative peripheral margins (Fig. 1). Adjuvant radiotherapy of the right
parietal area was started at two months post-surgery, with a β-line
dosage of 5,800 cGy/29 fractions for six weeks. Adjuvant
chemotherapy was refused by the patient for personal reasons.
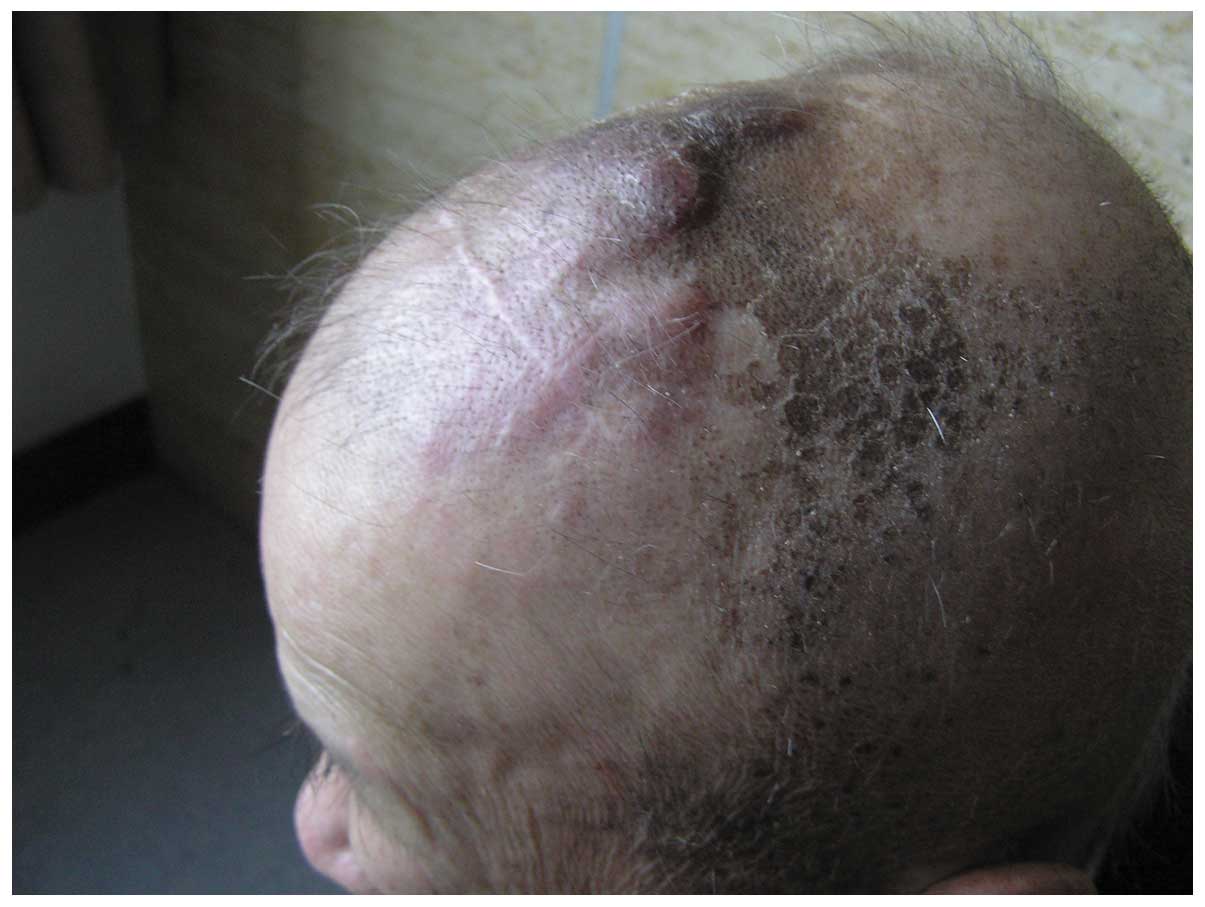
In June 2008, more than seven months after the
surgery, the patient was readmitted to the Department of
Neurosurgery presenting with multiple scalp masses (Fig. 2). This was diagnosed as a
post-operative recurrence of the scalp angiosarcoma. No evident
distant metastasis was documented by chest and abdominal CT scans.
The patient underwent an extended resection of the frontal and
occipital scalp masses, measuring 6.0×2.5 cm and 1.0×1.0 cm,
respectively, and a bilateral neck nodal dissection. The
post-operative histopathological examination showed two scalp
angiosarcomas with negative resection margins, and two out of eight
lymph nodes were metastatic. Again, the patient refused
post-operative chemotherapy.
Three months later in September 2008, a mass
measuring 3×4 cm was found close to the left pulmonary hilum in a
routine chest X-ray. A chest CT scan revealed a lobulated hilar
mass of 2.5×2.5 cm in size in the left upper lobe, with enlargement
of the mediastinal lymph nodes. A subsequent CT-guided core needle
biopsy of the lung mass indicated metastatic angiosarcoma. The
patient experienced no discomfort, such as coughing, dyspnea or
chest distress. Following a diagnosis of post-operative lung
metastasis of the scalp angiosarcoma, the patient received six
cycles of first-line palliative chemotherapy consisting of 750
mg/m2 cyclophosphamide on day 1, 60 mg/m2
epirubicin on day 1, 1.4 mg/m2 vincristine on day 1 and
250 mg/m2 dacarbazine from day 1–5, repeated every three
weeks. Chest CT showed disappearance of the lung mass, which
indicated a complete response (CR) according to to the Response
Evaluation Criteria in Solid Tumors (7). The progression-free survival (PFS)
time following first-line chemotherapy was eight months, and the
patient tolerated the chemotherapy extremely well. Grade 2 nausea
and leucopenia were observed according to Common Terminology
Criteria for Adverse Events (8). No
severe adverse reactions, such as febrile neutropenia, or cardiac
and renal dysfunction, were documented during the treatment
process.
In April 2009, four months after the end of
first-line chemotherapy, the patient presented with waist ache and
numbness of the lower limbs. Brain MRI revealed a mass in the left
parietal lobe. Whole-body bone single-photon emission CT showed
abnormally enhanced metabolism in the vertebral joint of the 8th
left rib, the 8th thoracic vertebra and the 1st lumbar vertebra.
Further spinal MRI indicated signal changes in these bones, which
were considered metastases. Chest CT showed no lesions.
Consequently, a clinical diagnosis of post-operative recurrence of
scalp angiosarcoma, with lung, brain and bone metastases, was
established. The patient was referred to a radiation oncologist to
receive brain, thoracic and lumbar vertebrae radiotherapy, with an
X-ray dosage of 6 MV, 4,000 cGy/20 fractions for four weeks. The
symptoms of waist ache and numbness of the lower limbs were greatly
relieved upon the conclusion of radiotherapy. However, during the
radiotherapy, a scalp nodule of 2 cm in diameter was found at the
edge of the previous surgery area and a mass of 1.0×1.0 cm in size
was found in the left parotid gland area, both without tenderness.
The disease was considered as having progressed, so second-line
chemotherapy was administered with a regimen of 75 mg/m2
docetaxel and 75 mg/m2 cisplatin on day 1, repeated
every three weeks. Severe myelosuppression and liver dysfunction
occurred following the first cycle of treatment. Therefore, the
regimen was changed to 60 mg/m2 docetaxel on day 1 plus
30 mg/m2 cisplatin from day 1–2. The patient tolerated
this well and received another five cycles of chemotherapy. In
addition, twice daily administration of 100 mg oral thalidomide was
prescribed throughout the treatment process and chemotherapy
intervals. The scalp nodule and left parotid mass significantly
reduced in size following the chemotherapy, and was considered to
be a partial response (PR). The patient again obtained a PFS time
of eight months for the second-line chemotherapy.
In March 2010, half a year after stopping the
chemotherapy, an abdominal CT scan revealed multiple liver
metastases during a routine follow-up. Chemotherapy was
administered again with a regimen of 1.0 g/m2
gemcitabine on days 1 and 8, 75 mg/m2 cisplatin on day 1
and 15 mg endostatin on days 1–14, repeated every three weeks.
Following six cycles of chemotherapy, the liver metastases were
greatly reduced in size, which was again considered to be a PR. The
patient continued to use endostatin as maintenance therapy. A PFS
time of nine months was obtained for this third-line
chemotherapy.
In December 2010, the disease progressed again. Best
supportive care was provided, and the patient finally succumbed in
February 2011, with an overall survival time of 38 months following
initial diagnosis. Written informed consent was obtained from the
patient’s family for publication of this case study and the
accompanying images.
Discussion
Angiosarcomas can occur in any region of the human
body, and are subdivided into groups, including
lymphoedema-associated angiosarcomas, cutaneous angiosarcomas,
radiation-induced angiosarcomas, soft-tissue angiosarcomas and
primary-breast angiosarcomas (9).
The most commonly affected area is the face and scalp region,
accounting for >50% of cutaneous angiosarcomas (3). As a rare and deadly malignant tumor,
angiosarcoma of the head and neck accounts for <0.1% of all head
and neck malignancies (10,11). Livingston and Klemperer first
reported this disease in 1926 (12), and 38 years later, Jones described a
series of nine cases occurring on the face and scalp (13). The prevalence of angiosarcomas is
highest in elderly patients, however, they can develop at any age,
and their distribution is similar between the two genders (5).
Due to the rarity and masquerading manifestations,
angiosarcoma is often diagnosed late. In the early stage, cutaneous
angiosarcoma can present as a bruise, or a typically multifocal
raised purple-red papule, which may be mistaken for a benign lesion
(9). With increasing tumor size,
tissue infiltration, edema, tumor fungation, ulceration and
hemorrhage can occur (9). If
untreated, the tumor can reach ≥20 cm in size. Local invasion into
the underlying calvarium and brain occurs frequently (14). Moreover, distant metastases often
occur via the hematogenous or lymphatic routes, with the lung being
the most commonly affected site (5,14).
Other common sites include the liver, bones, soft-tissue structures
and lymph nodes (1,4,15–18).
The patient in the current study initially presented with a scalp
mass. In the follow-up visits, distant metastases in the lung,
brain and bones were sequentially detected.
The diagnosis of angiosarcoma mainly depends on the
pathology. Under the microscope, pleomorphic and malignant
endothelial cells are apparent in typical angiosarcoma tissues. In
areas that are well differentiated, abnormal endothelial cells form
functioning vascular sinusoids continuous with normal vascular
channels. However, in areas of poor differentiation, the malignant
endothelial cells form continuous sheets, usually with necrosis and
hemorrhage (9,19,20).
As it is difficult to diagnose angiosarcoma by its morphology,
immunohistochemistry plays an important role in confirming the
diagnosis. Typically, endothelial markers, including CD34, CD31,
von Willebrand factor, Ulex europaeus agglutinin 1 and vascular
endothelial growth factor are expressed (5). Von Willebrand factor, Ulex europaeus
agglutinin 1 and CD31 are the most useful markers in
poorly-differentiated cases (21).
Treatment modalities for angiosarcoma include
resection with a wide margin, radiotherapy, immunotherapy and
chemotherapy. Combination treatment is recommended to obtain an
improved prognosis (22). For local
disease, radical surgery in the form of a complete resection and
adjuvant radiotherapy are suggested. However, clear margins are
rarely obtained for scalp angiosarcoma (~20% cases) despite large
resection areas (1,17). For this reason, post-operative
radiotherapy is recommended. For metastatic angiosarcomas, however,
chemotherapy is the primary treatment choice (5), although there have been no large
randomized trials to confirm a standard chemotherapy regimen. In
soft-tissue sarcomas, the main chemotherapy drugs are
anthracyclines, ifosfamide and taxanes (5). Van Glabbeke et al performed a
large meta-analysis of the effect of anthracycline-based
chemotherapy on patients with soft-tissue sarcomas, and found an
overall response rate of 26% and a median overall survival time of
51 weeks (23). Certain studies
have reported similar response rates and worse survival times in
angiosarcoma patients (4,24). Liposomal doxorubicin and paclitaxel
have also been reported to be useful in angiosarcoma in a number of
retrospective studies (25–28).
As aforementioned, angiosarcoma is derived from
vascular endothelial cells. Based on the pathogenesis of
angiosarcoma, certain antiangiogenic drugs, such as thalidomide
(29–31), bevacizumab (32,33)
and sorafenib (34), have been used
and have shown promising therapeutic effects in angiosarcoma
patients. The biological activities of thalidomide include
antiangiogenesis, tumor necrosis factor-α (TNF-α) inhibition and
immune stimulation. It is has previously been reported to be useful
in angiosarcoma of the breast, small intestine and scalp (29–31).
In the present case, chemotherapy combined with thalidomide and
endostatin (Endostar) was used to control the disease. Endostar
(YH-16), is a novel recombinant human endostatin that is expressed
and purified in Escherichia coli, and acts specifically on
neovascular endothelial cells to induce cell apoptosis, thereby
playing an antiangiogenic role in treating cancer (35).
As the patient in the present study developed lung
metastasis after surgery, first-line systemic chemotherapy
consisting of cyclophosphamide, epirubicin, vincristine and
dacarbazine was administered. The patient tolerated treatment well
and achieved a CR after six cycles, proving that the combination
regimen was effective for angiosarcoma. The PFS time for this
first-line treatment was approximately eight months. Docetaxel plus
cisplatin, combined with oral thalidomide were administered as
second-line chemotherapy, and a PR was achieved with a PFS time of
approximately eight months. Gemcitabine plus cisplatin combined
with endostatin were administered as the third-line treatment, and
a PR was achieved again with a PFS time of approximately nine
months. The overall survival time of the patient was 38 months
following initial diagnosis.
Angiosarcoma is a rare malignant sarcoma that is
prone to local recurrence and distal metastasis. Radical surgery is
the most useful method for treating this disease. Combination
treatment based on chemotherapy is strongly recommended for
advanced angiosarcoma, however, there is no standard chemotherapy
regimen at present. The present study indicates that the three
regimens of cyclophosphamide, epirubicin, vincristine and
dacarbazine, docetaxel, cisplatin and thalidomide, and gemcitabine,
cisplatin and endostatin are effective for treating angiosarcoma.
Angiogenesis agents such as thalidomide and endostatin may be of
potential use. Nevertheless, further large-scale prospective
studies are required to improve the treatment of angiosarcoma.
Abbreviations:
|
MRI
|
magnetic resonance imaging
|
|
CT
|
computed tomography
|
|
CR
|
complete response
|
|
PFS
|
progression-free survival
|
|
PR
|
partial response
|
References
|
1
|
Mark RJ, Poen JC, Tran LM, et al:
Angiosarcoma. A report of 67 patients and a review of the
literature. Cancer. 77:2400–2406. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cafiero F, Gipponi M, Peressini A, et al:
Radiation-associated angiosarcoma: diagnostic and therapeutic
implications: two case reports and a review of the literature.
Cancer. 77:2496–2502. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aust MR, Olsen KD, Lewis JE, et al:
Angiosarcomas of the head and neck: clinical and pathologic
characteristics. Ann Otol Rhinol Laryngol. 106:943–951. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fury MG, Antonescu CR, Van Zee KJ, et al:
A 14-year retrospective review of angiosarcoma: clinical
characteristics, prognostic factors and treatment outcomes with
surgery and chemotherapy. Cancer J. 11:241–247. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Young RJ, Brown NJ, Reed MW, et al:
Angiosarcoma. Lancet Oncol. 11:983–991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fayette J, Martin E, Piperno-Neumann S, et
al: Angiosarcomas, a heterogeneous group of sarcomas with specific
behavior depending on primary site: a retrospective study of 161
cases. Ann Oncol. 18:2030–2036. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trotti A, Colevas AD, Setser A, et al:
CTCAE v3.0: development of a comprehensive grading system for the
adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weiss SW and Goldblum JR: Enzinger and
Weiss’s Soft Tissue Tumors. 5th edition. Mosby Elsevier; St. Louis,
MO: 2008
|
|
10
|
Wolov RB, Sato N, Azumi N and Lack EE:
Intra-abdominal ‘angiosarcomatosis’ report of two cases after
pelvic irradiation. Cancer. 67:2275–2279. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Slingluff CL Jr, Hendrix J and Seigler HF:
Melanoma and cutaneous malignancies. Sabiston Textbook of Surgery:
The Biological Basis of Modern Surgical Practice. 16th edition.
Townsend CM Jr: W.B. Saunders Company; Philadelphia: pp. 487–510.
2001
|
|
12
|
Livingston SF and Kemperer P: Malignant
angiomas; with reference to the question of sarcoma due to roentgen
ray. Arch Pathol Lab Med. 1:899–910. 1926.
|
|
13
|
Jones EW: Malignant angioendothelioma of
the skin. Br J Dermatol. 76:21–39. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Obeng MK, Hernandez A, Dastgir A, et al:
Angiosarcoma of the scalp with calvarium involvement in a
50-year-old African-American man. J Natl Med Assoc. 96:1507–1512.
2004.PubMed/NCBI
|
|
15
|
Abraham JA, Hornicek FJ, Kaufman AM, et
al: Treatment and outcome of 82 patients with angiosarcoma. Ann
Surg Oncol. 14:1953–1967. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naka N, Ohsawa M, Tomita Y, et al:
Angiosarcoma in Japan: a review of 99 cases. Cancer. 75:989–996.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pawlik TM, Paulino AF, McGinn CJ, et al:
Cutaneous angiosarcoma of the scalp: a multidisciplinary approach.
Cancer. 98:1716–1726. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lahat G, Dhuka AR, Lahat S, et al: Outcome
of locally recurrent and metastatic angiosarcoma. Ann Surg Oncol.
16:2502–2509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Calonje E and Fletcher CDM: Vascular
tumors. Diagnostic Histopathology of Tumors. Fletcher CDM: 3rd
edition. Churchill Livingstone; pp. 41–76. 2007
|
|
20
|
Weiss SW, Lasota J and Miettinen MM:
Angiosarcoma of soft tissue. World Health Organization
Classification. Pathology and Genetics of Tumours of Soft Tissue
and Bone. Fletcher CDM, Unni KK and Mertens F: IARC Press; Lyon:
pp. 175–177. 2002
|
|
21
|
Ohsawa M, Naka N, Tomita Y, Kawamori D,
Kanno H and Aozasa K: Use of immunohistochemical procedures in
diagnosing angiosarcoma. Evaluation of 98 cases. Cancer.
75:2867–2874. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Naka N, Ohsawa M, Tomita Y, et al:
Prognostic factors in angiosarcoma: a multivariate analysis of 55
cases. J Surg Oncol. 61:170–176. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Van Glabbeke M, van Oosterom AT,
Oosterhuis JW, et al: Prognostic factors for the outcome of
chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185
patients treated with anthracycline containing first-line regimens
- a European Organization for Research and Treatment of Cancer Soft
Tissue and Bone Sarcoma Group Study. J Clin Oncol. 17:150–157.
1999.PubMed/NCBI
|
|
24
|
Budd GT: Management of angiosarcoma. Curr
Oncol Rep. 4:515–519. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mathew P, Vakar-Lopez F and Trocoso P:
Protracted remission of metastatic epithelioid angiosarcoma with
weekly infusion of doxorubicin, paclitaxel and cisplatin. Lancet
Oncol. 7:92–93. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eiling S, Lischner S, Busch JO, et al:
Complete remission of a radio-resistant cutaneous angiosarcoma of
the scalp by systemic treatment with liposomal doxorubicin. British
J Derm. 147:150–153. 2002. View Article : Google Scholar
|
|
27
|
Verdier E, Carvalo P, Young P, et al:
Lymphangiosarcoma treated with liposomal doxorubicin (Caelyx). Ann
Dermatol Venereol. 134:760–763. 2007.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Verweij J, Lee SM, Ruka W, et al:
Randomized phase II study of docetaxel versus doxorubicin in first-
and second-line chemotherapy for locally advanced or metastatic
soft tissue sarcomas in adults: a study of the european
organization for research and treatment of cancer soft tissue and
bone sarcoma group. J Clin Oncol. 18:2081–2086. 2000.PubMed/NCBI
|
|
29
|
Fraiman G, Ganti AK, Potti A and Mehdi S:
Angiosarcoma of the small intestine: a possible role for
thalidomide? Med Oncol. 20:397–402. 2003. View Article : Google Scholar
|
|
30
|
Ventura GJ and Roberts SC: Response of
metastatic angiosarcoma to thalidomide: Possible synergism with
radiation therapy. Proc Am Soc Clin Oncol. 19:575a2000.
|
|
31
|
Raina V, Sengar M, Shukla NK, et al:
Complete response from thalidomide in angiosarcoma after treatment
of breast cancer. J Clin Oncol. 25:900–901. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koontz BF, Miles EF, Rubio MA, et al:
Preoperative radiotherapy and bevacizumab for angiosarcoma of the
head and neck: two case studies. Head Neck. 30:262–266. 2008.
View Article : Google Scholar
|
|
33
|
Agulnik M, Yarber JL, Okuno SH, et al: An
open-label, multicenter, phase II study of bevacizumab for the
treatment of angiosarcoma and epithelioid hemangioendotheliomas.
Ann Oncol. 24:257–263. 2013. View Article : Google Scholar
|
|
34
|
Maki RG, D’Adamo DR, Keohan ML, et al:
Phase II study of sorafenib in patients with metastatic or
recurrent sarcomas. J Clin Oncol. 27:3133–3140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhuang HQ and Yuan ZY: Process in the
mechanisms of endostatin combined with radiotherapy. Cancer Lett.
282:9–13. 2009. View Article : Google Scholar : PubMed/NCBI
|