Introduction
Prostate cancer (PCa) is one of the most common
non-cutaneous malignancies in males. Since the introduction of the
prostate specific antigen (PSA)-based screening strategy in
clinical practice, a marked increase in the incidence of PCa has
been observed (1). Although the use
of this screening strategy has resulted in a 40% reduction in
PCa-associated mortality, the majority of patients succumb to the
disease once metastasis has occurred. In addition, overtreatment of
indolent PCa has emerged. This phenomenon may account for the
deficiencies in accurate diagnosis and risk stratification.
Therefore, the identification and validation of novel biomarkers
for PCa should be considered a priority (2).
Cofilin 1 (CFL1) is the non-muscle isoform of the
product of the CFL1 gene (Gene ID, 1072). CFL1 is a small
ubiquitous protein that is able to bind monomeric globular (G) and
filamentous actin and inhibits the polymerization of monomeric
G-actin in a pH-dependent manner (3),
playing a key role in cell migration and cytokinesis (4). This protein is reported to be directly
associated with the invasion, metastasis and chemoresistance of
various human malignant solid tumors (5,6). However,
no previous studies regarding CFL1 expression and its association
with clinicopathological features in PCa are available in the
literature. The expression of CFL1 and its clinical implications in
PCa are investigated in the present study.
Materials and methods
Patient characteristics and
specimens
In total, 111 patients with histologically confirmed
prostatic adenocarcinoma were enrolled in the present study. The
patients had undergone open radical prostatectomy in the Department
of Urology in The Affiliated Hospital of Zunyi Medical College
(Zunyi, Guizhou) between January 2002 and September 2012. No
patients received adjuvant androgen deprivation therapy prior to
surgery. The histological analysis of all cancer specimens was
conducted according to the Gleason score (GS) grading system
(7) prior to immunohistochemical
analysis. In addition to the PCa samples, 47 corresponding benign
prostatic hyperplasia (BPH) tissues were selected as controls. The
mean age of patients at the time of diagnosis was 69 years (range,
51–81 years). In total, 89 patients possessed no lymph node
metastases and the mean pre-operative PSA level was measured as
19.97 ng/ml (range, 0.14–98 ng/ml). For the 47 patients diagnosed
with BPH, the mean age was 68 years (range, 52–79 years) and the
mean PSA level was 11.0 ng/ml (range, 0.3–25.4 ng/ml).
The use of the aforementioned tissues was approved
by the Institutional Review Board of The Affiliated Hospital of
Zunyi Medical College, and written informed consent was obtained
from all patients.
Histological staining and
immunohistochemical analysis
Paraffin-embedded 4-mm thick tissue sections were
prepared from all samples for histological analysis. The tissue
sections were stained with hematoxylin and eosin prior to the
immunohistochemical detection of the CFL1 protein using a rabbit
polyclonal anti-human CFL1 primary antibody (bs-2759R, dilution,
1:200; Bioss, Inc., Woburn, MA, USA).
All tissue sections were dewaxed, rehydrated and
incubated in 3% hydrogen peroxide for 10 min at room temperature to
quench endogenous peroxidase activity. The sections were then
incubated overnight with the CFL1 antibody at 4°C in phosphate
buffered saline (PBS) containing 1% bovine serum albumin. Staining
was detected using an EnVision kit (ZSGB-Bio, Beijing, China) and
3,3′-diaminobenzidine (DAB; ZSGB-Bio) with 0.3%
H2O2 in PBS was used as the chromogen.
Subsequent to staining, the sections were counterstained using
hematoxylin and then dehydrated using ethanol and xylene, and
Permount mounting medium was applied to the coverslips (all from
Nanjing KeyGen Biotech. Co. Ltd., Shanghai, China). Rat
immunoglobulin G primary antibody (CB3560554, dilution, 1:200;
Biomeda Corporation, Foster City, CA, USA) was used as the negative
control.
Imaging and statistical analysis
Histological analysis was redetermined
simultaneously by two investigators using a double-headed light
microscope. Evaluation of CFL1 expression was scored according to
the percentage of positively stained cells in the field of view, as
follows: Negative (0), no staining; weak (+), 0–33% of cells
stained; moderate (++), 34–66% of cells stained; and strong (+++)
67–100% of cells stained. The association between CFL1 expression
and clinicopathological parameters was analyzed by χ2 or
Fisher's exact tests. Factors corresponding with the GS grouping or
extraprostatic extension were analyzed using the logistic
regression method. SPSS software version 13.0 (SPSS, Inc., Chicago,
IL, USA) was used for the statistical analyses. P<0.05 was
considered to indicate a statistically significant difference.
Results
CFL1 expression in PCa and BPH
samples
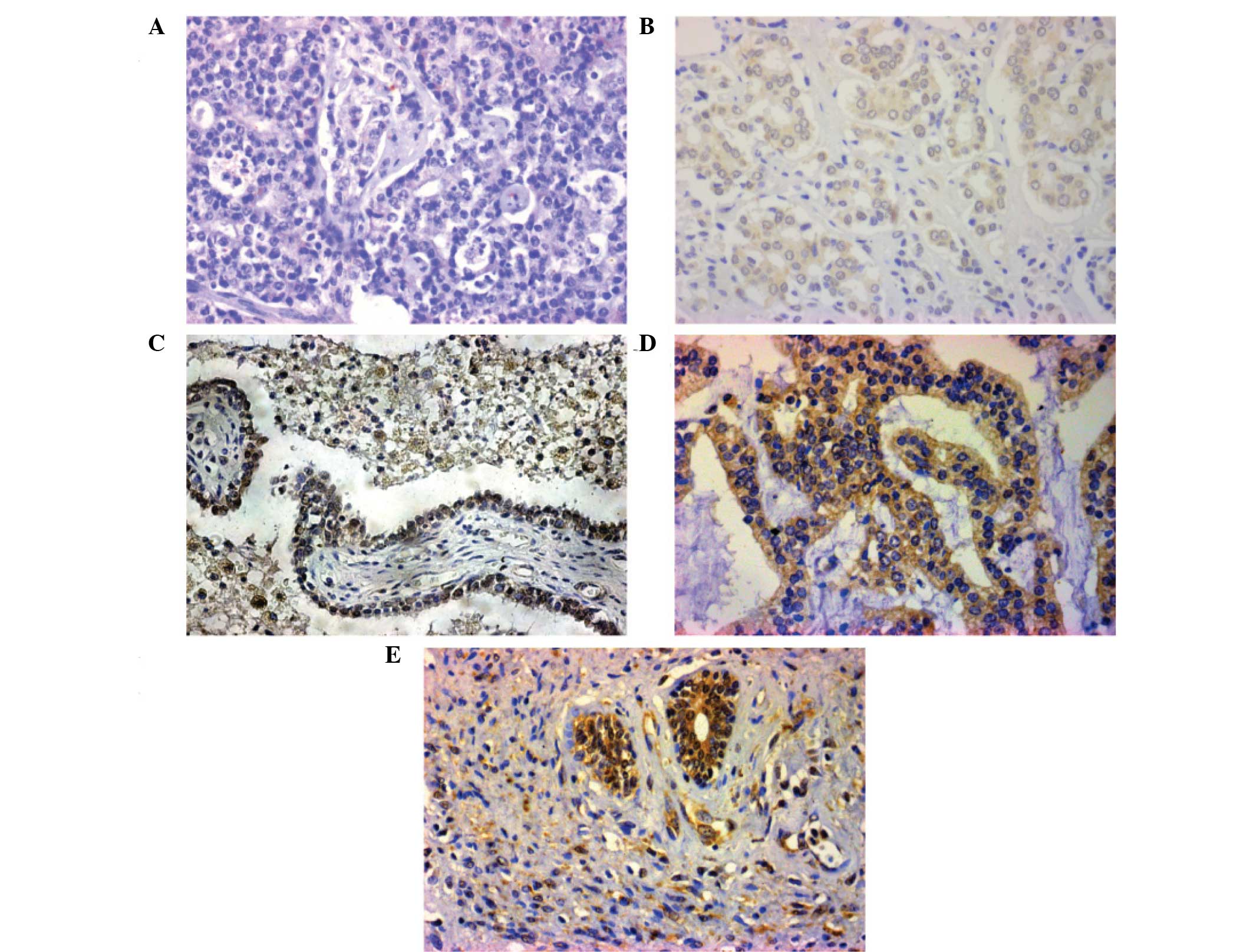
Expression of CFL1 was observed in 78 (70.3%) PCa
tumors, and no CFL1 overexpression was detected in BPH samples.
Additionally, 35 lesions (31.6%) exhibited light staining, 40
lesions (36.0%) exhibited moderate staining and three lesions
(2.7%) exhibited strong staining for CFL1. Microscopically, it was
observed that CFL1 was expressed in the cytoplasm, with low to high
expression in PCa cancer cells. The expression of CFL1 was also
observed in PCa cells located in the mesenchyme (Fig. 1B–E): CFL1 expression was increased in
patients that underwent lymph node metastasis (62.9%; 22 out of 35
patients; Table I). The distribution
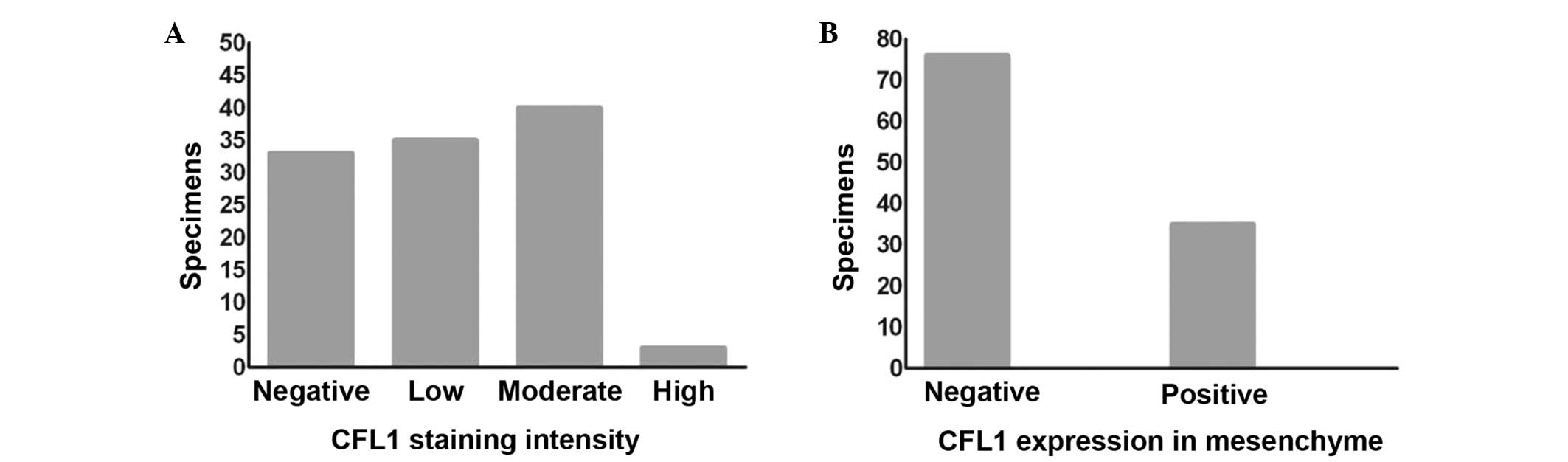
of the CFL1 staining intensity and the expression of CFL1 in the
mesenchyme of all PCa samples is shown in Fig. 2A and B.
 | Table I.Clinicopathological parameters of
patients from whom samples were obtained. |
Table I.
Clinicopathological parameters of
patients from whom samples were obtained.
| Variables | PCa, n | CFL1 positive in PCa,
n (%) | P-value |
|---|
| Age, years |
|
| 0.54 |
|
<69 | 59 | 43 (72.9) |
|
| ≥69 | 52 | 35 (67.3) |
|
| Preoperative PSA,
ng/ml |
|
| 0.45 |
|
<20 | 75 | 51 (68.0) |
|
| ≥20 | 36 | 27 (75.0) |
|
| Pathological
stage |
|
| 0.055 |
|
T2a/2b/2c | 68 | 43 (63.2) |
|
| ≥T3a | 43 | 35 (81.4) |
|
| Postoperative GS |
|
| <0.0001 |
|
<7 | 50 | 25 (50.0) |
|
| ≥7 | 61 | 53 (86.9) |
|
| Lymph node
metastasis |
|
|
<0.0001 |
| No | 89 | 56 (62.9) |
|
| Yes | 22 | 22 (100.0) |
|
Association between the expression of
CFL1 and clinicopathological features
Overexpression of CFL1 was revealed to differ
significantly between patients with a post-operative GS <7 and
patients with a GS ≥7 (50 vs. 86.9%, respectively; P<0.0001). A
similar incidence of overexpression was observed in patients with
lymph node metastasis compared with those without (100 vs. 62.9%,
respectively; P<0.0001). The CFL1 expression rate in patients
≥69 years of age was not significantly different from that of
patients aged <69 years (67.3 vs. 72.9%, respectively; P=0.54).
Overexpression of CFL1 also did not differ between patients with a
PSA level of <20 ng/ml and those with a level of ≥20 ng/ml (68
vs. 75%, respectively; P=0.45), or between patients with stage pT2
tumors and those with pT3–4 (extraprostatic extension) tumors (63.2
vs. 81.4%, respectively; P=0.055) (Table
I).
Binary logistic analysis was further conducted for
the predictive ability of all factors in order to analyze whether
CFL1 expression was significantly associated with
clinicopathological features (Table
II). PSA level [relative risk, 1.076; 95% confidence interval
(CI), 1.034–1.121; P<0.0001], CFL1 overexpression (relative
risk, 6.625; 95% CI, 2.621–16.747; P<0.0001) and CFL1-positive
mesenchyme cells (relative risk, 6.646; 95% CI, 2.469–17.885;
P<0.0001) were all significantly associated with a high GS at
the univariate level. Similarly, a strong association was observed
between extraprostatic extension (≥pT3a) and PSA level (relative
risk, 1.095; 95% CI, 0.935–1.053; P<0.0001), post-operative GS
(relative risk, 2.731; 95% CI, 1.917–3.890; P<0.0001), CFL1
expression (relative risk, 2.820; 95% CI, 1.133–7.019; P=0.026) and
CFL1-positive mesenchyme cells (relative risk, 10.875; 95% CI,
4.207–28.114; P<0.0001).
 | Table II.Variables associated with GS and
pathological stage stratification for prostate cancer. |
Table II.
Variables associated with GS and
pathological stage stratification for prostate cancer.
|
| GS, <7 vs. ≥7 | Pathological stage,
T2 vs. ≥T3a |
|---|
|
|
|---|
| Variables | P-value | RR | 95% CI | P-value | RR | 95% CI |
|---|
| Univariate
analysis |
|
|
|
|
|
|
| Age,
<69 vs. ≥69 years | 0.240 | 0.965 | 0.908–1.024 | 0.800 | 0.992 | 0.935–1.053 |
| PSA,
<20 vs. ≥20 ng/ml | <0.0001 | 1.076 | 1.034–1.121 | <0.0001 | 1.095 | 0.935–1.053 |
| Post-op
GS, <7 vs. ≥7 | – | – | – | <0.0001 | 2.731 | 1.917–3.890 |
| Cofilin
1, pos vs. neg | <0.0001 | 6.625 | 2.621–16.747 | 0.026 | 2.820 | 1.133–7.019 |
| Mesen
status, pos vs. neg | <0.0001 | 6.646 | 2.469–17.885 | <0.0001 | 10.875 | 4.207–28.114 |
| Multivariate
analysis |
|
|
|
|
|
|
| Age,
<69 vs. ≥69 years | 0.334 | 0.962 | 0.890–1.041 | 0.510 | 1.033 | 0.938–1.136 |
| PSA,
<20 vs. ≥20 ng/ml | 0.001 | 1.087 | 1.034–1.144 | 0.002 | 1.070 | 1.024–1.118 |
| Post-op
GS, <7 vs. ≥7 | – | – | – | <0.0001 | 2.280 | 1.516–3.430 |
| Cofilin
1, pos vs. neg | 0.006 | 5.287 | 1.627–17.177 | 0.184 | 0.347 | 0.073–1.654 |
| Mesen
status, pos vs. neg | 0.105 | 2.619 | 0.817–8.399 | 0.002 | 9.143 | 2.187–38.228 |
Multivariate analysis revealed that the PSA level
(relative risk, 1.087; 95% CI, 1.034–1.144; P<0.0001) and CFL1
expression (relative risk, 5.287; 95% CI, 1.627–17.177; P=0.006)
were independent predictors of high GS, regardless of age and
mesenchymal CFL1 expression. In addition, PSA (relative risk,
1.070; 95% CI, 1.024–1.118; P=0.002), post-operative GS (relative
risk, 2.280; 95% CI, 1.516–3.430; P<0.0001) and mesenchymal CFL1
status (relative risk, 9.143; 95% CI, 2.187–38.228; P=0.002) were
found to be independent factors predictive of extraprostatic
extension (≥T3a stage) at the multivariate level, while age was not
a significant predictor of extraprostatic extension.
Discussion
The exploration of novel biomarkers is of practical
significance for PCa, as it may inform physicians and surgeons of
which patients require radical surgery or active surveillance. It
may also facilitate improved screening, diagnosis, clinical outcome
prediction and decision making prior to surgery (8). Due to the high incidence of PCa
worldwide, there is an urgent demand for the identification of
robust biomarkers. Although PSA remains as the most widely used
biomarker for the diagnosis and screening of PCa, it demonstrates a
number of limitations, including the occurrence of false-positive
diagnosis and over-treatment due to the poor sensitivity and
specificity of PSA level testing (9–11).
In the present study, 70.3% of PCa cases were found
to be positive for CFL1 expression, with expression predominantly
observed in the cytoplasm of cancer cells. CFL1-positive cancer
cells were also observed in the mesenchyme in all cases with lymph
node metastasis. The rate of positive CFL1 expression was increased
significantly in poorly-differentiated PCa, defined by a GS≥7 or
the presence of lymph node metastasis. Furthermore, CFL1 expression
was absent in BPH tissues. Therefore, CFL1 immunohistochemical
expression is specific to PCa, and is associated with the
aggressiveness of the phenotype. This is consistent with a number
of studies that have reported CFL1 to be associated with a more
aggressive phenotype and with tumor progression in various solid
tumor tissues (12–15). For instance, CFL1 has been reported to
play a major role in tumor progression in ovarian carcinomas, as
nearly 64% of all ovarian tumors are positive for CFL1 (16), upregulation of phosphorylated CFL1
levels result in increased chemoresistance (17) and the patients with CFL1-positive
tumors demonstrate decreased progression-free survival rates
compared with the patients with CFL1-negative lesions (18). A similar association is observed in
urological carcinoma. Chung et al (19) reported that the invasiveness of
bladder carcinomas is markedly enhanced in vitro subsequent
to CFL1 phosphorylation by endothelial growth factor. Furthermore,
in PCa, CFL1 may also inhibit cancer cell growth by inducing the
formation of cofilin-actin rods within the cancer cells (20), and knockdown of CFL1 results in the
increased sensitivity of PCa to certain chemotherapeutic agents,
including docetaxel (21). CFL1 is
also important in the regulation of cancer cell migration and
invasion capability (6,22,23).
Hotulainen et al (4) reported
that the inhibition of cofilin activity was able to inhibit cell
motility, while the overexpression of cofilin increased the
velocity of cell migration in human glioblastoma cells (24).
Based on the logistic analysis, PSA and CFL1 were
identified as the most important predictive factors for patients
with a GS≥7 subsequent to surgery. Similarly, the findings
indicated that extra-prostatic extension in PCa was predicted by
PSA levels, post-operative GS, CFL1 expression and CFL1 status in
the mesenchyme. In general, PSA is positively associated with a
higher GS and continues to be a strong predictor of extraprostatic
extension (25,26). However, factors such as race or
ethnicity may significantly affect PSA values, even after
adjustment for age and prostate volume (27–29). A
number of studies have also demonstrated that a high GS is useful
for predicting extraprostatic extension (30–32), and
the present findings were consistent with these findings. As CFL1
expression was an independent prognostic factor in PCa,
immunohistochemical detection of this marker in cancer tissue
samples may aid in decision making. However, the exact mechanism of
CFL1 in tumor pathogenesis and invasion requires additional
investigation.
In conclusion, the present findings of the
evaluation of CFL1 as a biomarker revealed that this molecule has
high specificity in distinguishing malignant prostate tissues from
BPH, which may help to avoid the misdiagnosis of BPH as PCa. CFL1
expression was also found to be strongly associated with aggressive
characteristics, and may occur even before cancer cell initiation
and invasion. Although further studies are necessary, CFL1 is a
promising target that may be used as biomarker for early diagnosis,
monitoring, and decision making for treatment.
Acknowledgements
The authors would like to thank the Department of
Pathology at The Affiliated Hospital of Zunyi Medical College.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kelloff GJ and Sigman CC: Cancer
biomarkers: selecting the right drug for the right patient. Nat Rev
Drug Discov. 11:201–214. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ono S: Mechanism of depolymerization and
severing of actin filaments and its significance in cytoskeletal
dynamics. Int Rev Cytol. 258:1–82. 2007.PubMed/NCBI
|
|
4
|
Hotulainen P, Paunola E, Vartiainen MK and
Lappalainen P: Actin-depolymerizing factor and cofilin-1 play
overlapping roles in promoting rapid F-actin depolymerization in
mammalian nonmuscle cells. Mol Biol Cell. 16:649–664. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu B, Fukada K, Zhu H and Kyprianou N:
Prohibitin and cofilin are intracellular effectors of transforming
growth factor beta signaling in human prostate cancer cells. Cancer
Res. 66:8640–8647. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang W, Mouneimne G, Sidani M, et al: The
activity status of cofilin is directly related to invasion,
intravasation and metastasis of mammary tumors. J Cell Biol.
173:395–404. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Epstein JI: An update of the Gleason
grading system. J Urol. 183:433–440. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prensner JR, Rubin MA, Wei JT and
Chinnaiyan AM: Beyond PSA: the next generation of prostate cancer
biomarkers. Sci Transl Med. 4:127rv32012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lilja H, Ulmert D and Vickers AJ:
Prostate-specific antigen and prostate cancer: prediction,
detection and monitoring. Nat Rev Cancer. 8:268–278. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wolf AM, Wender RC, Etzioni RB, et al:
American Cancer Society Prostate Cancer Advisory Committee:
American Cancer Society guideline for the early detection of
prostate cancer: update 2010. CA Cancer J Clin. 60:70–98. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balk SP, Ko YJ and Bubley GJ: Biology of
prostate-specific antigen. J Clin Oncol. 21:383–391. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Castro MA, Dal-Pizzol F, Zdanov S, et al:
CFL1 expression levels as a prognostic and drug resistance marker
in nonsmall cell lung cancer. Cancer. 116:3645–3655. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang LH, Xiang J, Yan M, et al: The
mitotic kinase Aurora-A induces mammary cell migration and breast
cancer metastasis by activating the Cofilin-F-actin pathway. Cancer
Res. 70:9118–9128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Polachini GM, Sobral LM, Mercante AM, et
al: Proteomic approaches identify members of cofilin pathway
involved in oral tumorigenesis. PLoS One. 7:e505172012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang ZL, Miao X, Xiong L, et al: CFL1 and
Arp3 are biomarkers for metastasis and poor prognosis of squamous
cell/adenosquamous carcinomas and adenocarcinomas of gallbladder.
Cancer Invest. 31:132–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou J, Wang Y, Fei J and Zhang W:
Expression of cofilin 1 is positively correlated with the
differentiation of human epithelial ovarian cancer. Oncol Lett.
4:1187–1190. 2012.PubMed/NCBI
|
|
17
|
Nishimura S, Tsuda H, Kataoka F, et al:
Overexpression of cofilin 1 can predict progression-free survival
in patients with epithelial ovarian cancer receiving standard
therapy. Hum Pathol. 42:516–521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li M, Yin J, Mao N and Pan L: Upregulation
of phosphorylated cofilin 1 correlates with taxol resistance in
human ovarian cancer in vitro and in vivo. Oncol Rep. 29:58–66.
2013.PubMed/NCBI
|
|
19
|
Chung H, Kim B, Jung SH, et al: Does
phosphorylation of cofilin affect the progression of human bladder
cancer? BMC Cancer. 13:452013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ren S, Ouyang DY, Saltis M, et al:
Anti-proliferative effect of 23,24-dihydrocucurbitacin F on human
prostate cancer cells through induction of actin aggregation and
cofilin-actin rod formation. Cancer Chemother Pharmacol.
70:415–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pérez-Martínez FC, Carrión B, Lucío MI, et
al: Enhanced docetaxel-mediated cytotoxicity in human prostate
cancer cells through knockdown of cofilin-1 by carbon nanohorn
delivered siRNA. Biomaterials. 33:8152–8159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Rheenen J, Song X, van Roosmalen W, et
al: EGF-induced PIP2 hydrolysis releases and activates cofilin
locally in carcinoma cells. J Cell Biol. 179:1247–1259. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oser M and Condeelis J: The cofilin
activity cycle in lamellipodia and invadopodia. J Cell Biochem.
108:1252–1262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yap CT, Simpson TI, Pratt T, Price DJ and
Maciver SK: The motility of glioblastoma tumour cells is modulated
by intracellular cofilin expression in a concentration-dependent
manner. Cell Motil Cytoskeleton. 60:153–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Partin AW, Carter HB, Chan DW, et al:
Prostate specific antigen in the staging of localized prostate
cancer: influence of tumor differentiation, tumor volume and benign
hyperplasia. J Urol. 143:747–752. 1990.PubMed/NCBI
|
|
26
|
Paquette EL, Connelly RR, Sun L, Paquette
LR and Moul JW: Predictors of extracapsular extension and positive
margins in African American and white men. Urol Oncol. 21:33–38.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Asbell SO and Vijayakumar S: Racial
differences in prostate-specific antigen levels in patients with
local-regional prostate cancer. Prostate. 31:42–46. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moul JW, Connelly RR, Mooneyhan RM, et al:
Racial differences in tumor volume and prostate specific antigen
among radical prostatectomy patients. J Urol. 162:394–397. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Resnick MJ, Canter DJ, Guzzo TJ, et al:
Does race affect postoperative outcomes in patients with low-risk
prostate cancer who undergo radical prostatectomy? Urology.
73:620–623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Magheli A, Rais-Bahrami S, Trock BJ, et
al: Prostate specific antigen versus prostate specific antigen
density as a prognosticator of pathological characteristics and
biochemical recurrence following radical prostatectomy. J Urol.
179:1780–1784. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Giannarini G, Scott CA, Moro U, Pertoldi
B, Beltrami CA and Selli C: Are PSA density and PSA density of the
transition zone more accurate than PSA in predicting the
pathological stage of clinically localized prostate cancer? Urol
Oncol. 26:353–360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nishimoto K, Nakashima J, Hashiguchi A, et
al: Prediction of extraprostatic extension by prostate specific
antigen velocity, endorectal MRI, and biopsy Gleason score in
clinically localized prostate cancer. Int J Urol. 15:520–523. 2008.
View Article : Google Scholar : PubMed/NCBI
|