Introduction
Lung cancer is the leading cause of death from
cancer in China. Non-small cell lung cancer (NSCLC) constitutes
~85% of all lung cancers (1), with
adenocarcinoma comprising the major histological type.
Platinum-based chemotherapeutic agents are the standard first-line
chemotherapeutic agents for advanced NSCLC (2). Cisplatin (CDDP), for example, is
widely used for the treatment of NSCLC. However, acquired
resistance develops during treatment, leading to tumor recurrence
and further progression. Therefore, understand the underlying
mechanism of drug resistance to CDDP and increasing the sensitivity
to therapeutic drugs are key steps towards the improved treatment
of lung cancer patients.
Studies suggest a direct molecular and phenotypic
association between resistance to chemotherapy and acquisition of
epithelial-mesenchymal transition (EMT) characteristics in cancer
cells (3–6). EMT is a cellular process during which
epithelial polarized cells become motile mesenchymal-appearing
cells (7–9) and gain increased cell motility and
invasiveness (10,11). EMT involves loss of epithelial
cell-cell junctions and expression of epithelial markers such as
E-cadherin, and gain in the expression of mesenchymal markers such
as vimentin, Snail and Slug.
Connexins are a group of homologous proteins that
form the inter-membrane channels of gap junctions (12). An abnormal expression level and
distribution of connexins are closely related to tumor formation
(13,14). Studies indicate that Cx genes,
including Cx43, are tumor-suppressor genes (15,16).
Cx43 is one of the most common of the connexins and the major Cx
homolog expressed in lung tissue (17–19).
Studies have shown that Cx43 plays important roles in cancer
development, cell proliferation, apoptosis, invasion and metastasis
in lung cancer (20–26). Most importantly, Cx43 is able to
sensitize NSCLC cells to CDDP and ionizing radiation (27,28).
In the present study, a CDDP-resistant human lung
adenocarcinoma A549 cell line (A549/CDDP) was established. We found
that Cx43 is involved in the acquisition of EMT in A549/CDDP cells.
By overexpression of Cx43 in A549/CDDP cells, the mesenchymal
phenotype was reversed and A549/CDDP cells were resensitized to
CDDP. Knockdown of Cx43 expression by siRNA induced EMT in A549
cells. These results suggest that the downregulation of Cx43
expression promotes lung cancer progression and may thus be a
therapeutic target for lung cancer.
Materials and methods
Materials
CDDP, anti-Cx43, anti-β-actin and dimethylsulfoxide
(DMSO) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Cell
culture reagents and Lipofectamine™ 2000 were obtained from
Invitrogen. Antibodies for E-cadherin, vimentin, Slug and Snail
were obtained from Cell Signaling Technology (Boston, MA, USA).
Secondary antibodies for western blotting were obtained from
Amersham Biosciences Corp. (Piscataway, NJ, USA). All other
reagents were obtained from Sigma-Aldrich unless stated
otherwise.
Overexpression of Cx43 and inhibition of
Cx43 expression by siRNA transfection
Cx43 was expressed in A549/CDDP cells with a
pcDNA3.1-Cx43 vector (Shanghai Jima Co. Ltd., Shanghai, China).
Cells were transfected with siRNA targeting the human Cx43 gene
(CAGUCUGCCUUUCGUUGUA) or a non-specific control siRNA (NC control
in the figures). Transfection into A549 cells was carried out using
Lipofectamine 2000 according to the manufacturer’s
instructions.
MTT assay
MTT assay was used to examine cell viability.
Briefly, cells were seeded in a 96-well plate at a density of
5×103/well. After incubation in 5% CO2 at
37°C for 24 h, cells were exposed to different concentrations of
CDDP for 48 h. Then 20 μl of MTT (5 mg/ml in PBS) was added to each
well and cells were incubated for 4 h at 37°C. After the medium was
removed, the dark blue formazan that formed was dissolved in 200 μl
DMSO. The absorbance at 570 nm was measured by a microplate reader
(Bio-Rad Laboratories, Hercules, CA, USA). Data presented represent
at least three separate repeated experiments.
Western blotting
Western blotting protocols were performed according
to our previous study (29).
Anti-Cx43, anti-E-cadherin, anti-vimentin, anti-Slug and anti-Snail
were used. All western blotting exposures were in the linear range
of detection, and the intensities of the resulting bands were
quantified by Quantity One software on a GS-800 densitometer
(Bio-Rad Laboratories).
Wound-healing assay
The cells were seeded into a 6-well plate and
incubated until they reached 80% confluency. A 200-μl pipette tip
was used to create a wound, and cells were washed twice with
serum-free culture media to remove floating cells and then replaced
with fresh medium without serum. Cells were subjected to the
indicated treatment for 24 h, and cells migrating from the leading
edge were photographed at 0 and 24 h.
Matrigel invasion assay
The assay for cell invasion was performed in a
24-well Transwell unit (8-μm pore size) which was coated with 1
mg/ml Matrigel matrix as described (30). Briefly, the cells were placed on the
Matrigel-coated Transwell (the upper compartment of the invasion
chamber) in the presence or absence of drugs. Conditioned medium
(500 μl) was added to the lower compartment of the invasion
chamber. After incubation at 37°C for 48 h, cells that had invaded
the lower surface of the membrane were fixed with methanol and
stained with hematoxylin and eosin. Cells in random fields were
counted by light microscopy.
Statistical analysis
Statistical analysis between groups was performed
using an unpaired Student’s t-test with SigmaPlot 10.0 software
(Jandel Scientific, San Rafael, CA, USA). Data are presented as
mean ± SEM. Differences with P<0.05 are considered to have
statistical significance.
Results
Establishing a CDDP-resistant A549 cell
line
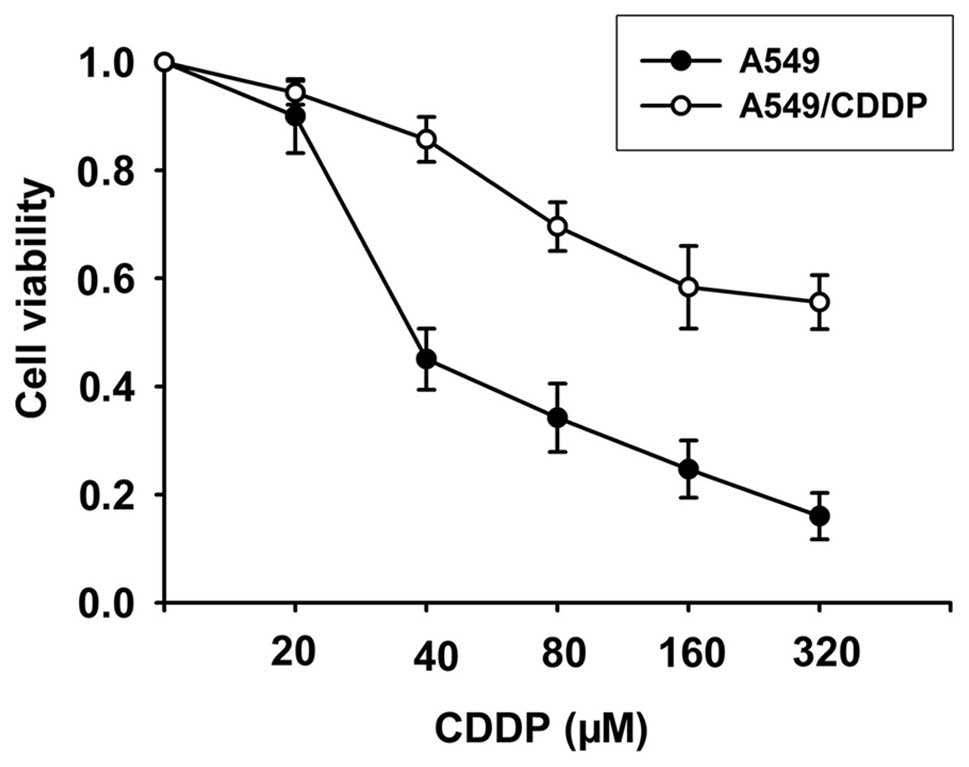
We generated a CDDP-resistant cell line A549/CDDP by
exposing these cells to cisplatin continuously. Cells were exposed
to CDDP for more than a year, at an initial concentration of 2.5 μM
CDDP reaching a final concentration of 40 μM CDDP. This cell line
was maintained for 6 months. As shown in Fig. 1, the A549/CDDP cell line
demonstrated a 5.8-fold higher resistance to CDDP than the A549
cell line.
Acquired resistance of A549/CDDP cells to
CDDP induces the cells to undergo EMT
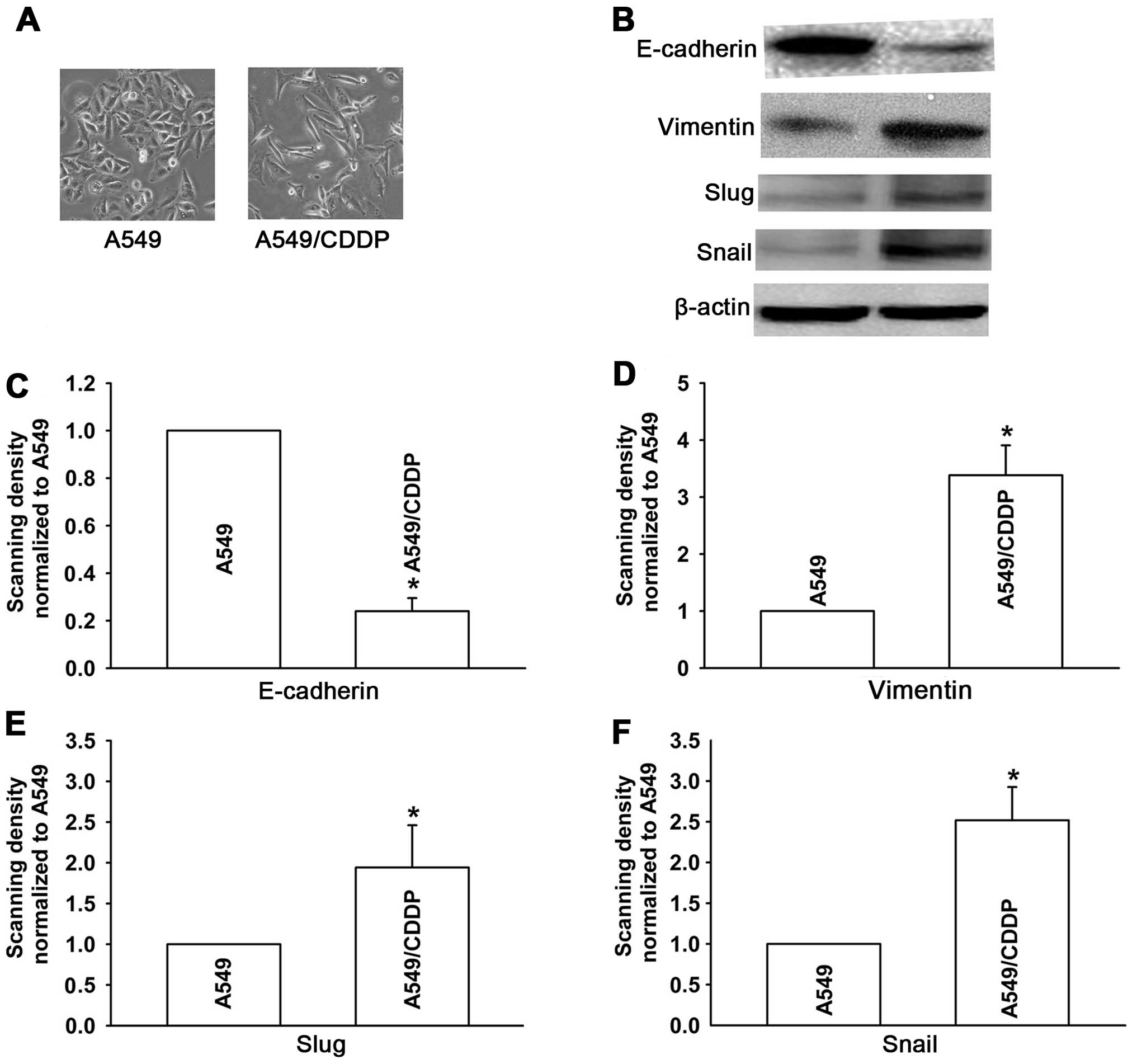
We first observed the morphological changes in
A549/CDDP cells. As shown in Fig.
2A, the A549 cells, which were sensitive to CDDP, displayed
classical epithelial morphology. In contrast, the A549/CDDP cells,
which were resistant to CDDP, showed a spindle-like fibroblastic
phenotype and increased formation of pseudopodia (Fig. 2A). These results suggest that the
A549/CDDP cells gained a mesenchymal phenotype. To further
determine the induction of EMT in A549/CDDP cells, we investigated
the expression of EMT markers: epithelial markers such as
E-cadherin, and mesenchymal markers such as vimentin, Snail and
Slug by western blotting. As shown in Fig. 2B, the expression of E-cadherin was
significantly less in the A549/CDDP cells than that in the parental
A549 cells. In contrast, the expression levels of vimentin, Snail
and Slug were largely increased. These results demonstrated that
the acquired resistance to CDDP induced A549/CDDP cells to undergo
EMT.
A549/CDDP cells display increased
potential for migration and invasion
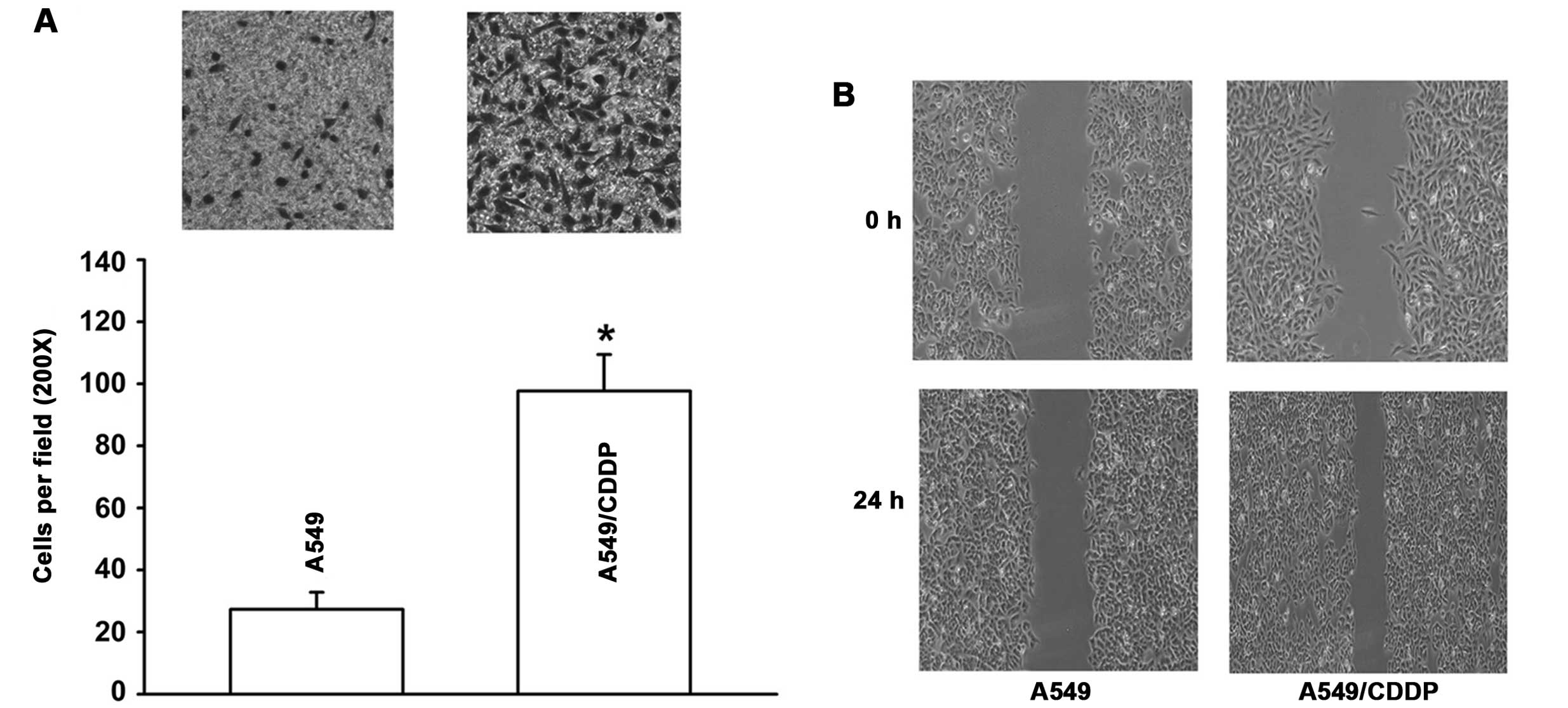
Cells that have undergone EMT display increased
migratory and invasive behaviors. Thus, in the following
experiment, we measured the invasive and migratory activity of the
cells by Transwell and wound-healing assays. We found that the
A549/CDDP cells displayed increased potential for migration and
invasion. A549/CDDP cells demonstrated a 3.6-fold increase in
invasive capability in the Matrigel-coated membrane when compared
with the A549 cells (Fig. 3A).
Also, as shown in Fig. 3B, the
number of cells migrating across the wound in the A549/CDDP cells
was significantly increased relative to the A549 cells, suggesting
that A549/CDDP cells showed enhanced migratory activity.
A549/CDDP cells show decreased expression
of Cx43
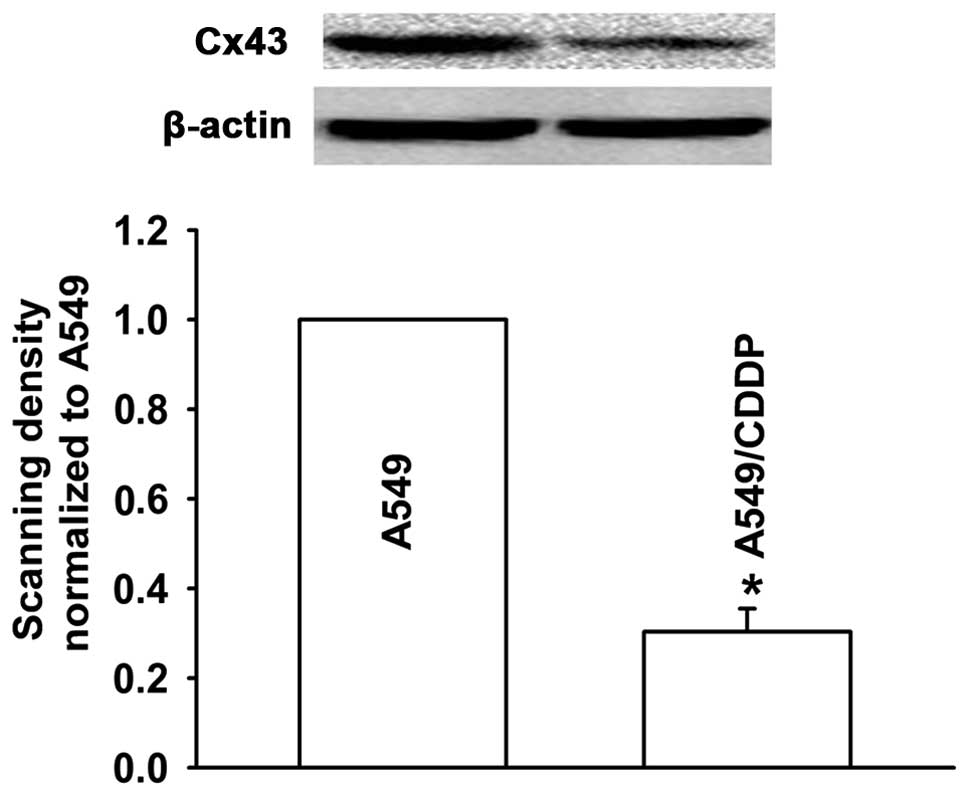
To explore the effect of Cx43 on the resistance of
lung adenocarcinoma cells to CDDP, we investigated the Cx43
expression level in the A549 cells and A549/CDDP cells by western
blotting. The expression level of Cx43 in the A549/CDDP cells was
significantly decreased when compared with that in the A549 cells
(Fig. 4). This suggests that Cx43
may be involved in CDDP-induced EMT in human lung cancer.
Cx43 is involved in the regulation of
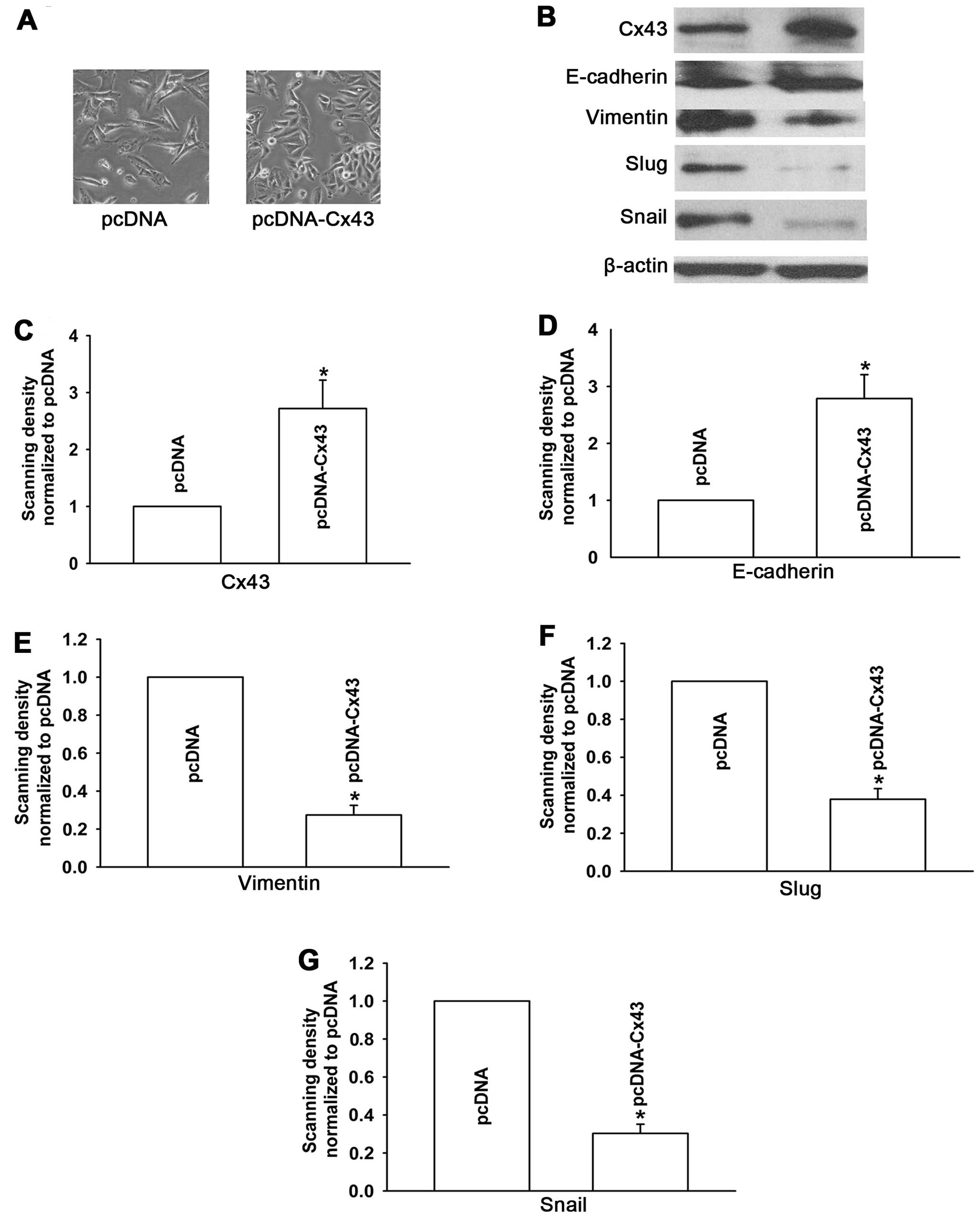
CDDP-induced EMT Overexpression of Cx43 reverses EMT in A549/CDDP
cells
Based on the results described above, we
hypothesized that Cx43 regulates EMT in A549 cells. Thus, the
downregulation of Cx43 may initiate EMT during the development of
the resistance of A549 cells to CDDP. To directly investigate the
role of Cx43 in CDDP-induced EMT, we manipulated Cx43 expression
levels in two ways: overexpression of Cx43 by transfection of
pcDNA-Cx43 and knockdown of Cx43 expression with siRNA-Cx43.
Firstly, we overexpressed Cx43 by transfection of pcDNA-Cx43 in
A549/CDDP cells. Western blotting was used to confirm this effect
(Fig. 5B). Consistent with the
previous experiment (Fig. 2), the
morphological changes and the marker proteins associated with EMT
(epithelial marker such as E-cadherin, and mesenchymal markers such
as vimentin, Snail and Slug) were observed in the subsequent
experimental study. Fig. 5A shows
that the pcDNA-transfected cells exhibited an elongated
fibroblast-like morphology, whereas Cx43-A549/CDDP cells, which had
a high Cx43 expression level, displayed epithelial morphology.
Moreover, compared with the pcDNA-transfected cells, the expression
level of epithelial marker E-cadherin was significantly increased,
while levels of mesenchymal markers vimentin, Snail and Slug were
decreased upon overexpression of Cx43 in the A549/CDDP cell line
(Fig. 5B). These results
demonstrate that upregulation of Cx43 by pcDNA-Cx43 converted EMT
to mesenchymal-epithelial transition (MET) in the A549/CDDP
cells.
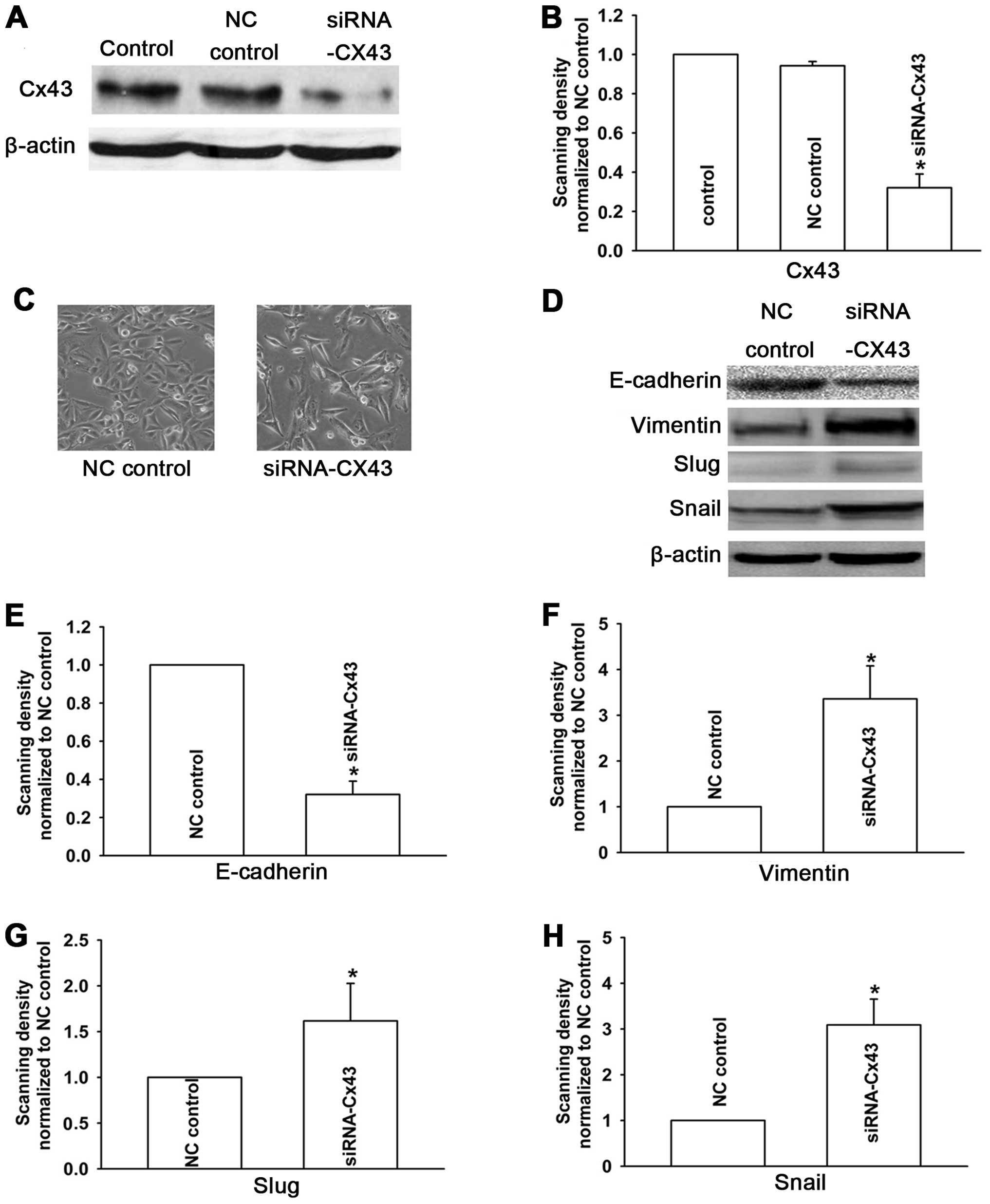
Knockdown of Cx43 expression induces EMT
in A549 cells
To further verify whether the EMT-associated
phenotypes were specifically regulated by Cx43, we downregulated
Cx43 expression in A549 cells which were sensitive to CDDP using
siRNA (Fig. 6A and B). The results
showed that A549 cells underwent EMT. Compared with the NC control
cells, the cells displayed a spindle-like fibroblastic phenotype
(Fig. 6C), and the expression of
E-cadherin was markedly decreased, while the expression levels of
vimentin, Snail and Slug were upregulated in A549 cells transfected
with Cx43 siRNA (Fig. 6D). These
results suggest that Cx43 is involved in the regulation of
CDDP-induced EMT in human lung cancer cells.
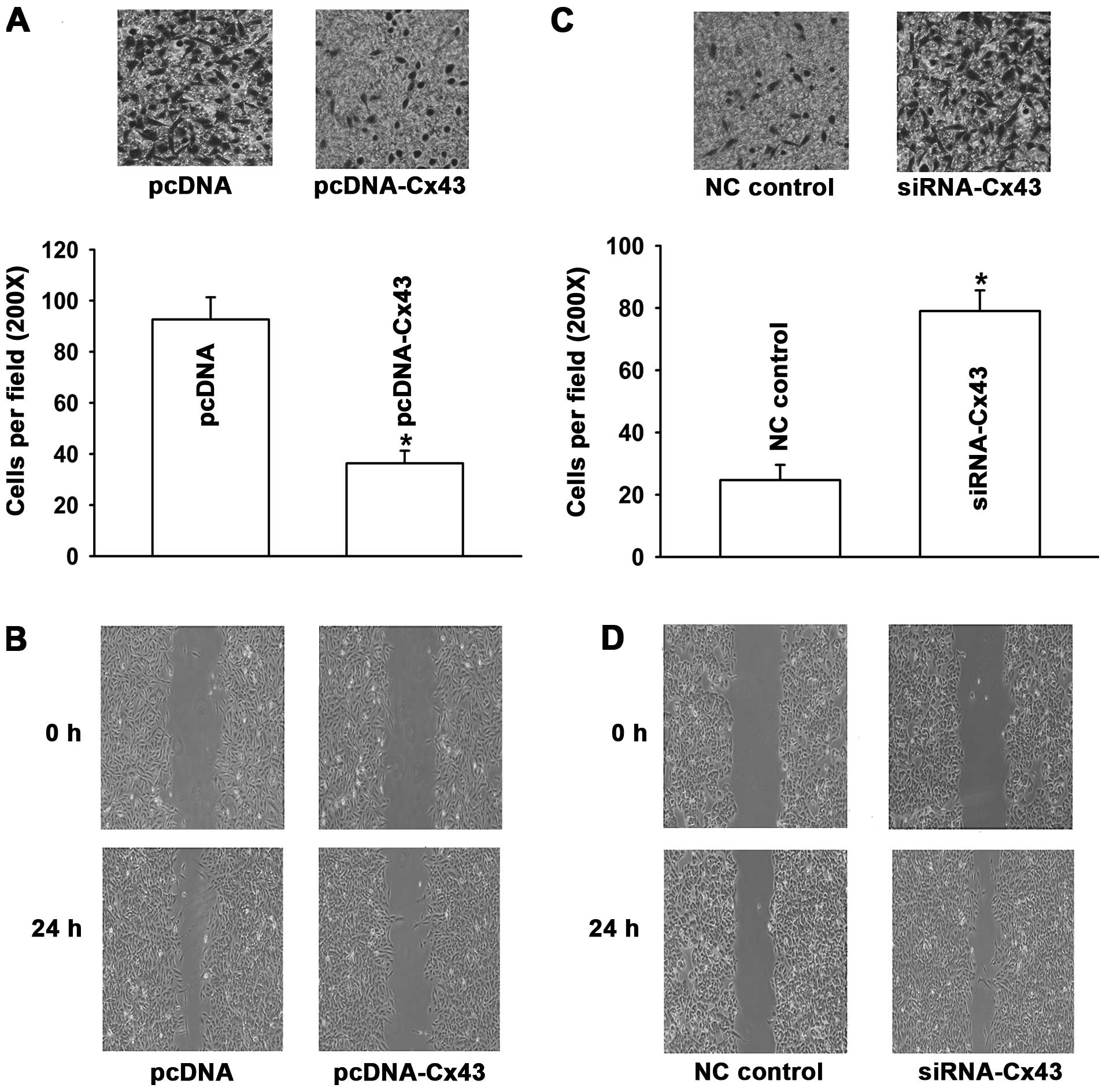
Cx43 regulates invasive and migratory
properties of cells
To further investigate the effect of Cx43 on EMT in
human lung cancer, we investigated the invasive and migratory
properties of the cells. The results in Fig. 7A and B revealed that when A549/CDDP
cells were transfected with Cx43, the capability of these cells to
migrate and invade were obviously reduced relative to the
pcDNA-transfected cells. In contrast, A549 cells transfected with
Cx43 siRNA showed significant enhancement in their invasive and
migratory properties (Fig. 7C and
D). These results provide further evidence that Cx43 is
involved in the regulation of CDDP-induced EMT in human lung cancer
cells.
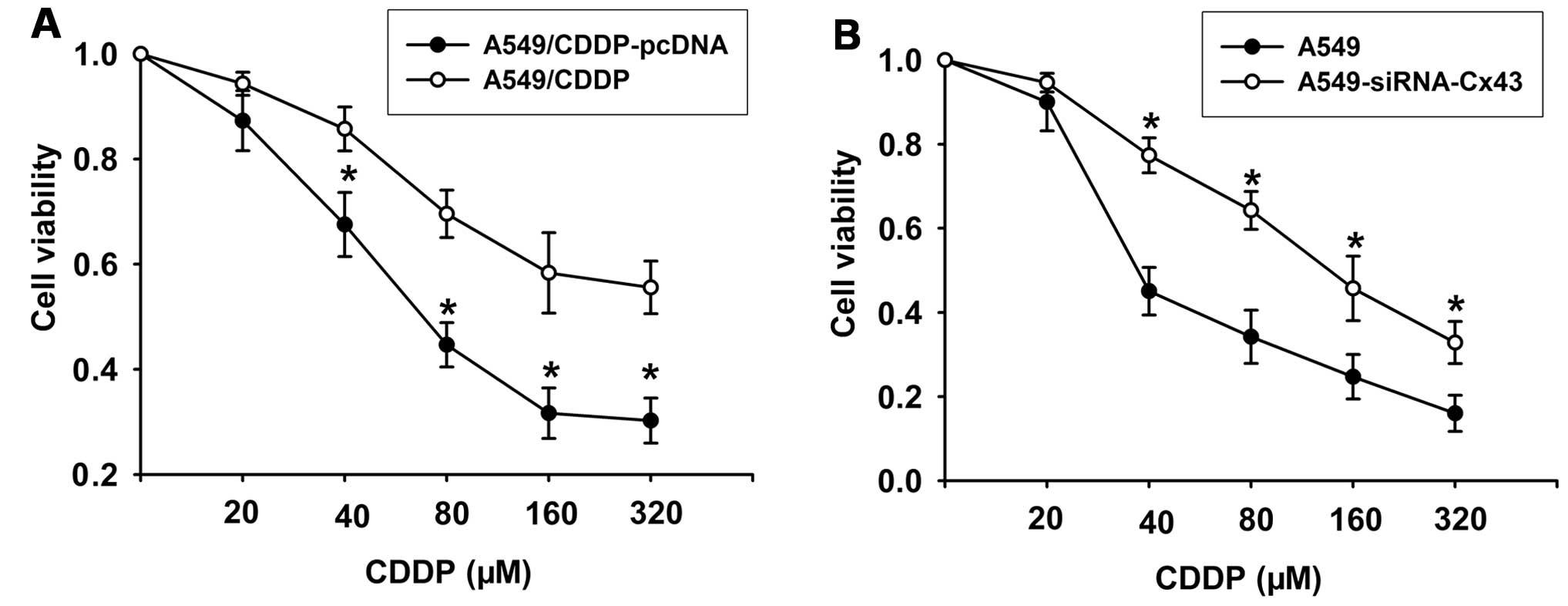
Cx43 regulates CDDP-induced cytoxicity in
human adenocarcinoma cells
To observe the effect of Cx43 on the cytotoxic
effect of CDDP, we manipulated Cx43 expression in two ways:
overexpression of Cx43 by transfection of pcDNA-Cx43 in A549/CDDP
cells and knockdown of Cx43 expression with siRNA-Cx43 in A549
cells. The results showed that compared with the pcDNA-transfected
cells, overexpression of Cx43 in the A549/CDDP cell line
significantly reversed resistance of the cells to CDDP (Fig. 8A). Conversely, knockdown of Cx43
expression with siRNA-Cx43 resulted in insensitivity of A549 cells
to CDDP (Fig. 8B). These results
suggest that downregulation of Cx43 which induces EMT may underlie
the resistance of A549 cells to CDDP.
Discussion
Emerging evidence suggests that chemo-resistance is
associated with the acquisition of EMT in cancer cells (3–5,31,32).
For example, gemcitabine-resistant pancreatic cancer (31), tamoxifen-resistant MCF7 breast
cancer (5), oxaliplatin-resistant
colorectal cancer (4),
paclitaxel-resistant ovarian cancer (3) and gefitinib-resistant lung cancer
cells (32) acquire EMT
characteristics. Novel research has demonstrated that targeting EMT
is a promising new therapeutic strategy (33–36).
Tan et al (33) reported
that Par-4 downregulation confers CDDP resistance in pancreatic
cancer cells by inducing PI3K/Akt pathway-dependent EMT; knockdown
of miR-134/487b/655 inhibited the EMT process and reversed
TGF-β1-induced resistance to gefitinib in lung adenocarcinoma cells
(34); downregulation of the PDGF-D
pathway reversed EMT in gemcitabine-resistant hepatocellular cancer
cells (35); and knockdown of Snail
and Slug reversed CDDP resistance in ovarian cancer (36). Thus, regulating EMT is a new way to
overcome drug resistance in cancer cells and to promote a better
treatment outcome for cancer patients.
Consistent with the above studies, when the human
lung adenocarcinoma cell line A549 became resistant to CDDP, these
cells underwent EMT with significant morphological changes such as
acquisition of a classical mesenchymal phenotype, downregulation of
epithelial marker E-cadherin, and upregulation of mesenchymal
markers vimentin, Snail and Slug. However, the exact mechanism
underlying the CDDP-induced EMT phenotype of A549 cancer cells is
still unclear.
Cx43 has been reported to play important roles in
cancer development, cell proliferation, apoptosis, invasion and
metastasis in lung cancer (20–26). A
study showed that concurrent reduction in the expression of Cx43
and E-cadherin may contribute to the development of lung cancer.
For example, Cx43 may induce E-cadherin expression and inhibit cell
proliferation and progression of lung cancer (20). Another report also confirmed that
transfection of Cx43 induced E-cadherin overexpression (23). Since E-cadherin is a typical
epithelial marker, based on these reports, Cx43 may influence lung
cancer development by regulating EMT. Indeed, the expression level
of Cx43 was significantly less in A549/CDDP cells than that in the
parental A549 cells. To further investigate the effect of Cx43
expression on CDDP-induced EMT in the human adenocarcinoma cell
line, firstly, Cx43 was overexpressed in A549/CDDP cells. This
resulted in A549/CDDP cells displaying classical epithelial
morphology, increased E-cadherin expression, decreased expression
of mesenchymal markers, reduced invasive and migratory activity and
resensitization to CDDP. This suggests that overexpression of Cx43
reversed CDDP-induced EMT and enhanced the cytotoxicity of CDDP.
Secondly, when Cx43 expression was knocked down in A549 cells
sensitive to CDDP exposure, the cells underwent EMT. Thus, the
present study is the first report to show that Cx43 is involved in
the regulation of CDDP-induced EMT in human lung adenocarcinoma
cells.
Cx43 could sensitize cancer cells to
chemotherapeutic agents, including CDDP (37,38).
Although the mechanisms underlying these effects are still not
clear, we can speculate the following scenarios: i) Cx43 could
improve cell resistance to CDDP which may be mediated by the
suppression of Src activity (37);
ii) Cx43 could enhance the cytotoxic effect of various
chemotherapeutic agents (etoposide, pacitaxel, doxorubicin) by
promoting apoptosis (39); and iii)
Cx43 enhanced the efficiency of CDDP in tumor testicular cells by
gap junctional intercellular communication (GJIC)-mediated toxic
bystander effects (38). The
present study provides the first evidence that Cx43 enhances the
cytotoxicity of CDDP by regulating EMT in human lung cancer
cells.
In summary, the present study showed that Cx43 plays
a critical role in promoting lung cancer progression and CDDP
resistance by regulating EMT. Overexpression of Cx43 in
CDDP-resistant A549 cells reversed EMT to MET and enhanced the
cytotoxity of CDDP. Thus, Cx43 may be a potential target for
overcoming CDDP resistance in lung cancer therapy.
Acknowledgements
The present study was supported by the China
Postdoctoral Science Foundation (no. 20090461139), the National
Natural Science Foundation of China (no. 81001457) and the
Foundation of Bengbu Medical College (no. Bykf13A11 and no.
Byycx1329).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Ota S, Ishii G, Goto K, et al:
Immunohistochemical expression of BCRP and ERCC1 in biopsy specimen
predicts survival in advanced non-small-cell lung cancer treated
with cisplatin-based chemotherapy. Lung Cancer. 64:98–104. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kajiyama H, Shibata K, Terauchi M, et al:
Chemoresistance to paclitaxel induces epithelial mesenchymal
transition and enhances metastatic potential for epithelial ovarian
carcinoma cells. Int J Oncol. 31:277–283. 2007.
|
|
4
|
Yang AD, Fan F, Camp ER, et al: Chronic
oxaliplatin resistance induces epithelial-to-mesenchymal transition
in colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hiscox S, Jiang WG, Obermeier K, et al:
Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and
involves modulation of β-catenin phosphorylation. Int J Cancer.
118:290–301. 2006.PubMed/NCBI
|
|
6
|
Hiscox S, Morgan L, Barrow D, Dutkowskil
C, Wakeling A and Nicholson RI: Tamoxifen resistance in breast
cancer cells is accompanied by an enhanced motile and invasive
phenotype: inhibition by gefitinib (‘Iressa’, ZD1839). Clin Exp
Metastasis. 21:201–212. 2004.PubMed/NCBI
|
|
7
|
Chang CJ, Chao CH, Xia W, et al: p53
regulates epithelial-mesenchymal transition and stem cell
properties through modulating miRNAs. Nat Cell Biol. 13:317–323.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: mechanism,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
10
|
Hugo H, Ackland ML, Blick T, et al:
Epithelial-mesenchymal and mesenchymal-epithelial transitions in
carcinoma progression. J Cell Physiol. 213:374–383. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Min C, Eddy SF, Sherr DH and Sonenshein
GE: NF-κB and epithelial to mesenchymal transition of cancer. J
Cell Biochem. 104:733–744. 2008.
|
|
12
|
Proksch E, Brandner JM and Jensen JM: The
skin: an indispensable barrier. Exp Dermatol. 17:1063–1072. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Laird DW: Life cycle of connexins in
health and disease. J Biochem. 394:527–543. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cronier L, Crespin S, Strale PO, Defamie N
and Mesnil M: Gap junctions and cancer: new functions for an old
story. Antioxid Redox Signal. 11:323–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Plante I, Stewart MK, Barr K, Allan AL and
Laird DW: Cx43 suppresses mammary tumor metastasis to the lung in a
Cx43 mutant mouse model of human disease. Oncogene. 30:1681–1692.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogawa K, Pitchakarn P, Suzuki S, et al:
Silencing of connexin 43 suppresses invasion, migration and lung
metastasis of rat hepatocellular carcinoma cells. Cancer Sci.
103:860–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Willecke K, Eiberger J, Degen J, et al:
Structural and functional diversity of connexin genes in the mouse
and human genome. Biol Chem. 383:725–737. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okuma A, Kuraoka A, Iida H, Inai T, Wasano
K and Shibata Y: Colocalization of connexin 43 and connexin 45 but
absence of connexin 40 in granulosa cell gap junctions of rat
ovary. J Reprod Fertil. 107:255–264. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Risley MS, Tan IP, Roy C and Saez JC:
Cell-, age- and stage dependent distribution of connexin43 gap
junctions in testes. J Cell Sci. 103:81–96. 1992.PubMed/NCBI
|
|
20
|
de Oliveira KD, Tedardi MV, Cogliati B and
Dagli ML: Higher incidence of lung adenocarcinomaa induced by DMBA
in Cx43 heterozygous knockout mice. Biomed Res Int.
2013:6184752013. View Article : Google Scholar
|
|
21
|
Zhao W, Han HB and Zhang ZQ: Suppression
of lung cancer cell invasion and metastasis by Cx43 involves the
secretion of follistatin-like 1 mediated via histone acetylation.
Int J Biochem Cell Biol. 43:1459–1468. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu HT, Li QC, Zhang YX, et al: Cx43
recruits E-cadherin expression and inhibits the malignant behaviour
of lung cancer cells. Folia Histochem Cytobiol. 46:315–321.
2008.PubMed/NCBI
|
|
23
|
Zhang YX, Xu HT, Qi FJ and Wang EH:
Expression of Cx43 in lung cancer and its correlation with
E-cadherin. Zhonghua Bing Li Xue Za Zhi. 35:339–343. 2006.(In
Chinese).
|
|
24
|
Chen Q, Qian B, Yang L and Jiang Z: The
expression of Cx43 protein in non-small cell lung cancer tissues
and its clinical significance. Zhongguo Fei Ai Za Zhi. 6:272–274.
2003.(In Chinese).
|
|
25
|
Cesen-Cummings K, Fernstrom MJ, Malkinson
AM and Ruch RJ: Frequent reduction of GJIC and Cx43 expression in
human and mouse lung carcinoma cells. Carcinogenesis. 19:61–67.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin Z, Zhang Z and Wang N: Inhibition of
in vivo growth of lung carcinoma cells after transfetion with gap
junction gene Cx43. Zhonghua Zhong Liu Za Zhi. 19:253–255. 1997.(In
Chinese).
|
|
27
|
Du G, Yang Y, Zhang Y, et al:
Thrombocytosis and immunohistochemical expression of connexin43 at
diagnosis predict survival in advanced non-small-cell lung cancer
treated with cisplatin-based chemotherapy. Cancer Chemother
Pharmacol. 71:893–904. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Sharma S, Hershman JM, Brent GA,
Dubinett SM and Huang M: Iodide sensitizes genetically modified
non-small cell lung cancer cells to ionizing radiation. Cancer Gene
Ther. 13:74–81. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu ML, Zhang CL, Yuan DD, Tong XH and Tao
L: Panax notoginseng saponins enhances the cytotoxicity of
cisplatin via increasing gap junction intercellular communication.
Biol Pharm Bull. 35:1230–1237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim KS, Yao L, Lee YC, et al:
Hyul-Tong-Ryung suppresses PMA-induced MMP-9 expression by
inhibiting AP-1-mediated gene expression via ERK1/2 signaling
pathway in MCF-7 human breast cancer cells. Immunopharmacol
Immunotoxicol. 32:600–606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Li Y, Kong D, et al: Acquisition
of epithelial-mesenchymal transition phenotype of
gemcitabine-resistant pancreatic cancer cells is linked with
activation of the notch signaling pathway. Cancer Res.
69:2400–2407. 2009. View Article : Google Scholar
|
|
32
|
Rho JK, Choi YJ, Lee JK, et al: Epithelial
to mesenchymal transition derived from repeated exposure to
gefitinib determines the sensitivity to EGFR inhibitors in A549, a
non-small cell lung cancer cell line. Lung Cancer. 63:219–226.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tan J, You Y, Xu T, et al: Par-4
downregulation confers cisplatin resistance in pancreatic
cancercells via PI3K/Akt pathway-dependent EMT. Toxicol Lett.
224:7–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kitamura K, Seike M, Okano T, et al:
MiR-134/487b/655 cluster regulates TGF-β-induced
epithelial-mesenchymal transition and drug resistance to gefitinib
by targeting MAGI2 in lung adenocarcinoma cells. Mol Cancer
Ther. 13:444–453. 2014.PubMed/NCBI
|
|
35
|
Wu Q, Wang R, Yang Q, et al:
Chemoresistance to gemcitabine in hepatoma cells induces
epithelial-mesenchymal transition and involves activation of PDGF-D
pathway. Oncotarget. 4:1999–2009. 2013.PubMed/NCBI
|
|
36
|
Haslehurst AM, Koti M, Dharsee M, et al:
EMT transcription factors snail and slug directly contribute to
cisplatin resistance in ovarian cancer. BMC Cancer. 12:912012.
View Article : Google Scholar : 2012.
|
|
37
|
Sato H, Iwata H, Takano Y, et al: Enhanced
effect of Cx43 on cisplatin-induced cytotoxicity in mesothelima
cells. J Pharmacol Sci. 110:466–475. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hong X, Wang Q, Yang Y, et al: Gap
junctions propagate opposite effects in normal and tumor testicular
cells in response to cisplatin. Cancer Lett. 317:165–171. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang RP, Hossain MZ, Huang R, Gano J, Fan
Y and Boynton AL: Connexin 43 (cx43) enhances chemotherapy-induce
apoptosis in human glioblastoma cells. Int J Cancer. 92:130–138.
2001. View Article : Google Scholar : PubMed/NCBI
|