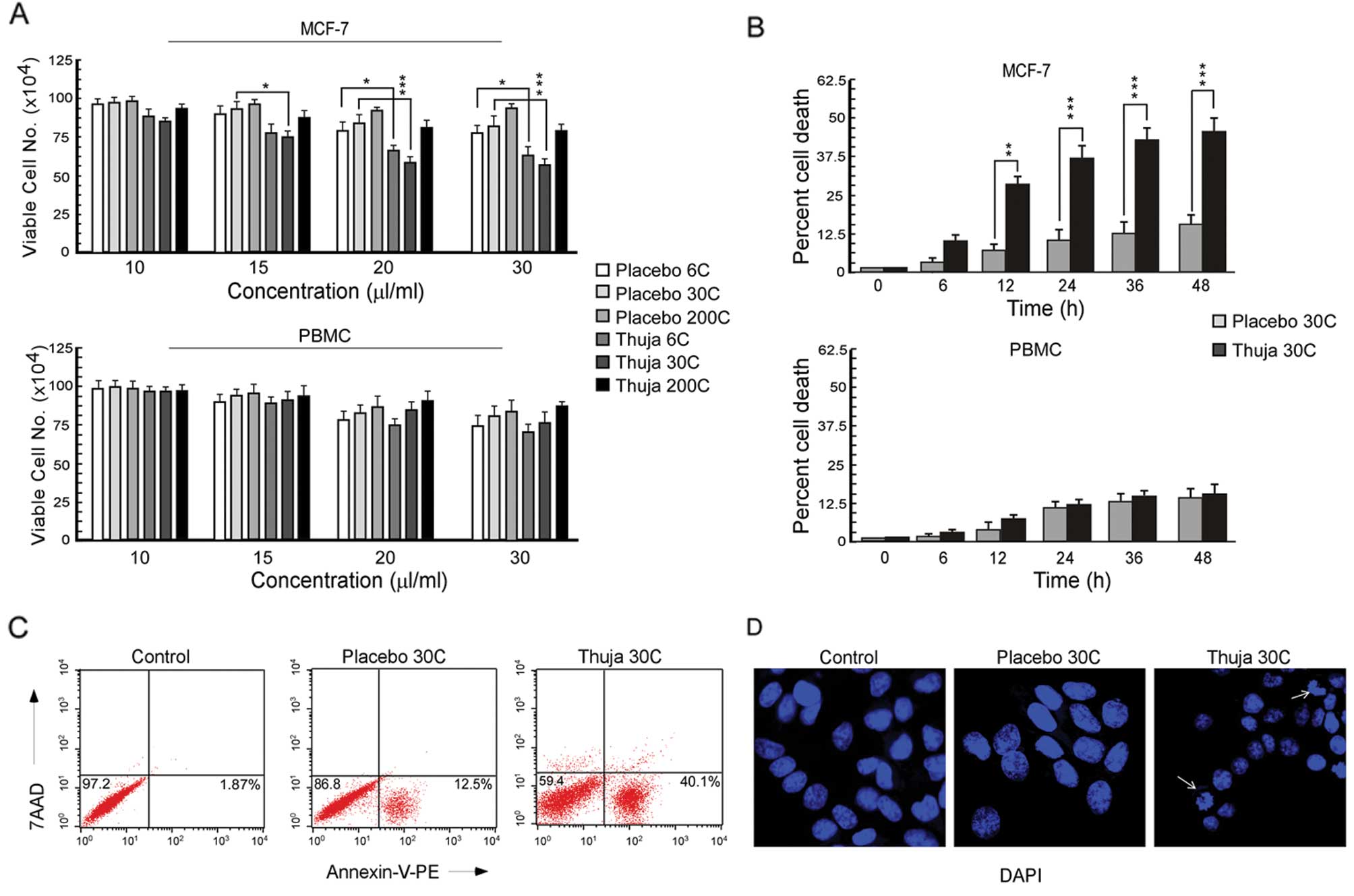
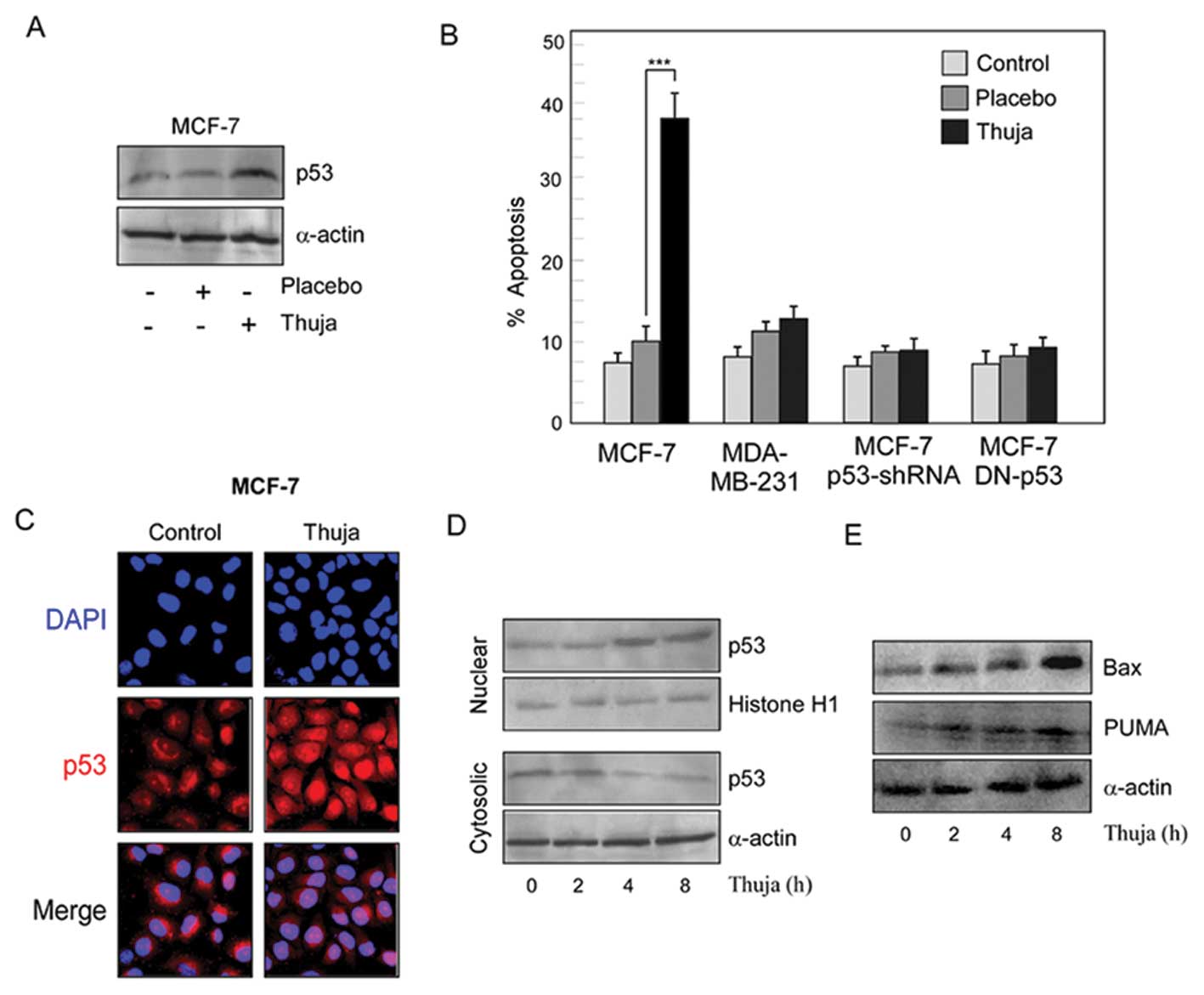
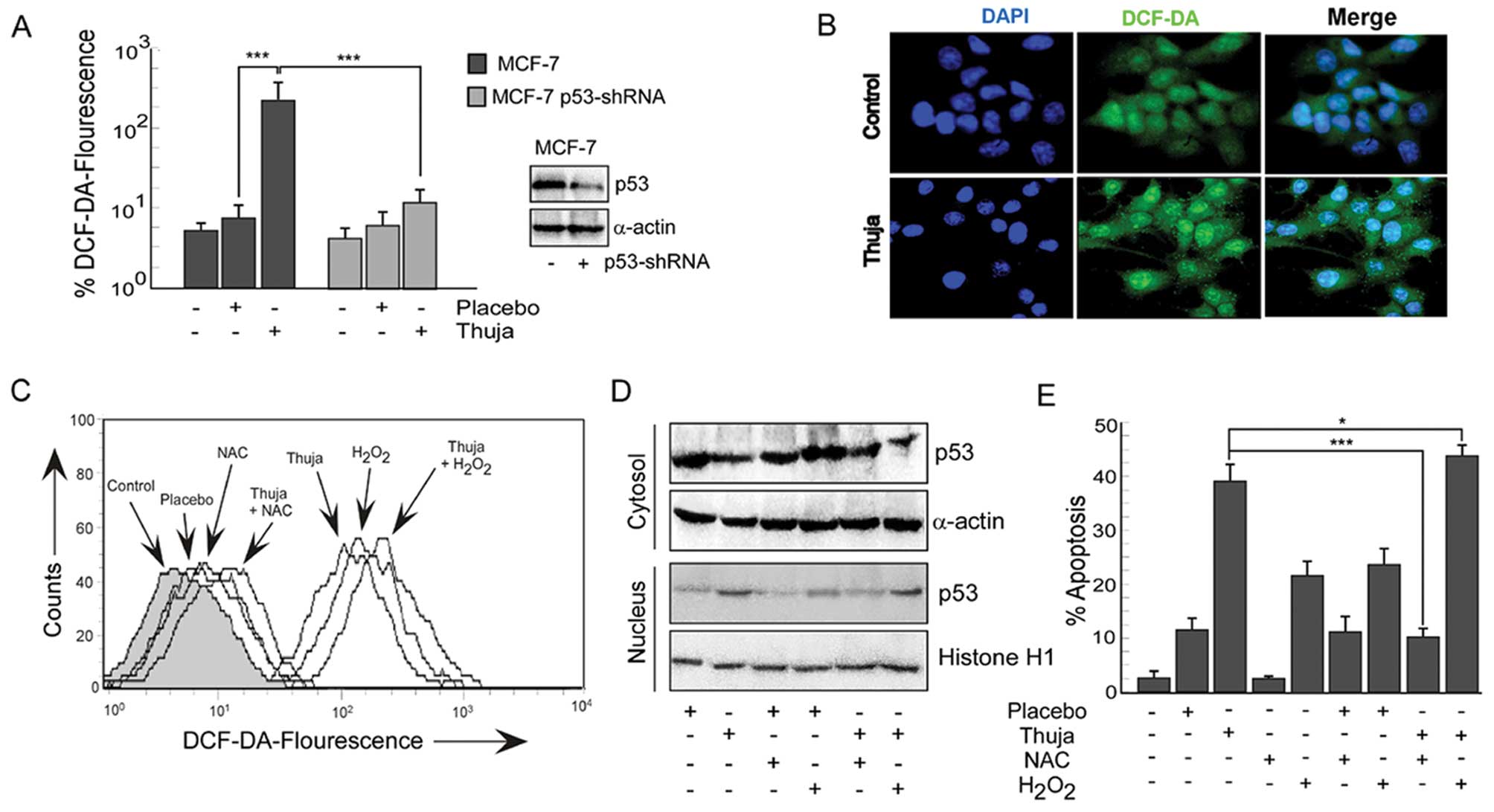
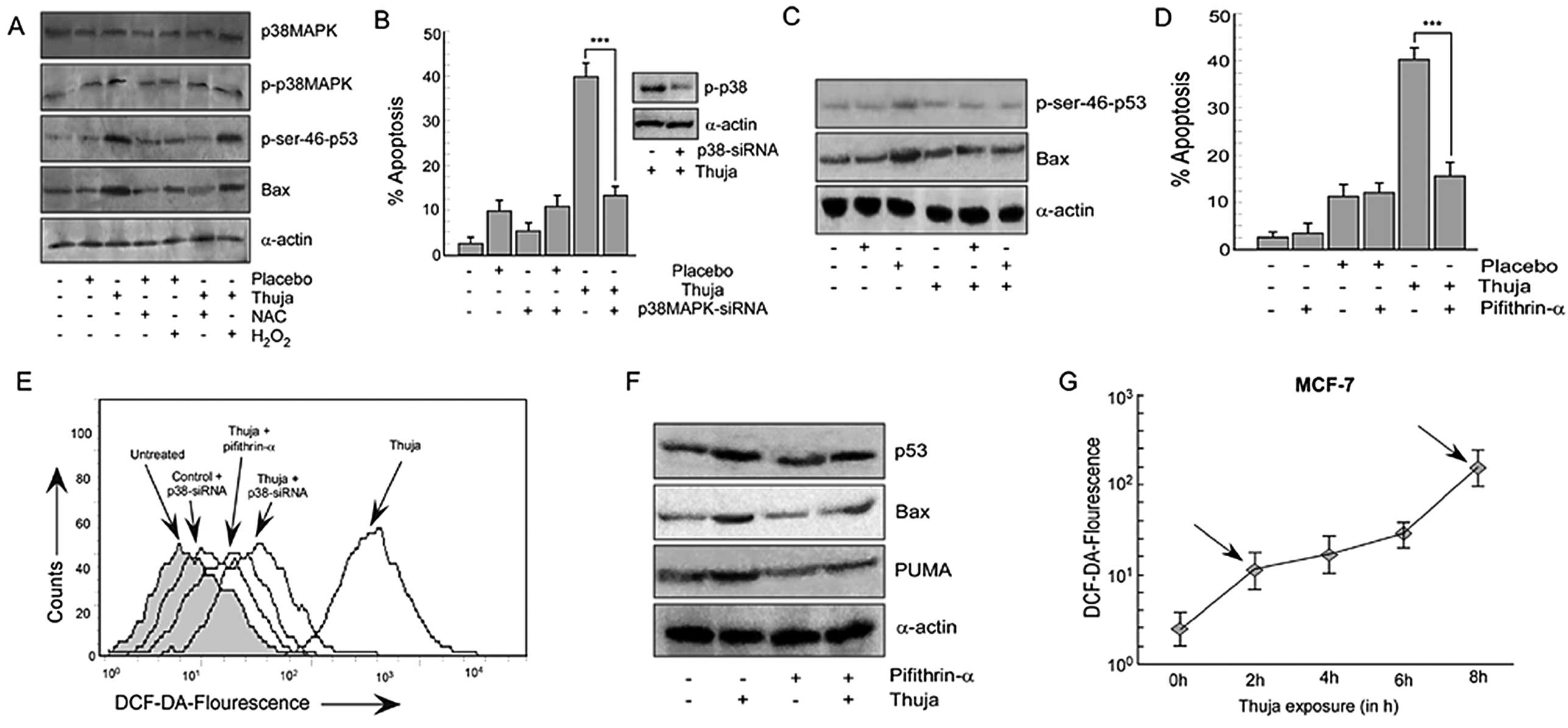
|
1
|
Hanf V and Gonder U: Nutrition and primary
prevention of breast cancer: foods, nutrients and breast cancer
risk. Eur J Obstet Gynecol Reprod Biol. 123:139–149. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cassileth BR and Vickers AJ: Complementary
and alternative therapies. Urol Clin North Am. 30:369–376. 2003.
View Article : Google Scholar
|
|
3
|
Flinn JE: Bromium in acute lymphatic
leukemia. J Am Inst Homeopath. 58:213–214. 1965.PubMed/NCBI
|
|
4
|
Gruchmann W: Arsenic: destroyer and
healer; a contribution to the management of carcinoma. Hippokrates.
27:444–445. 1956.(In German).
|
|
5
|
Pathak S, Multani AS and Banerji P and
Banerji P: Ruta 6 selectively induces cell death in brain cancer
cells but proliferation in normal peripheral blood lymphocytes: a
novel treatment for human brain cancer. Int J Oncol. 23:975–982.
2003.PubMed/NCBI
|
|
6
|
Pathak S, Kumar Das J, Jyoti Biswas S and
Khuda-Bukhsh AR: Protective potentials of a potentized homeopathic
drug, Lycopodium-30, in ameliorating azo dye induced
hepatocarcinogenesis in mice. Mol Cell Biochem. 285:121–131. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frenkel M, Mishra BM, Sen S, Yang P,
Pawlus A, Vence L, Leblanc A, Cohen L and Banerji P and Banerji P:
Cytotoxic effects of ultra-diluted remedies on breast cancer cells.
Int J Oncol. 36:395–403. 2010.PubMed/NCBI
|
|
8
|
Ojeswi BK, Khoobchandani M, Hazra DK and
Srivastava MM: Protective effect of Thuja occidentalis
against DMBA-induced breast cancer with reference to oxidative
stress. Hum Exp Toxicol. 29:369–375. 2010.
|
|
9
|
Biswas R, Mandal SK, Dutta S,
Bhattacharyya SS, Boujedaini N and Khuda-Bukhsh AR: Thujone-rich
fraction of Thuja occidentalis demonstrates major
anti-cancer potentials: evidences from in vitro studies on
A375 cells. Evid Based Complement Alternat Med.
2011:5681482011.PubMed/NCBI
|
|
10
|
Naser B, Bodinet C, Tegtmeier M and
Lindequist U: Thuja occidentalis (Arbor vitae): a review of
its pharmaceutical, pharmacological and clinical properties. Evid
Based Complement Alternat Med. 2:69–78. 2005. View Article : Google Scholar
|
|
11
|
Sunila ES and Kuttan G: Protective effect
of Thuja occidentalis against radiation-induced toxicity in
mice. Integr Cancer Ther. 4:322–328. 2005.
|
|
12
|
Sunila ES and Kuttan G: A preliminary
study on antimetastatic activity of Thuja occidentalis L. in
mice model. Immunopharmacol Immunotoxicol. 28:269–280. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dubey SK and Batra A: Hepatoprotective
activity from ethanol fraction of Thuja occidentalis Linn.
Asian J Res Chem. 1:32–35. 2008.
|
|
14
|
Dubey SK and Batra A: Antioxidant activity
of Thuja occidentalis Linn. Asian J Pharm Clin Res. 2:73–76.
2009.
|
|
15
|
Efeyan A and Serrano M: p53: guardian of
the genome and policeman of the oncogenes. Cell Cycle. 6:1006–1010.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu B, Chen Y and St Clair DK: ROS and
p53: a versatile partnership. Free Radic Biol Med. 44:1529–1535.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pervaiz S and Clement MV: Tumor
intracellular redox status and drug resistance - serendipity or a
causal relationship? Curr Pharm Des. 10:1969–1977. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mandal D, Lahiry L, Bhattacharyya A,
Chattopadhyay S, Siddiqi M, Sa G and Das T: Black tea protects
thymocytes in tumor-bearing animals by differential regulation of
intracellular ROS in tumor cells and thymocytes. J Environ Pathol
Toxicol Oncol. 24:91–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Adhikary A, Mohanty S, Lahiry L, Hossain
DM, Chakraborty S and Das T: Theaflavins retard human breast cancer
cell migration by inhibiting NF-κB via p53-ROS cross-talk. FEBS
Lett. 584:7–14. 2010.PubMed/NCBI
|
|
20
|
Mazumdar M, Adhikary A, Chakraborty S,
Mukherjee S, Manna A, Saha S, Mohanty S, Dutta A, Bhattacharjee P,
Ray P, Chattopadhyay S, Banerjee S, Chakraborty J, Ray AK, Sa G and
Das T: Targeting RET to induce medullary thyroid cancer cell
apoptosis: an antagonistic interplay between PI3K/Akt and
p38MAPK/caspase-8 pathways. Apoptosis. 18:589–604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lahiry L, Saha B, Chakraborty J,
Bhattacharyya S, Chattopadhyay S, Banerjee S, Choudhuri T, Mandal
D, Bhattacharyya A, Sa G and Das T: Contribution of p53-mediated
Bax transactivation in theaflavin-induced mammary epithelial
carcinoma cell apoptosis. Apoptosis. 13:771–781. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saito S, Yamaguchi H, Higashimoto Y, Chao
C, Xu Y, Fornace AJ Jr, Appella E and Anderson CW: Phosphorylation
site interdependence of human p53 post-translational modifications
in response to stress. J Biol Chem. 278:37536–37544. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Freund A, Patil CK and Campisi J: p38MAPK
is a novel DNA damage response-independent regulator of the
senescence-associated secretory phenotype. EMBO J. 30:1536–1548.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murphy PJ, Galigniana MD, Morishima Y,
Harrell JM, Kwok RP, Ljungman M and Pratt WB: Pifithrin-α inhibits
p53 signaling after interaction of the tumor suppressor protein
with hsp90 and its nuclear translocation. J Biol Chem.
279:30195–30201. 2004.
|
|
25
|
Chang LC, Song LL, Park EJ, Luyengi L, Lee
KJ, Farnsworth NR, Pezzuto JM and Kinghorn AD: Bioactive
constituents of Thuja occidentalis. J Nat Prod.
63:1235–1238. 2000. View Article : Google Scholar
|
|
26
|
British Herbal Pharmacopoeia. Thuja,
British HerbalMedicine Association; West Yorks, UK: 1983
|
|
27
|
Thangapazham RL, Gaddipati JP, Rajeshkumar
NV, Sharma A, Singh AK, Ives JA, Maheshwari RK and Jonas WB:
Homeopathic medicines do not alter growth and gene expression in
prostate and breast cancer cells in vitro. Integr Cancer Ther.
5:356–361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
MacLaughlin BW, Gutsmuths B, Pretner E,
Jonas WB, Ives J, Kulawardane DV and Amri H: Effects of homeopathic
preparations on human prostate cancer growth in cellular and animal
models. Integr Cancer Ther. 5:362–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Preethi K, Ellanghiyil S, Kuttan G and
Kuttan R: Induction of apoptosis of tumor cells by some potentiated
homeopathic drugs: implications on mechanism of action. Integr
Cancer Ther. 11:172–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bishayee K, Paul A, Ghosh S, Sikdar S,
Mukherjee A, Biswas R, Boujedaini N and Khuda-Bukhsh AR:
Condurango-glycoside-A fraction of Gonolobus condurango
induces DNA damage associated senescence and apoptosis via
ROS-dependent p53 signalling pathway in HeLa cells. Mol Cell
Biochem. 82:173–183. 2013.
|
|
31
|
Huschtscha L, Bartier W, Malmstrom A and
Tattersall M: Cell-death by apoptosis following anticancer
drug-treatment in vitro. Int J Oncol. 6:585–593.
1995.PubMed/NCBI
|
|
32
|
Gu B and Zhu WG: Surf the
post-translational modification network of p53 regulation. Int J
Biol Sci. 8:672–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bulavin DV, Saito S, Hollander MC,
Sakaguchi K, Anderson CW, Appella E and Fornace AJ Jr:
Phosphorylation of human p53 by p38 kinase coordinates N-terminal
phosphorylation and apoptosis in response to UV radiation. EMBO J.
18:6845–6854. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sanchez-Prieto R, Rojas JM, Taya Y and
Gutkind JS: A role for the p38 mitogen-activated protein kinase
pathway in the transcriptional activation of p53 on genotoxic
stress by chemotherapeutic agents. Cancer Res. 60:2464–2472.
2000.PubMed/NCBI
|
|
35
|
Oda K, Arakawa H, Tanaka T, Matsuda K,
Tanikawa C, Mori T, Nishimori H, Tamai K, Tokino T, Nakamura Y and
Taya Y: p53AIP1, a potential mediator of p53-dependent apoptosis,
and its regulation by Ser-46-phosphorylated p53. Cell. 102:849–862.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim H, You S, Farris J, Foster LK and
Foster DN: Post-transcriptional inactivation of p53 in immortalized
murine embryo fibroblast cells. Oncogene. 20:3306–3310. 2001.
View Article : Google Scholar : PubMed/NCBI
|