Introduction
Infiltrating cribriform carcinoma (ICC) of the
breast, which is characterized by a predominant cribriform growth
pattern of its invasive component, is a distinct histological type
of invasive carcinoma first described by Page et al in 1983
(1). The incidence of ICC is
reported to range from 0.3 to 3.5% (1–4). The
carcinoma has a low frequency of axillary nodal metastases and a
favorable prognosis (2). Previous
immunohistochemical studies (2,5–7)have
revealed that the majority of patients with ICC exhibited estrogen
receptor (ER) and progesterone receptor (PR) positive tumors, while
human epidermal growth factor receptor 2 (HER2) amplification was
rarely observed (5). For this
reason, some people recommended that this favorable histotype with
luminal tumor may be suitable for no therapy or endocrine therapy
alone (8). However, no standard
treatment guidelines exist for ICC and thus, treatment is mostly
based on that for invasive ductal carcinoma (IDC). Clinically, the
tumor usually presents as a mass, but is frequently asymptomatic.
The tumor is not usually detected by mammography, but may be
identified as a non-malignant mass on sonography. However, few
studies have described its radiological features. In the present
study, nine cases of ICC of the breast are presented in order to
provide additional clinicopathological data; in particular, imaging
findings for its diagnosis and treatment.
Patients and methods
Patient selection
Nine patients diagnosed with ICC were treated at the
Department of Breast Surgery, Yantai Yuhuangding Hospital
Affiliated to the Medical College of Qingdao University (Yantai,
Shandong, China) between 2007 and 2012. Based on the new definition
by the World Health Organization (WHO) (9) and the description by Page et al
in 1983, all cases were pure ICC. The study protocol was approved
by the Human Ethics Committee of the Yantai Yuhuangding Hospital
Affiliated to the Medical College of Qingdao University. Informed
consent was obtained from all patients prior to the surgery and the
examination of the specimens.
Patient history
The medical records of the patients were retrieved
from the hospital registry and the clinicopathological
characteristics, including age, menopausal status, family history,
laterality, tumor size, lymph node status, hormone receptor status,
HER2 status and radiological examinations, were analyzed, as well
as the treatment and outcome.
Pathological examination
The histology of the primary tumor was reviewed by
an expert pathologist. The pathological tumor stage
(tumor-node-metastasis stage) was assessed according to the
criteria established by the sixth edition of the American Joint
Committee on Cancer staging manual (10). Specimens were considered to be
HER2-positive when they scored +3 by immunohistochemistry or were
shown to be positive by fluorescent in situ hybridization
(9).
Results
Patient characteristics
The patient characteristics are shown in Table I. All nine cases were of females.
The median age was 55 years (range, 40–79 years) at diagnosis. Four
cases were premenopausal and the rest were menopausal. A mass or
lump in the breast was the main symptom in all patients. The tumor
involved the left breast in four patients and the right breast in
five patients. The tumors were mainly located in the upper outer
quadrant (5/9). The median size of the tumors was 1.7 cm (range,
1.0–3.2 cm).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Case no. | Age, years | Menopause | Family history | Laterality | Location
(quadrant) | Tumor size, cm | Surgery | Metastatic LNs,
n | TNM stage |
Immunohistochemistry | Chemotherapy | Radiotherapy | Endocrine
therapy | Follow-up,
months |
|---|
| 1 | 49 | No | Yes | Right | Superior
external | 1.5 | BCS+ALND | 5 | IIIA | ER(++), PR(+),
HER2(+) | CEFb | Yes | None | 28 |
| 2 | 40 | Yes | Yes | Right | Superior
external | 1.7 | BCS+SLNB | 0 | I | ER(++), PR(++),
HER2(−) | None | Yes | Tamoxifen (6
months) | 70 |
| 3 | 41 | No | No | Right | Superior
external | 1.1 | BCS+SLNB+ALND | 1 | IIA | ER(++), PR(++),
HER2(−) | None | None | None | 69 |
| 4 | 69 | Yes | No | Right | Superior
internal | 1.0 | BCS+SLNB | 0 | I | ER(+++), PR(+++),
HER2(−), p53(−), Ki-67 3%, CK5/6(−), CD10, p63 focal(+),
34βE12(+) | None | None | None | 48 |
| 5 | 68 | Yes | No | Right | Superior
external | 3.2 | BCS+ALND | 0 | IIA | ER(+++), PR(+++),
HER2(++)a, p53 2%, Ki-67 5%,
CK5/6(−), CD56(−), CgA(−), Syn(−) | None | Yes | Letrozole (17
months) | 17 |
| 6 | 46 | No | Yes | Left | Superior
internal | 2.5 | M+SLNB+ALND | 1 | IIB | ER(+), PR(+++),
HER2(−) | TEb | None | Unknown | 4 |
| 7 | 79 | Yes | No | Left | Superior
external | 3.0 | M+ALND | 1 | IIB | ER(+), PR(++),
HER2(−) | None | None | None | Unknown |
| 8 | 55 | No | Yes | Left | Inferior
external | 2.0 | M+SLNB+ALND | 2 | IIA | ER(+), PR(++),
HER2(−), p53(−), Ki-67 5% | CEFc | None | None | 53 |
| 9 | 71 | Yes | Yes | Left | Inferior
internal | 1.2 | M+SLNB | 0 | I | ER(+), PR(+),
HER2(−), p53 focal(+), Ki-67 2% | None | None | Arimidex (25
months) | 25 |
Examination findings
Tumor marker detection revealed that the level of
carcinoembryonic antigen in the blood serum was high in one patient
(4.01 ng/ml; normal range, 0–3.4 ng/ml) and that the ferritin level
was high in two patients (193.0 and 181.7 ng/ml; normal range,
13–150 ng/ml). Pre-operative sonographic findings were available
for review for all patients. Sonograms revealed that all masses
exhibited a hypoechoic internal echo texture (9/9) and that a
number of masses presented with an irregular shape (8/9), obscure
boundary (5/9), partially microlobulated (5/9) or
well-circumscribed (4/9) margins, and an inhomogeneous echo (8/9).
Color Doppler flow imaging revealed markedly increased flow signals
in two patients and a resistance index of 0.77 in one patient.
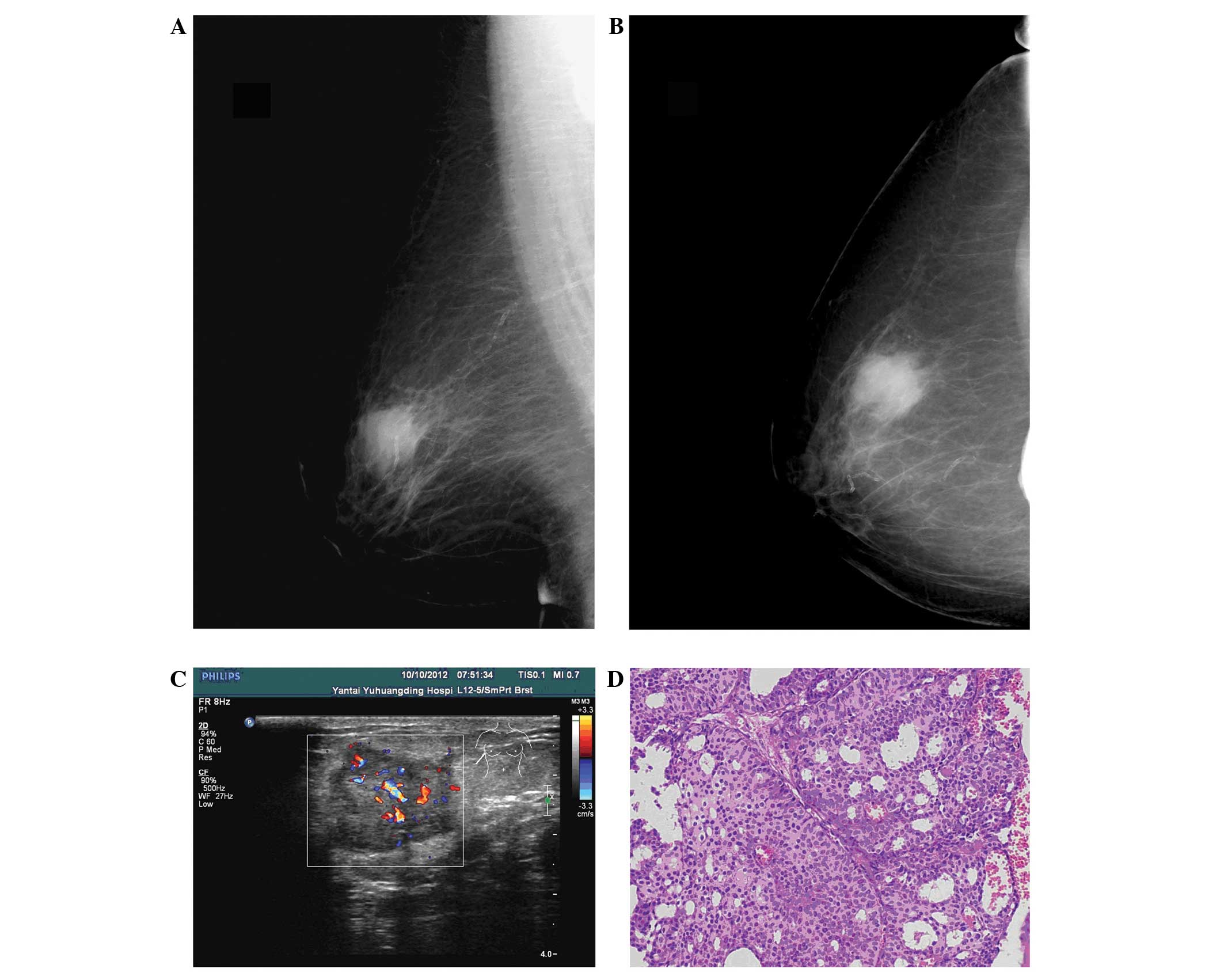
Pre-operative mammographic imaging was available for review for
eight patients. The tumors in two patients were mammographically
occult. The other six tumors that were visible exhibited increased
radiological density masses, and sand-like calcification was not
observed in all patients (Fig. 1).
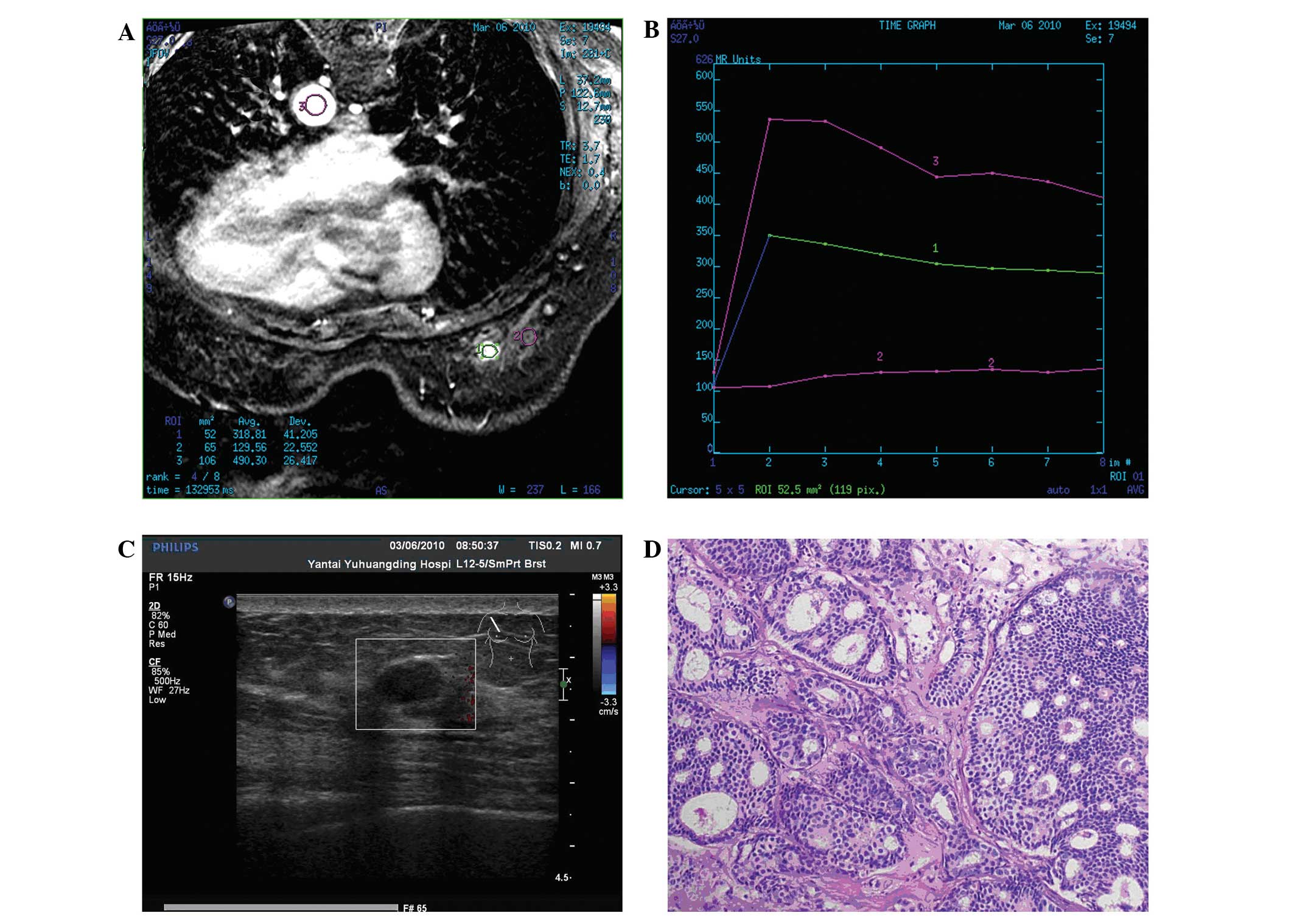
Magnetic resonance imaging performed in one patient revealed that
the mass exhibited a slightly high signal intensity on
fat-saturated T1- and T2-weighted images. Following contrast
enhancement, a homogeneous early enhancement was revealed with a
quick ascent and quick descent time-density curve (Fig. 2).
Pathological findings
Surgery was the primary treatment for all patients.
The frozen section technique was used for intraoperative
evaluation, but only 50% (4/8) of patients could be accurately
diagnosed. Hematoxylin and eosin (H&E) staining and
immunohistochemistry confirmed all cases. Six clinically
node-negative patients underwent sentinel lymph node biopsy; three
of whom exhibited lymph node metastasis and then received axillary
lymph node dissection. Four patients were treated with mastectomy
and the others with breast-conserving surgery. The pathological
findings revealed five cases with lymph node involvement, one of
which exhibited metastasis in more than three lymph nodes.
Immunohistochemistry revealed that all ICCs expressed ER and PR,
but that none were positive for HER2. An average of 3.75% (range,
2–5%) tumor cells exhibited nuclear staining for Ki-67 (n=4).
Treatment and follow-up
Following surgery, three patients received adjuvant
chemotherapy, three patients received radiotherapy and three
patients received adjuvant hormonal therapy. Follow-up was
undertaken for eight cases. With a median follow-up time of 38
months (range, 4–70 months), all cases survived without axillary
lymph node or distant metastases. However, one patient (case 2;
Table I) who received radiotherapy
and tamoxifen for six months without chemotherapy developed local
recurrence 49 months after breast-conserving surgery.
Breast-conserving surgery was performed again for local recurrence
at Qilu hospital of Shandong University (Jinan, China). Following
surgery, the patient received six cycles of chemotherapy (the
specific chemotherapy regimen is unknown) and adjuvant hormonal
therapy (letrozole). Subsequent to being followed up for 22 months,
the patient was alive with a disease-free status.
Discussion
ICC of the breast is characterized by a predominant
cribriform growth pattern of its invasive component according to
the new definition by the WHO (9)
and the description by Page et al from 1983 (1). Pure ICC is defined as being almost
entirely (>90%) of an invasive cribriform pattern, while lesions
that demonstrate a predominantly cribriform differentiation with
the remaining component limited to a tubular carcinoma (TC) pattern
are also included in the category of ICC. Cases with a component
that is <50% of a carcinoma type other than TC should be
regarded as a mixed type of ICC. In histopathological specimens,
this type of carcinoma must be distinguished from other invasive
breast carcinomas that exhibit a cribriform pattern, including
adenoid cystic carcinoma. Immunocytochemical staining for basement
membrane materials or an ultrastructural examination is recommended
when accurate diagnosis is difficult (12). In the cases of the present study, it
was observed that 50% (4/8) of patients could not be correctly
diagnosed by intraoperative frozen section, while H&E staining
and immunohistochemistry were able to confirm all cases.
The radiological findings of ICC are not well known,
since few studies have described the disease. Stutz et al
(4) reported that the tumor was
mammographically occult in four (50%) cases. The other four tumors
all showed as large (20–35-mm) spiculated masses and two contained
a few flecks of punctate calcification. Another case report
described a circumscribed high-density mass with
microcalcifications on mammography, which was described as a
borderline lesion on sonography (13). In the present series, all tumors
demonstrated an increased radiological density mass with the
exception of two (25%), which were mammographically occult, but
sand-like calcification was not observed. The ultrasound
appearances were not all entirely typical of breast carcinoma. The
study by Stutz et al revealed that the majority of tumors
(3/4) presented as an ill-defined, inhomogeneous solid mass, but
without distal acoustic attenuation (4). Another study revealed masses with an
oval (2/3) or irregular (1/3) shape, partially microlobulated (2/3)
or well-circumscribed (1/3) margins, and a hypoechoic (2/3) or an
isoechoic (1/3) internal echo texture. Sonographic assessments were
classified as Breast Imaging Reporting and Data System category 4A
in two cases and 4C in one case (n=3) (13). In the cases of the present study,
all masses exhibited a hypoechoic internal echo texture (9/9) and a
number of masses presented with an irregular shape (8/9), obscure
boundary (5/9), partially microlobulated (5/9) or
well-circumscribed (4/9) margins, and an inhomogeneous echo (8/9).
Although the sonographic findings are usually highly suggestive of
malignancy, this type of carcinoma may also be shown as a
non-malignant mass on sonography. Magnetic resonance imaging is
also an essential examination technique for breast cancer. In the
present study, the tumor revealed slightly high signal intensity on
fat-saturated T1- and T2-weighted images in one patient. Following
contrast enhancement, a homogeneous early enhancement was revealed
with a quick ascent and quick descent time-density curve. However,
homogeneous early enhancement with a delayed wash-out kinetic
pattern was also reported (6).
ICC is a well-differentiated neoplasm
architecturally, cytologically, ultrastructurally and functionally.
The nuclear grade is usually low or moderate, and the
ultrastructural features suggest a high degree of differentiation
(12). In a previous study,
immunohistochemistry revealed that all patients with pure ICC were
ER-positive, compared with 20/21 (95.2%) among the mixed ICC cases.
PR expression was positive in 26/30 (86.7%) pure ICC cases and
19/21 (90.5%) mixed ICC cases. However, HER2 amplification was
rarely observed (5). As in the
present cases, all ICCs expressed ER and PR, but none were positive
for HER2. Intraductal carcinoma, generally of the cribriform type,
and mutifocality are often observed in cases of ICC (1,2,9).
Clinically, the tumor usually presents as a mass,
but is frequently occult. For this reason, the lesions are usually
larger at presentation, although they grow slowly the majority of
the time (3). However, in the
present cases, the median size of the tumors (1.7 cm) was not
large. The axillary lymph nodes are less frequently involved in ICC
than in IDC (1). Venable et
al (2) reported that the
maximal number of metastatic lymph nodes in pure ICC was not more
than three, and that there was no marked difference in the positive
lymph node rate among the pure ICC, mixed ICC and IDC control
groups. As in the present cases, 55.6% (5/9) of patients presented
with lymph node metastasis, but only one case involved more than
three lymph nodes. Internal mammary node metastasis has also been
reported in pure ICC (15).
ICC manifests a better prognosis than that of IDC.
The 10-year overall survival rate for ICC is 90–100%, and the
outcome of mixed ICC is reported to be less favorable than that of
the pure form, but better than that of common ductal carcinoma
(1,3,16). In the present study, ICC was identified to be associated
with a series of favorable prognostic factors, including a smaller
tumor size, less frequent axillary lymph node metastasis, a higher
positive rate of ER and PR expression, no HER2 expression and a
lower proliferation index. Sand-like calcification, which may be
the result of an active secretory process by the tumor cells, was
not observed in any of the cases. We believe that these factors
lead to its excellent prognosis. Zhang et al observed a
significantly higher number of positive lymph nodes in mixed ICC
than in the pure form. In addition, mixed ICC cases exhibited a
higher proliferation index than pure ICC cases, regardless of the
positivity rate or the average Ki-67 percentage (5). This result demonstrated that the
prognosis of mixed ICC is less favorable than that of the pure form
(1,3,15).
However, pure ICC could also result in distant (bone) metastasis if
untreated (7). In the present
study, one patient who received radiotherapy and short-term
hormonal therapy without chemotherapy developed local recurrence
following breast-conserving surgery, which indicated that adjuvant
chemotherapy may play an essential role following surgery.
Surgery was the primary treatment for ICC. Sentinel
lymph node biopsy was more suitable due to its lower rate of lymph
node metastasis. A molecular classification of breast cancer has
been proposed for a better understanding of its biology and
treatment. Using immunohistochemistry, the present study found all
cases to be of the luminal subtype. The study by Colleoni et
al (8) recommended that
favorable histotypes (e.g., the tubular, cribriform, mucinous and
papillary types) with luminal tumors may be suitable for no therapy
or endocrine therapy alone. However, such decisions must be made
cautiously, as it is still possible to have local recurrence, as in
the present case, or distant (bone) metastasis if not treated
(7). We therefore hypothesize that
chemotherapy and radiotherapy remain necessary for high-risk
patients. Endocrine therapy has a significant effect, since the
majority of tumors are positive for ER or PR expression.
In summary, the present study described the
clinicopathological features, in particular the imaging findings,
of nine cases of ICC of the breast. ICC was found to exhibit unique
biological characteristics and manifested a good prognosis, as it
revealed more favorable prognostic factors. Although local
recurrence is possible, when considering the benign course of pure
ICC, chemotherapy and radiotherapy may not be indicated in all
cases. In the future, larger samples and an increased follow-up
time will be required when further examining these findings.
References
|
1
|
Page DL, Dixon JM, Anderson TJ, Lee D and
Stewart HJ: Invasive cribriform carcinoma of the breast.
Histopathology. 7:525–536. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Venable JG, Schwartz AM and Silverberg SG:
Infiltrating cribriform carcinoma of the breast: a distinctive
clinicopathologic entity. Hum Pathol. 21:333–338. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ellis IO, Galea M, Broughton N, Locker A,
Blamey RW and Elston CW: Pathological prognostic factors in breast
cancer. II Histological type Relationship with survival in a large
study with long-term follow-up. Histopathology. 20:479–489. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stutz JA, Evans AJ, Pinder S, et al: The
radiological appearances of invasive cribriform carcinoma of the
breast. Nottingham Breast Team Clin Radiol. 49:693–695. 1994.
|
|
5
|
Zhang W, Zhang T, Lin Z, et al: Invasive
cribriform carcinoma in a Chinese population: comparison with
low-grade invasive ductal carcinoma-not otherwise specified. Int J
Clin Exp Pathol. 6:445–457. 2013.PubMed/NCBI
|
|
6
|
Lim HS, Jeong SJ, Lee JS, et al:
Sonographic findings of invasive cribriform carcinoma of the
breast. J Ultrasound Med. 30:701–705. 2011.PubMed/NCBI
|
|
7
|
Zhang W, Lin Z, Zhang T, Liu F and Niu Y:
A pure invasive cribriform carcinoma of the breast with bone
metastasis if untreated for thirteen years: a case report and
literature review. World J Surg Oncol. 10:2512012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Colleoni M, Russo L and Dellapasqua S:
Adjuvant therapies for special types of breast cancer. Breast.
20(Suppl 3): 153–157. 2011. View Article : Google Scholar
|
|
9
|
Rakha E, Pinder SE, Shin SJ and Tsuda H:
Tubular carcinoma and cribriform carcinoma. WHO Classification of
Tumours of the Breast. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH and
van de Vijver MJ: 4th edition. IARC Press; Lyon: pp. 43–45.
2012
|
|
10
|
Greene FL, Page DL, Fleming ID, et al:
Breast. American Joint Committee on Cancer Cancer Staging Manual.
6th edition. Springer; New York, NY: pp. 223–240. 2002
|
|
11
|
Wolff AC, Hammond ME, Hicks DG, et al;
American Society of Clinical Oncology; College of American
Pathologists. Recommendations for human epidermal growth factor
receptor 2 testing in breast cancer: American Society of Clinical
Oncology/College of American Pathologists clinical practice
guideline update. J Clin Oncol. 31:3997–4013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wells CA and Ferguson DJ: Ultrastructural
and immunocytochemical study of a case of invasive cribriform
breast carcinoma. J Clin Pathol. 41:17–20. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishimura R, Ohsumi S, Teramoto N,
Yamakawa T, Saeki T and Takashima S: Invasive cribriform carcinoma
with extensive microcalcifications in the male breast. Breast
Cancer. 12:145–148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gatti G, Pruneri G, Gilardi D, Brenelli F,
Bassani G and Luini A: Report on a case of pure cribriform
carcinoma of the breast with internal mammary node metastasis:
description of the case and review of the literature. Tumori.
92:241–243. 2006.PubMed/NCBI
|
|
15
|
Colleoni M, Rotmensz N, Maisonneuve P, et
al: Outcome of special types of luminal breast cancer. Ann Oncol.
23:1428–1436. 2012. View Article : Google Scholar
|