Introduction
Lung cancer is the leading cause of
cancer-associated mortality among males, and has recently surpassed
breast cancer as the most common cause of cancer-associated
mortality among females (1).
Histologically, human lung cancers can be categorized into
small-cell lung cancer (SCLC) and non-SCLC (NSCLC). NSCLC comprises
~80% of all lung cancer cases, and mainly constitutes
adenocarcinomas and squamous cell carcinomas (2). Notable reductions in smoking, and
advances in early detection and treatment have been reported;
however, the 5-year survival rate of patients with NSCLC is ≤18%.
This is mainly due to the majority of patients that are diagnosed
at advanced and later stages of cancer, and following the
occurrence of metastasis (1).
Therefore, understanding the molecular mechanisms that underlie the
metastasis of NSCLC is necessary for the development of diagnostic
technologies and novel treatment methods. Tumor metastasis is a
complicated process involving numerous steps. The initial and most
critical stage of metastasis includes the detachment of malignant
cells from the primary tumor and invasion for the formation of a
new lesion (3). Cancer cells promote
their invasive potential by undergoing a phenotypic conversion
referred to as the epithelial-mesenchymal transition (EMT), in
which epithelial cells lose polarity and their cell-cell adhesion
ability, acquiring a mesenchymal phenotype. This process involves a
variety of signaling pathways and is dominated by several
transcription factors (4,5). The numerous signaling pathways
participating in this transition involve transforming growth factor
β (TGFβ), bone morphogenetic protein, Wnt/β-catenin, Notch,
Hedgehog and receptor tyrosine kinases (6). In particular, activation of the
Wnt/β-catenin signaling pathway was reported to promote EMT in
various tumors (7).
Nemo-like kinase (NLK), initially identified as the
photosensitive cluster required for the rotation of cells during
Drosophila eye development, is an evolutionarily conserved
mitogen-activated protein kinase (MAPK)-associated kinase (8). As a serine/threonine protein kinase that
serves as an important negative regulatory molecule in the
Wnt/β-catenin signaling pathway (9,10), NLK
phosphorylates T cell factor/lymphoid enhancer factor proteins,
inhibiting their binding to transcriptional response elements
(11). Accumulating evidence has
demonstrated that NLK serves a pivotal role in cell proliferation,
migration, invasion and apoptosis via regulation of a variety of
transcription components. For example, in human breast cancer
cells, NLK was reported to associate with heat shock protein in the
inhibition of apoptosis (12). NLK
was revealed to also negatively regulate glioblastoma, in part via
the inhibition of Wnt/β-catenin signaling (13). Additionally, NLK-mediated
phosphorylation of histone deacetylase 1 was revealed to negatively
regulate Wnt/β-catenin signaling to prevent the aberrant
proliferation of non-transformed primary fibroblast cells (14). In addition, the negative modulation of
the Wnt/β-catenin signaling pathway suppressed the progression of
NSCLC (15). Therefore, it was
proposed that NLK interacts with β-catenin in modulating EMT in
NSCLC.
In the present study, the expression of NLK was
analyzed via immunohistochemistry (IHC) and western blotting of
fresh-frozen NSCLC tissue samples, and NSCLC tissue microarrays
(TMAs). In addition, the association between NLK expression and the
clinicopathological features of NSCLC, and the effects of altering
NLK expression on NSCLC metastasis were determined. Furthermore,
immunofluorescence staining and a co-immunoprecipitation assay were
conducted to investigate the underlying mechanism.
Materials and methods
Sections and tissue samples
The present study included 159 NSCLC patients who
had consented and enrolled before surgery (30 women and 121 men).
Patient ages ranged from 39–83 years (median, 63.2 years). A total
of 151 NSCLC sections for immunohistochemical analysis were
obtained from patients with newly diagnosed NSCLC between
2005–2009, along with corresponding clinicopathological data. Eight
fresh NSCLC samples and corresponding para-cancerous tissues from
patients who underwent surgery but had received no chemotherapy or
radiation therapy before sample collection were also collected for
western blotting analysis between 2015–2016. All samples were
collected at the Affiliated Hospital of Nantong University, Jiangsu
Province, China. The study protocol was approved by the Human
Research Ethics Committee of the Affiliated Hospital of Nantong
University (Nantong, China). The collection of fresh-frozen human
NSCLC tissue samples met the requirement of an institutional review
board protocol approved by the Partners Human Research Committee
Affiliated Hospital of Nantong University, Nantong, China). All
tissue sections used for immunohistochemistry were formalin-fixed,
and paraffin-embedded. Tumor staging was in accordance with the
guidelines of the 7th edition of TNM staging in lung cancer
(16). The 5-year actual overall
survival time was calculated from the date of surgery until the
date of death or last follow-up appointment. Representative 2.0-mm
tissue core samples from each patient were used to conduct TMA
analysis (Shanghai Outdo Biotech Co., Ltd.). All tissues for
immunoblot analysis were frozen immediately after surgery, then
manually homogenized with a homogenizer in RIPA lysis buffer (cat.
no. P0013B; Beyotime Institute of Biotechnology, Nantong, China).
After centrifugation at 10,000 × g, 4°C for 15 min, the supernatant
was extracted immediately for western blot analysis or stored at
−80°C.
Immunohistochemistry (IHC)
Immunostaining was performed using the
avidin-biotin-peroxidase complex. Tissue sections were
deparaffinized using a graded ethanol series, and endogenous
peroxidase activity was blocked by soaking in 3% hydrogen peroxide
(H2O2) for 10 min. The sections were then
processed in 10 mmol/l citrate buffer (pH 6.0) and heated to 121°C
in an autoclave for 20 min to retrieve the antigen. After rinsing
in PBS (pH 7.2), the sections were incubated with anti-NLK (diluted
1:1,00; cat. no. ab116715) and anti-vimentin antibodies (diluted
1:1,00; cat. no. ab92547; both from Abcam) for 1 h at room
temperature. All slides were processed using the
peroxidase-anti-peroxidase method (Dako, Hamburg, Germany). After
being rinsed in PBS, the peroxidase reaction was visualized by
incubating the sections with a liquid mixture (0.02%
diaminobenzidine tetrahydrochloride, 0.1% phosphate buffer
solution, and 3% H2O2). After rinsing in
water, the sections were counterstained with hematoxylin,
dehydrated, and cover-slipped. Stained sections were observed under
a microscope. The extent of immunostaining was evaluated and scored
separately by two independent investigators with no prior knowledge
of the clinical or pathological parameters of the patients. This
TMA followed the Tissue Microarray System Quick-Ray manual (UT06;
Unitma Co., Ltd.). For the semi-quantification of positive
staining, both the intensity (0,1,2 or 3) and proportion of
positive cells (0 to 100) were noted. Thus, the score ranged from 0
(no. staining) to 300 (all cells strongly stained). X-tile software
(Rimm Laboratory, Yale University; http://www.tissuearray.org/rimmlab) (17) was used to divide the protein
expression into two categories (low expression and high
expression); high expression represented a microscopic score of
70–300, and low expression was from 0–70.
Western blotting
Tissues and cell protein were promptly homogenized
after collection in a homogenization buffer containing 50 mM
Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% NP-40, 5 mM EDTA, 60 mM
β-glycerophosphate, 0.1 mM sodium orthovanadate, 0.1 mM NaF, and
complete protease inhibitor cocktail (Roche Diagnostics), then
centrifuged at 12,000 × g for 15 min to collect the supernatant.
Protein concentrations were measured with a BioRad protein assay
(BioRad Laboratories, Inc.). The supernatant was diluted in 2X SDS
loading buffer and boiled for 15 min. Proteins were then separated
with 10% SDS-polyacrylamide gel electrophoresis, and transferred to
polyvinylidene difluoride filter membranes (EMD Millipore). Then,
the mass of protein loaded per lane was initially set at 200 µg,
and the loading amount was adjusted according to the measured
protein concentration.
The membranes were blocked with 5% non-fat milk in
TBST (150 mM NaCl, 20 mM Tris, 0.05% Tween-20) for 2 h at room
temperature. They were then washed three times with TBST and
incubated overnight with primary antibodies anti-β-actin (dilution
1:500, cat. no. 4970, Cell Signaling Technology, Inc.), anti-NLK
(dilution 1:500; cat. no. ab116715), anti-β-catenin (dilution
1:500; cat. no. ab22656), anti-E-cadherin (dilution 1:500; cat. no.
ab1416), and anti-vimentin (dilution 1:500; cat. no. ab92547; all
from Abcam). Horseradish peroxidase-linked IgG secondary antibodies
(cat. no. A0208; Beyotime Institute of Biotechnology) at 1:5,000
dilution were added for 1 h incubation at room temperature. The
band intensity was measured by the ImageJ analysis system (National
Institutes of Health, USA) (18) and
normalized against β-actin levels. Experiments were carried out on
three separate occasions.
Cell cultures and transfection
The human NSCLC cell lines NCI-H1975, NCI-H1299,
NCI-H1650, and A549 were purchased from the Shanghai Institute of
Cell Biology Academia Sinica. All cancer cell lines were grown in
RPMI-1640 medium supplemented with 10% fetal bovine serum, and 100
U/ml penicillin-streptomycin (Gibco-BRL; Thermo Fsiher Scientific,
Inc.) at 37°C and 5% CO2. The medium was changed after
24 h and replaced with fresh medium for transfection. Full-length
NLK (Gene ID: 51701) was isolated from the human cDNA library.
NLK-shRNA and control-shRNA lentiviruses were obtained from
GeneChem Technologies. The shRNA target sequences were:
5′-GAATATCCGCTAAGGATGC-3′ and 5′-CAGATCCAAGAGATGGAAA-3′. A549 cells
were infected with control-shRNA or NLK-shRNA lentiviruses
according to the manufacturer's protocol.
Wound healing assay
A549 cells were seeded in 6-well plates (Corning,
Inc.) and transfected with NLK-shRNA, control-shRNA and full-length
NLK according to the manufacturer's instructions (19). When they reached ~80% confluency 48 h
post-transfection, cells were serum-starved for 12 h, then
scratched using the fine end of a 100-µl pipette tip. Wound healing
was observed at different time-points within the scrape line.
Representative scrape line images were captured using an inverted
Leica phase contrast microscope (Leica DFC 300 FX) under a 20×
objective lens every 24 h. Image Processing and Analysis in Java
was used to measure the wound healing assays. Duplicate wells for
each condition were examined, and each experiment was repeated
three times.
Invasion assay
The cell invasion assay was performed in a Transwell
chamber (24-well type, 8 µm pore size; Corning, Inc.) with the BD
Matrigel Basement Membrane Matrix according to the manufacturer's
recommended protocol. Serum-free RPMI-1640 medium (0.5 ml) was
placed in the upper chamber, and DMEM with 10% FBS was added to the
bottom chambers. An equal amount (1×105) of cells were
plated in the upper chambers of the quadruplicate wells and
incubated at 37°C for 72 h. Cells were then fixed with methanol and
stained with 3% crystal violet at 37°C to visualize the nuclei. The
results of three independent experiments were averaged. Image
Processing and Analysis in Java was used to assess the Transwell
assay.
Immunofluorescence staining
Cells were seeded on coverslips in 24-well plates
and cultured overnight. They were then washed with PBS, fixed with
4% paraformaldehyde for 1 h, and incubated at 4°C overnight with
anti-β-catenin (dilution 1:100; cat. no. ab32572) and anti-vimentin
(dilution 1:100; cat. no. ab92547; both from Abcam) antibodies.
After three washes with PBS, the cells were incubated with Alexa
Fluor-conjugated secondary antibodies (Alexa Fluor 594 (cat. no.
R37117; dilution 1:200), Alexa Fluor 488 (cat. no. R37114; dilution
1:500; Molecular Probe, Inc.), and counterstained with DAPI.
Fluorescence was detected using a Leica fluorescence microscope
(Leica Microsystems GmbH). All assays were performed three
times.
Co-immunoprecipitation
Immunoprecipitation was performed as previously
described (20). Briefly, total cell
lysates were incubated with an anti-NLK antibody, anti-β-catenin
antibody or control IgG at 4°C overnight. Protein A/G
(Sigma-Aldrich; Merck KGaA) was then added for 2 h at 4°C with
gentle shaking. The precipitates were washed three times with
homogenization buffer and boiled for another 15 min with SDS sample
buffer followed by western blotting.
Statistical analyses
Values are presented as the means ± standard
deviations (SDs) (21). Each
experiment was repeated at least three times per condition. The
Chi-square method was used to determine statistical significance.
One-way analysis of variance (ANOVA) followed by Bonferroni post
hoc test was used for the comparison of multiple groups. And SPSS
20.0 statistical software (IBM Corp.) was used for statistical
analysis. Survival analysis was performed using the Kaplan-Meier
method with the log-rank test. Univariate and multivariate analyses
were based on the Cox proportional hazards regression model. A
P-value <0.05 was considered statistically significant for all
analyses.
Results
Expression of NLK in NSCLC
tissues
To investigate the expression of NLK, E-cadherin and
vimentin protein in NSCLC tissues, western blotting was performed
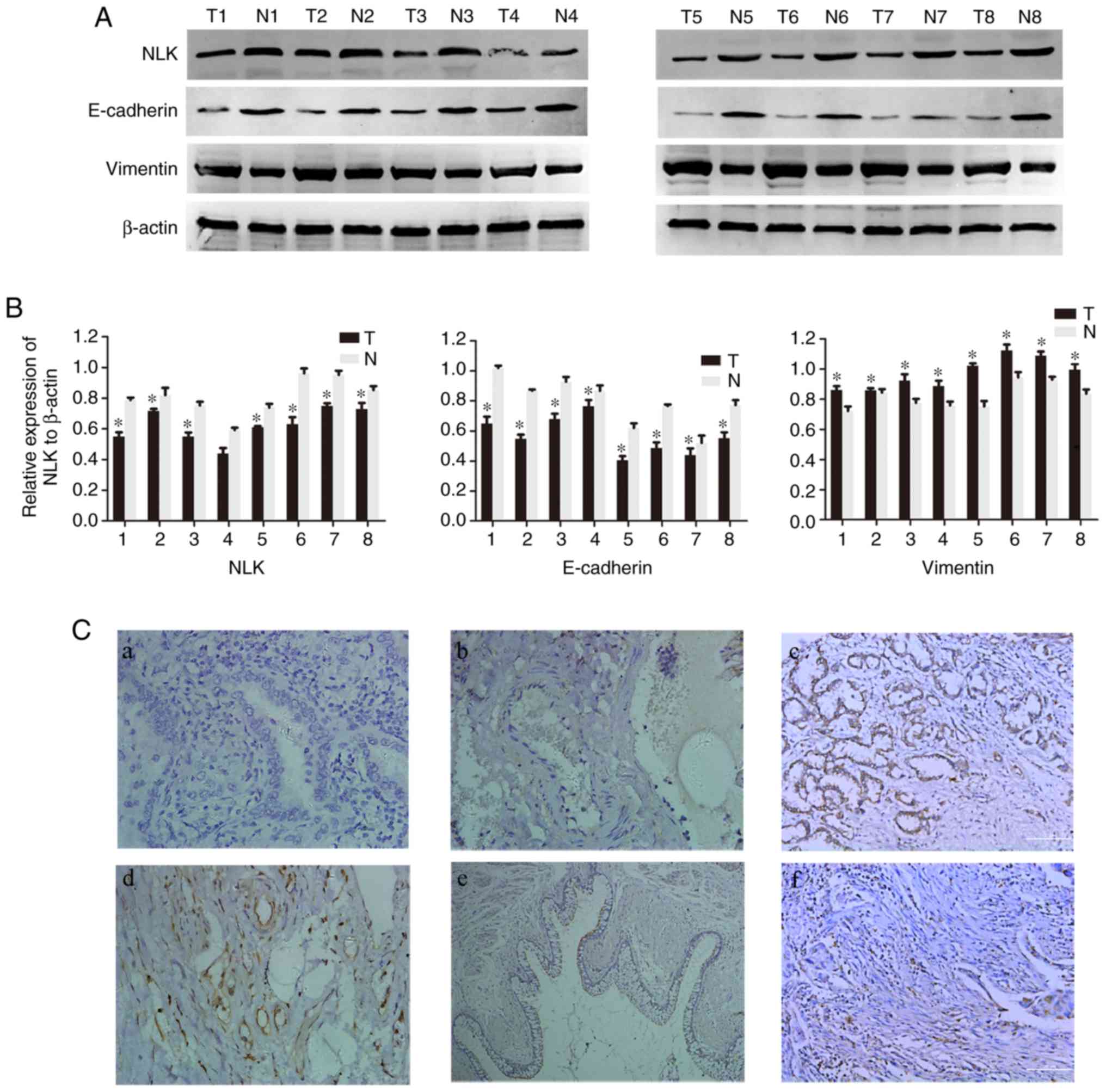
in 8 pairs of fresh NSCLC and non-tumorous adjacent tissues. As
presented in Fig. 1A and B, the
expression levels of NLK and E-cadherin protein were significantly
decreased in tumor tissues, while vimentin was upregulated compared
with matched healthy tissues (P<0.05).
To further determine the association between NLK,
E-cadherin and vimentin in NSCLC tissues, IHC analysis of 151 NSCLC
patient samples was conducted. NLK was observed to be expressed
mainly in the membrane and cytoplasm, E-cadherin was located in the
membrane and vimentin was detected in the cytoplasm. NLK and
E-cadherin were notably upregulated, while vimentin expression was
decreased in healthy lung tissues compared with NSCLC tissues
(Fig. 1C).
Analysis of the association between
NLK and the clinicopathological features of NSCLC by TMA-IHC
Statistical analysis was performed to determine the
association between NLK and the clinicopathological features of
NSCLC (Table I) in 151 NSCLC
specimens. A total of 89 (58.9%) patients exhibited low or
undetectable expression of NLK, while 62 (41.1%) presented NLK
upregulation. NLK was significantly associated with TNM stage
grouping (χ2=7.885, P=0.019), primary tumor
(χ2=8.966, P=0.011), lymph node metastasis
(χ2=19.085, P<0.001), and E-cadherin
(χ2=47.111, P=0.001) and vimentin expression
(χ2=52.847, P<0.001). A notable association was
observed between NLK expression and patient age, sex, smoking
status, tumor size and histological type.
 | Table I.Correlation of NLK expression in
tumorous tissues with clinicopathological characteristics in NSCLC
patients. |
Table I.
Correlation of NLK expression in
tumorous tissues with clinicopathological characteristics in NSCLC
patients.
|
| NLK |
|---|
|
|
|
|---|
| Clinicopathological
characteristics | n | Low or no
expression | High
expression | Pearson
χ2 | P-value |
|---|
| Total | 151 | 89 | 62 |
|
|
| Age at diagnosis
(years) |
|
|
| 0.663 | 0.416 |
|
<60 | 48 | 26 (54.2) | 22 (45.8) |
|
|
|
≥60 | 103 | 63 (61.2) | 40 (38.2) |
|
|
| Sex |
|
|
| 0.299 | 0.585 |
|
Male | 121 | 70 (57.9) | 51 (42.1) |
|
|
|
Female | 30 | 19 (63.3) | 11 (36.7) |
|
|
| Smoking |
|
|
| 6.785 | 0.376 |
|
Yes | 57 | 31 (54.4) | 26 (45.6) |
|
|
| No | 94 | 58 (61.7) | 36 (38.3) |
|
|
| Histopathology
grading |
|
|
| 2.118 | 0.347 |
|
Adenocarcinoma | 97 | 53 (54.6) | 44 (45.4) |
|
|
|
Squamous cell carcinoma | 40 | 27 (67.5) | 13 (32.5) |
|
|
|
Others | 14 | 9 (64.3) | 5 (35.7) |
|
|
| Primary tumor |
|
|
| 8.966 | 0.011a |
| T1 | 55 | 41 (74.5) | 14 (25.5) |
|
|
| T2 | 76 | 39 (51.3) | 37 (48.77) |
|
|
|
T3+T4 | 20 | 9 (45) | 11 (55) |
|
|
| Stage grouping with
TNM |
|
|
| 7.885 | 0.019a |
| I | 52 | 25 (48.1) | 27 (51.9) |
|
|
| II | 57 | 32 (56.1) | 25 (43.9) |
|
|
|
III | 42 | 32 (76.2) | 10 (23.8) |
|
|
|
Differentiation |
|
|
| 6.896 | 0.032a |
| Low
grade | 43 | 20 (46.5) | 23 (53.5) |
|
|
| Middle
grade | 95 | 58 (61.1) | 37 (38.9) |
|
|
| High
grade | 13 | 11 (84.6) | 2 (15.4) |
|
|
| Lymph node
metastasis |
|
|
| 19.085 |
<0.001a |
|
Yes | 66 | 52 (78.8) | 14 (21.2) |
|
|
| No | 85 | 37 (43.5) | 48 (56.5) |
|
|
| E-cadherin
expression |
|
|
| 47.111 |
<0.001a |
|
Low | 82 | 69 (84.1) | 13 (15.9) |
|
|
|
High | 69 | 20 (29.0) | 49 (71.0) |
|
|
| Vimentin
expression |
|
|
| 52.847 |
<0.001a |
|
Low | 64 | 16 (25.0) | 48 (75.0) |
|
|
|
High | 87 | 73 (83.9) | 14 (16.1) |
|
|
Survival analysis
A Cox proportional hazards model was constructed to
perform univariate and multivariate analyses of all prognostic
variables for the 5-year survival rate of patients with NSCLC
(Table II). Univariate Cox
regression analyses revealed that low NLK expression [hazard ratio
(HR) 0.341, 95% confidence interval (CI) 0.219–0.531, P<0.001],
lymph node metastasis (HR=5.732, 95% CI=3.732–8.803, P<0.001),
advanced TNM stage (HR=3.214, 95% CI=2.341–3.941, P<0.001), and
E-cadherin (HR=0.239, 95% CI=0.158–0.363, P<0.001) and vimentin
expression (HR=5.820, 95% CI=3.644–9.295, P<0.001) were
significantly associated with the survival of patients with NSCLC
for all variables. In addition, multivariate analysis indicated NLK
expression (HR=0.387, 95% CI=0.248–0.605, P<0.001) and TNM
(HR=3.037, 95% CI=2.341–3.941, P<0.001) as independent
prognostic factors in patients with NSCLC. Collectively, the
results inidcated that reduced expression of NLK could be a
potential indicator of poor prognosis in NSCLC.
 | Table II.Univariate and multivariate analyses
of overall survival in 151 NSCLC specimens. |
Table II.
Univariate and multivariate analyses
of overall survival in 151 NSCLC specimens.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | HR | P-value | 95% CI | HR | P-value | 95% CI |
|---|
| NLK expression | 0.341 |
<0.001a | 0.219–0.531 | 0.387 |
<0.001a | 0.248–0.605 |
| High
vs. low |
|
|
|
|
|
|
| Sex | 0.848 | 0.507 | 0.521–1.380 |
|
|
|
| Male
vs. female |
|
|
|
|
|
|
| Age (years) | 1.141 | 0.536 | 0.751–1.733 |
|
|
|
| ≤60 vs.
>60 |
|
|
|
|
|
|
| Smoking | 0.878 | 0.507 | 0.597–1.290 |
|
|
|
| Yes vs.
no |
|
|
|
|
|
|
| Histopathology
grading | 1.124 | 0.397 | 0.857–1.475 |
|
|
|
| Ad vs.
Sq vs. others |
|
|
|
|
|
|
|
Differentiation | 0.841 | 0.049a | 0.708–0.999 |
|
|
|
| Low vs.
middle vs. high grade |
|
|
|
|
|
|
| Primary tumor | 1.118 | 0.431 | 0.847–1.476 |
|
|
|
| T1 vs.
T2 vs. T3+T4 |
|
|
|
|
|
|
| Lymph node
metastasis | 5.732 |
<0.001a | 3.732–8.803 |
|
|
|
| Yes vs.
no |
|
|
|
|
|
|
| TNM stage | 3.214 |
<0.001a | 2.475–4.173 | 3.037 |
<0.001a | 2.341–3.941 |
| I vs.
II vs. III+IV |
|
|
|
|
|
|
| E-cadherin
expression | 0.239 |
<0.001a | 0.158–0.363 |
|
|
|
| High
vs. low |
|
|
|
|
|
|
| Vimentin
expression | 5.820 |
<0.001a | 3.644–9.295 |
|
|
|
| High
vs. low |
|
|
|
|
|
|
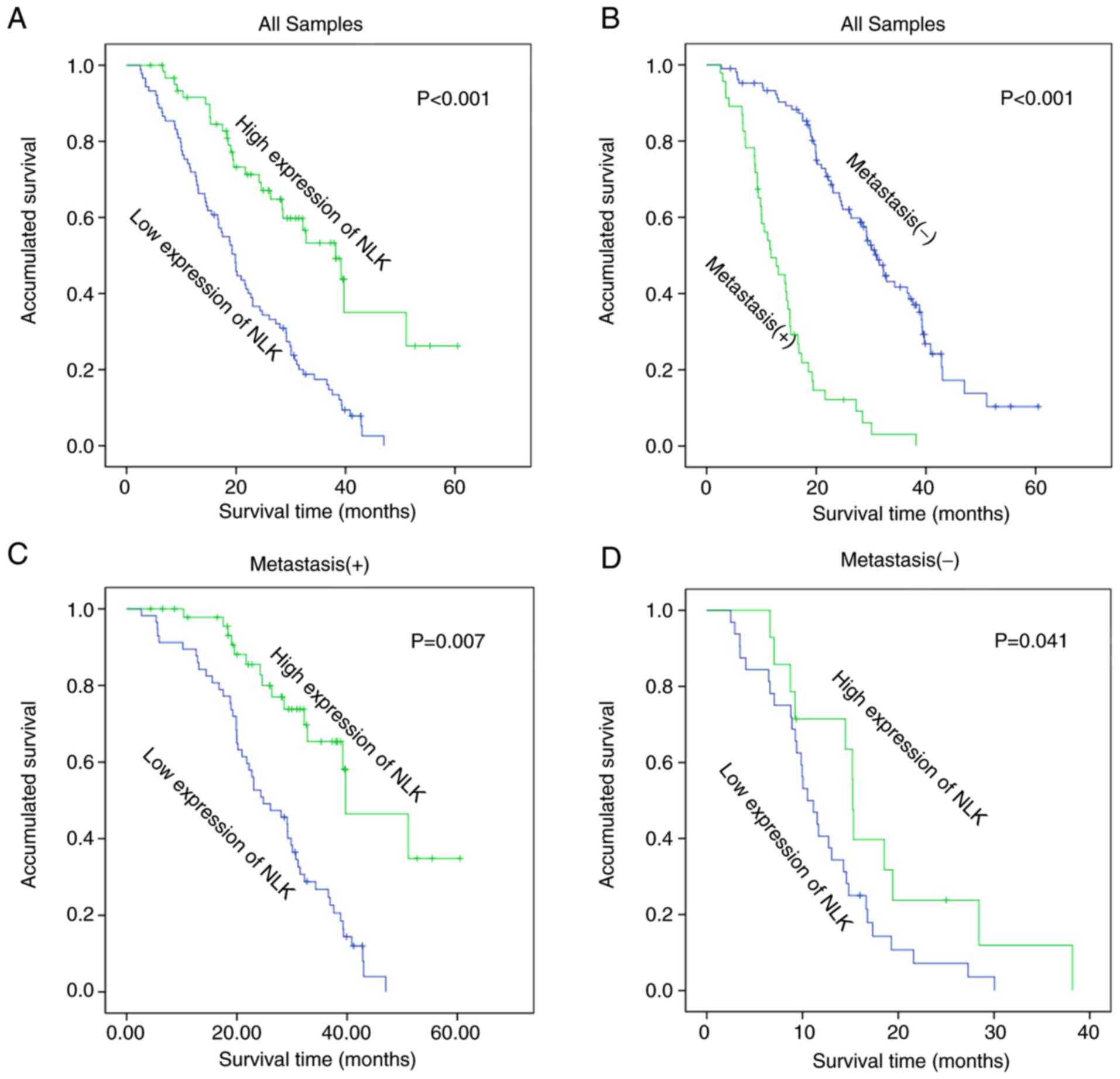
Kaplan-Meier survival curves further confirmed that
the reduction of NLK protein expression was significantly
associated with poor prognosis in patients with NSCLC (log-rank
test, P<0.001; Fig. 2A). In
accordance with NLK expression, the survival curve demonstrated a
high degree of discrimination, indicating that the score standard
for assessing NLK staining was suitable and that NLK was a reliable
prognostic factor. Kaplan-Meier analysis also revealed that
patients with lymph node metastasis had lower overall survival
rates than patients without metastasis (log-rank test, P<0.001;
Fig. 2B). Additionally, patients with
lymph node metastasis and upregulated NLK expression demonstrated
significantly better prognosis than those with lymph node
metastasis and downregulated NLK expression (P<0.05; Fig. 2B). Patients with increased NLK
expression and no metastasis had a significantly higher overall
survival rate than those with downregulated NLK expression and no
metastasis (P=0.007; Fig. 2D).
Expression of NLK in NSCLC cell
lines
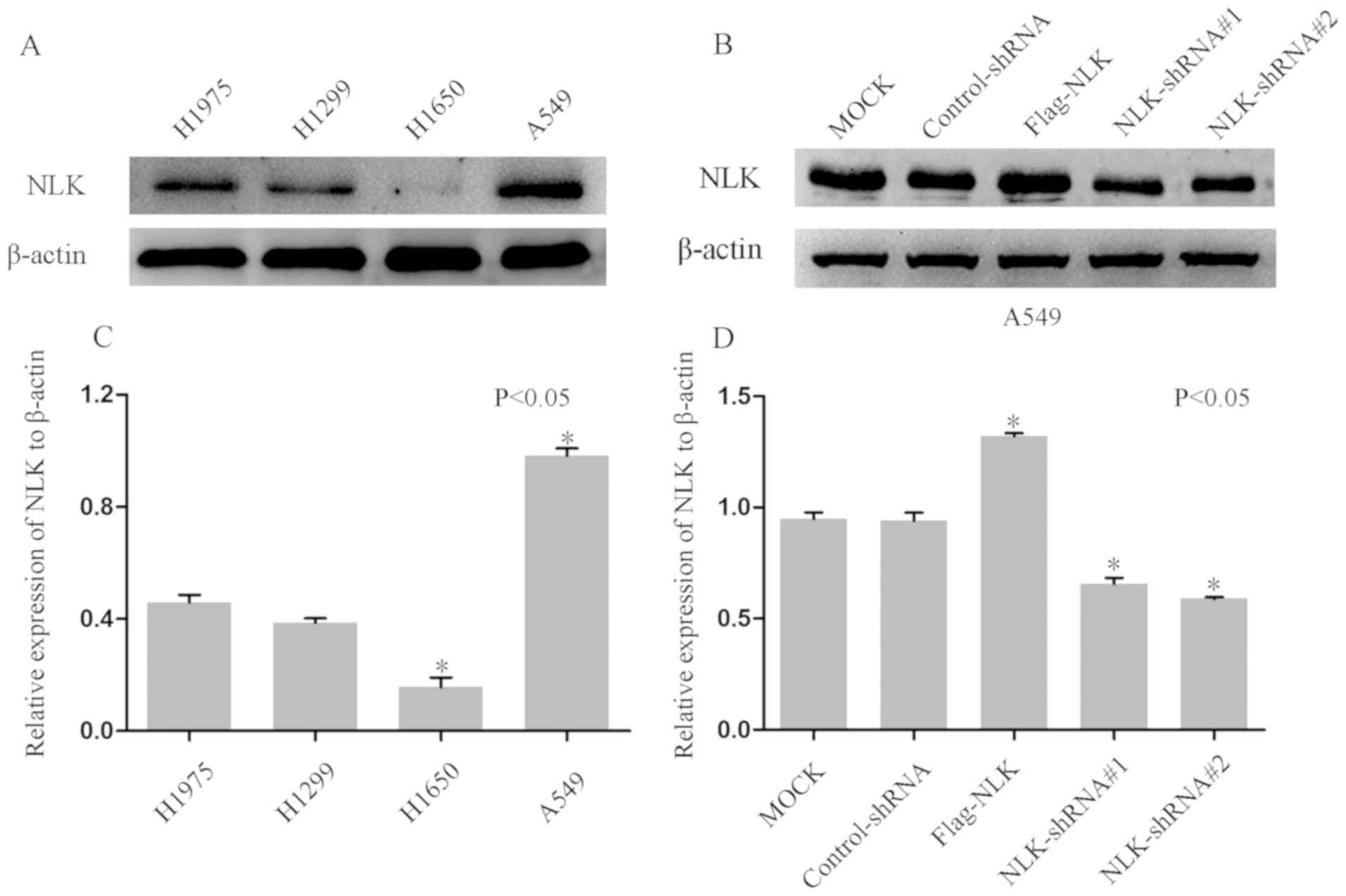
To investigate the function of NLK in the
carcinogenesis of NSCLC, NLK expression was determined in four
NSCLC cell lines. Western blotting demonstrated upregulated NLK
expression in A549 cells and downregulated NLK expression in H1650
cells compared with H1975 and H1299 and (Fig. 3A and C). Subsequently, cells were
transfected with NLK-shRNAs to downregulate the expression of NLK.
As presented in Fig. 3B and D,
western blotting revealed notable reductions in NLK protein
expression in cells transfected with NLK-shRNAs compared with
non-transfected or control-shRNA-transfected cells. In addition, a
Flag-NLK vector was constructed to overexpress NLK in A549
cells.
NLK inhibits the migration and
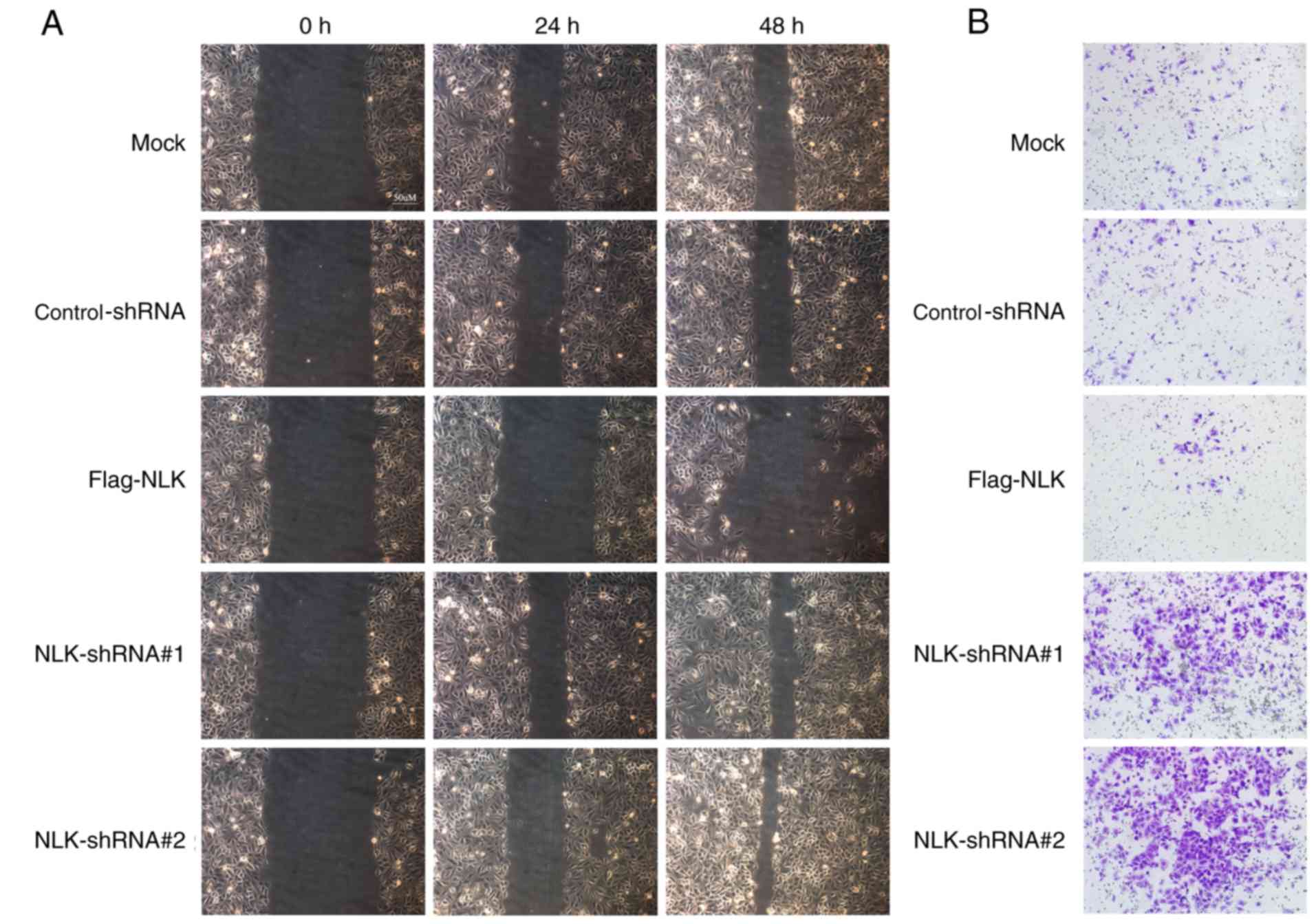
invasion of NSCLC cell lines
In advanced stages of cancer or postoperative
recurrence, NSCLC can be characterized by increased metastatic
potential. Therefore, wound healing and Transwell assays were
performed to investigate whether NLK affects NSCLC cell migration
and invasion. In the wound healing assay, it was observed that the
migration ability of the NLK-Flag group was decreased compared with
the control group. Conversely, cells transfected with NLK-shRNA
exhibited increased metastatic potential compared with the negative
group; the migration ability of the NLK-shRNA group was promoted by
reducing the expression of NLK (Fig.
4A). Similarly, the Transwell assay revealed that upregulating
the expression of NLK suppressed cell migration compared with the
control and negative groups (Fig.
4B).
NLK interacts with β-catenin in
regulating EMT
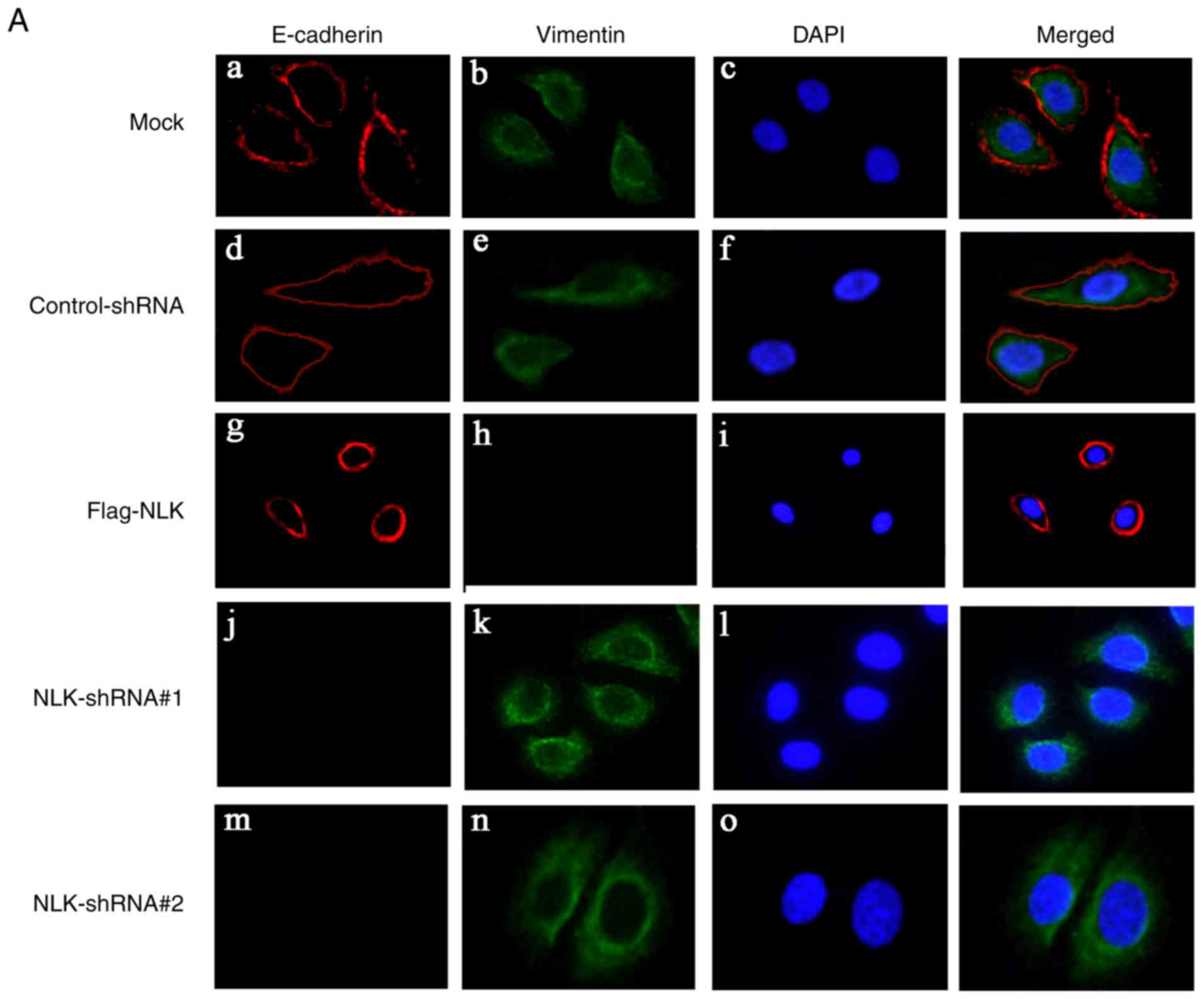
To determine whether NLK affects EMT in NSCLC cells,
immunofluorescence staining was conducted. A549 cells were
transfected with NLK-shRNA and Flag-NLK; alterations in the
expression of EMT markers were then analyzed. As presented in
Fig. 5A, upregulating NLK expression
via a Flag-NLK vector resulted in increased expression of the
epithelial marker E-cadherin in the cell membrane, while the
expression of the mesenchymal marker vimentin was decreased in the
cytoplasm. Following NLK downregulation by NLK-shRNA, E-cadherin
expression was notably undetected in the cell membrane, while
vimentin expression was upregulated in the cytoplasm.
Western blotting revealed that upregulation of NLK
led to increased E-cadherin expression, while that of vimentin and
β-catenin was downregulated. Similarly, the expression of vimentin
and β-catenin was increased, while that of E-cadherin was decreased
following NLK downregulation (Fig. 5B and
C). Immunoprecipitation of A549 cell protein revealed that NLK
could co-immunoprecipitate with an anti-β-catenin antibody, but not
under control conditions, indicating a naturally occurring
interaction between endogenous NLK and β-catenin in vivo and
vice versa. Thus, this could be a novel mechanism underlying the
role of the tumor suppressor gene NLK in the progression of NSCLC
(Fig. 5D and E).
Discussion
Since there is a lack of specific clinical features
in early stage NSCLC, patients may possess an advanced stage
disease prior to definitive diagnosis. NSCLC often metastasizes to
the brain, bone, adrenal gland or liver tissues, which limits the
use of optimal surgical procedures, and can lead to treatment
failure and tumor-associated mortality. The invasion-metastasis
cascade is comprised of two key steps: i) The spread of cancer
cells from the primary tumor to distant tissues; and ii) the
development of micro-metastases from cells that migrate to distant
organs (22). According to research
statistics, distant metastasis accounts for >90% of
tumor-associated mortalities (23).
Studies into the metastasis, survival and treatment of NSCLC have
revealed that >50% of patients with NSCLC present with
metastatic disease at the time of diagnosis, and only ~1% of
patients survive for ≥5 years, with an intermediate survival of 7
months (24). Therefore, further
investigation is required to determine the specific mechanism
underlying NSCLC metastasis.
NLK was initially identified as a regulator of
Drosophila photoreceptor development (8). NLK is an important member of the MAPK
subfamily, and serves important roles in cell proliferation,
invasion, metastasis, apoptosis and other biological processes. In
the present study, 8 pairs of fresh NSCLC tissues and corresponding
adjacent normal tissues were collected for immunoblotting, which
demonstrated that NLK expression was significantly reduced in NSCLC
tissues than corresponding adjacent normal tissues. To further
analyze NLK expression in NSCLC tissues, paraffin-fixed samples
from 151 patients with NSCLC were studied via IHC. The association
between NLK expression and the clinicopathological features of
NSCLC were statistically analyzed. The results demonstrated that
NLK expression in NSCLC tissues was significantly decreased than in
adjacent tissues, consistent with the findings from fresh tissues.
Statistical analysis revealed that low NLK expression was
associated with pathological classification, clinical stage and
lymph node metastasis in NSCLC. Cox multivariate regression
analysis indicated low NLK expression as an independent factor
affecting the prognosis of patients with NSCLC. Kaplan-Meier
survival curves also indicated that the overall survival of
patients with NSCLC and high NLK expression was significantly
increased than those with low NLK expression. NLK may be involved
in the pathophysiology of NSCLC. Emami et al (25) reported low NLK expression in prostate
cancer, which could inhibit androgen receptor expression and
promote the apoptosis of prostate cancer cells. Cui et al
(26) reported that NLK expression in
highly differentiated human glioblastoma was significantly lower
than in poorly differentiated glioblastoma; the survival rate of
patients with glioblastoma exhibiting low NLK expression was
significantly increased than those with high expression levels. In
nasopharyngeal carcinoma, calcium channel, voltage-dependent α 2/δ
subunit 3 could induce mitochondrial-mediated apoptosis and
activate NLK via the Wnt/Ca2+ pathway. This can
antagonize Wnt signaling-mediated, anchorage-dependent and
independent cell proliferation via cyclin D1 and CMYC, and
respectively suppress invasion and EMT via matrix metalloproteinase
7 and snail family transcriptional repressor 1 (Snai1) (27). These results indicated that NLK may be
a tumor suppressor in cell proliferation, EMT and tumor metastasis.
Investigations into gallbladder cancer demonstrated that NLK was
upregulated in cancer tissues, which was associated with the poor
prognosis of patients (28). Notably,
Lv et al reported that increased NLK expression in SCLC
could promote invasion and metastasis (29). Conversely, the present study observed
reduced NLK expression in NSCLC; this may reflect the differential
expression of NLK in organs, tissues and the internal environment
of cells (30).
The present study reported that the expression of
E-cadherin in the NSCLC cell membrane was reduced, while that of
vimentin was increased compared in normal tissue. Statistical
analysis revealed that NLK expression was associated with
E-cadherin and vimentin. In addition, the expression of E-cadherin
and vimentin in A549 cells following transfection was determined.
Immunofluorescence analysis demonstrated that E-cadherin expression
was significantly reduced, while that of vimentin was increased
following NLK knockdown compared with the control group. Opposing
findings were obtained when NLK was overexpressed in cells. This
indicated that NLK serves an important role in EMT, which is
associated with tumor metastasis (31–35). In
NSCLC, forkhead box Q1 could promote tumor metastasis by inducing
EMT, leading to adverse prognosis (36). In colorectal cancer, Wnt can activate
pre-B-cell leukemia transcription factor 3, and promote tumor
proliferation and metastasis by inducing EMT via Snail and zinc
finger E-box-binding homeobox 1 (37).
To determine the role of NLK in the metastasis of
NSCLC, NLK expression and its effects on NSCLC invasion and
metastasis were investigated at the cellular level. NLK expression
was analyzed in four NSCLC cell lines via immunoblotting in the
present study. A549 cells were selected for further analysis as
these cells exhibited relatively higher NLK expression. Following
the transfection of A549 cells with an NLK-overexpression plasmid,
the expression of NLK increased significantly, and decreased after
shRNA-mediated interference. The scratch-wound and Transwell assays
demonstrated that the viability, and invasive and metastatic
potentials of A549 cells transfected with the NLK-overexpression
plasmid were significantly reduced than the control group.
Conversely, the invasive and metastatic potentials of A549 cells
transfected with NLK-shRNA were significantly promoted compared to
the control group. Therefore, the present study proposed that NLK
may serve a role in the metastatic process of NSCLC. In the past
decade, a number of studies have reported that a quantity of
transcription factors and co-factors have been demonstrated to be
phosphorylated and regulated by NLK (38). Previous studies found that NLK
participated in NSCLC cell proliferation by modulating the Wnt
signaling pathway (5). Moreover,
aberrant expression of NLK was correlated with proliferation and
apoptosis in hepatocellular carcinoma (39), as well as prostate (25) and colon cancer (40). The present study revealed that NLK had
impacts on metastasis of cells via regulation of EMT in NSCLC. As
is recognized, EMT is closely related to tumor metastasis. Hence,
the hypothesis that high-expression of NLK may inhibit NSCLC
metastasis was put forward. However, it is still unclear whether
the effects of NLK on proliferation and apoptosis are related to
cell migration and invasion in NSCLC, this requires further
study.
TGFβ, Wnt/β-catenin, nuclear factor-κB and Hedgehog
serve important roles in regulating EMT. In several tumor models,
the Wnt/β-catenin signaling pathway has been reported to regulate
EMT (41–47). In oral squamous cell carcinoma,
SRY-box 8 regulates stem cell-like protein and platinum-induced EMT
by activating the Wnt/β-catenin signaling pathway. In breast
cancer, microRNA-23a activates the Wnt/β-catenin signaling pathway
via direct interaction with cadherin-1, and promotes TGFβ 1-induced
EMT and tumor metastasis (43). Yang
et al (7) and Liang et
al (48) reported that
Wnt/β-catenin promoted the metastasis of NSCLC by inducing EMT, and
revealed that NLK antibodies could be used to form an
NLK-interacting β-catenin immunoprecipitation complex. These
protein complexes suggest that NLK may interact directly or
indirectly with β-catenin. Therefore, low levels of NLK expression
in NSCLC may induce EMT by regulating the Wnt/β-catenin signaling
pathway.
In conclusion, NSCLC was reported to exhibit reduced
NLK expression levels, which could be associated with metastasis
and the poor prognosis of NSCLC. Dysregulating the expression of
NLK in A549 cells significantly altered the invasive and migration
potentials of tumor cells. In addition, NLK was proposed to be
involved in EMT in NSCLC, and serve an important role in the
Wnt/β-catenin signaling pathway in this process.
Acknowledgements
We gratefully acknowledge Dr Lili Ji, Dangping Wang,
Donghua Niu, Mengyuan Lv and Caixin Zhang.
Funding
The present study was supported by the Project of
Jiangsu Provincial Six Talent Peak (2014-WSN-028), the Youth Fund
of Nantong Health and Family Planning Commission (WQ2016077), the
Graduate Science and Technology Innovation Program of Nantong
University (YKC16103).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
CS and LX completed most of the experimental part
and the writing of the article. JF is the head of the project,
responsible for the project design, normal operation and
coordination of the project. The other co-authors participated in
some experiments of this study. CS, JN, YW and SZ were responsible
for collecting cases. JN and YW directed the experimental design
and technical guidance for the immunofluorescence experiment, and
they completed most of the related data analysis. ZT and WZ
assisted CS and LX in molecular biology experiments.
Ethics approval and consent to
participate
The study protocol was approved by the Human
Research Ethics Committee of the Affiliated Hospital of Nantong
University (Nantong, China) and patients provided consent and
enrolled before surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NLK
|
Nemo-like kinase
|
|
NSCLC
|
non-small cell lung cancer
|
|
TNM
|
tumor node metastasis
|
|
SCLC
|
small cell lung cancer
|
|
EMT
|
epithelial-mesenchymal transition
|
|
MAPK
|
mitogen-activated protein kinase
|
|
TCF/LEF
|
T cell factor/lymphoid enhancer
factor
|
|
HSP27
|
heat shock protein 27
|
|
IHC
|
immunohistochemistry
|
|
TMAs
|
tissue microarrays
|
|
HR
|
hazard ratio
|
|
95% CI
|
95% confidence interval.
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grunert S, Jechlinger M and Beug H:
Diverse cellular and molecular mechanisms contribute to epithelial
plasticity and metastasis. Nat Rev Mol Cell Biol. 4:657–665. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bogenrieder T and Herlyn M: Axis of evil:
Molecular mechanisms of cancer metastasis. Oncogene. 22:6524–6536.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang S, Liu Y, Li MY, Ng CSH, Yang SL,
Wang S, Zou C, Dong Y, Du J, Long X, et al: FOXP3 promotes tumor
growth and metastasis by activating Wnt/β-catenin signaling pathway
and EMT in non-small cell lung cancer. Mol Cancer. 16:1242017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi KW and Benzer S: Rotation of
photoreceptor clusters in the developing Drosophila eye
requires the nemo gene. Cell. 78:125–136. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takada I, Mihara M, Suzawa M, Ohtake F,
Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y,
et al: A histone lysine methyltransferase activated by
non-canonical Wnt signalling suppresses PPAR-gamma transactivation.
Nat Cell Biol. 9:1273–1285. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishitani T, Kishida S, Hyodo-Miura J, Ueno
N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J and
Matsumoto K: The TAK1-NLK mitogen-activated protein kinase cascade
functions in the Wnt-5a/Ca(2+) pathway to antagonize
Wnt/beta-catenin signaling. Mol Cell Biol. 23:131–139. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamada M, Ohnishi J, Ohkawara B, Iemura S,
Satoh K, Hyodo-Miura J, Kawachi K, Natsume T and Shibuya H: NARF,
an nemo-like kinase (NLK)-associated ring finger protein regulates
the ubiquitylation and degradation of T cell factor/lymphoid
enhancer factor (TCF/LEF). J Biol Chem. 281:20749–20760. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shaw-Hallgren G, Chmielarska Masoumi K,
Zarrizi R, Hellman U, Karlsson P, Helou K and Massoumi R:
Association of nuclear-localized Nemo-like kinase with heat-shock
protein 27 inhibits apoptosis in human breast cancer cells. PLoS
One. 9:e965062014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sa JK, Yoon Y, Kim M, Kim Y, Cho HJ, Lee
JK, Kim GS, Han S, Kim WJ, Shin YJ, et al: In vivo RNAi screen
identifies NLK as a negative regulator of mesenchymal activity in
glioblastoma. Oncotarget. 6:20145–20159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masoumi KC, Daams R, Sime W, Siino V, Ke
H, Levander F and Massoumi R: NLK-mediated phosphorylation of HDAC1
negatively regulates Wnt signaling. Mol Biol Cell. 28:346–355.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lv L, Wan C, Chen B, Li M, Liu Y, Ni T,
Yang Y, Liu Y, Cong X, Mao G and Xue Q: Nemo-like kinase (NLK)
inhibits the progression of NSCLC via negatively modulating WNT
signaling pathway. J Cell Biochem. 115:81–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (Eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhai X, Xu L, Zhang S, Zhu H, Mao G and
Huang J: High expression levels of MAGE-A9 are correlated with
unfavorable survival in lung adenocarcinoma. Oncotarget.
7:4871–4881. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang Z, Li J, Shen Q, Feng J, Liu H, Wang
W, Xu L, Shi G, Ye X, Ge M, et al: Contribution of upregulated
dipeptidyl peptidase 9 (DPP9) in promoting tumoregenicity,
metastasis and the prediction of poor prognosis in non-small cell
lung cancer (NSCLC). Int J Cancer. 140:1620–1632. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Zhu J, Yang L, Guan C, Ni R, Wang
Y, Ji L and Tian Y: Interaction with CCNH/CDK7 facilitates CtBP2
promoting esophageal squamous cell carcinoma (ESCC) metastasis via
upregulating epithelial-mesenchymal transition (EMT) progression.
Tumour Biol. 36:6701–6714. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang S, Shi W, Chen Y, Xu Z, Zhu J, Zhang
T, Huang W, Ni R, Lu C and Zhang X: Overexpression of SYF2
correlates with enhanced cell growth and poor prognosis in human
hepatocellular carcinoma. Mol Cell Biochem. 410:1–9. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xue Q, Lv L, Wan C, Chen B, Li M, Ni T,
Liu Y, Liu Y, Cong X, Zhou Y, et al: Expression and clinical role
of small glutamine-rich tetratricopeptide repeat (TPR)-containing
protein alpha (SGTA) as a novel cell cycle protein in NSCLC. J
Cancer Res Clin Oncol. 139:1539–1549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lovly CM and Carbone DP: Lung cancer in
2010: One size does not fit all. Nat Rev Clin Oncol. 8:68–70. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Emami KH, Brown LG, Pitts TE, Sun X,
Vessella RL and Corey E: Nemo-like kinase induces apoptosis and
inhibits androgen receptor signaling in prostate cancer cells.
Prostate. 69:1481–1492. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cui G, Li Z, Shao B, Zhao L, Zhou Y, Lu T,
Wang J, Shi X, Wang J, Zuo G, et al: Clinical and biological
significance of nemo-like kinase expression in glioma. J Clin
Neurosci. 18:271–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Peng C, Wu G, Wang Y, Liu R, Yang
S, He S, He F, Yuan Q, Huang Y, et al: Expression of NLK and its
potential effect in ovarian cancer chemotherapy. Int J Gynecol
Cancer. 21:1380–1387. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li M, Zhang S, Wang Z Zhang B, Wu X, Weng
H, Ding Q, Tan Z, Zhang N, Mu J, et al: Prognostic significance of
nemo-like kinase (NLK) expression in patients with gallbladder
cancer. Tumour Biol. 34:3995–4000. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lv M, Li Y, Tian X, Dai S, Sun J, Jin G
and Jiang S: Lentivirus-mediated knockdown of NLK inhibits
small-cell lung cancer growth and metastasis. Drug Des Devel Ther.
10:3737–3746. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ishitani T and Ishitani S: Nemo-like
kinase, a multifaceted cell signaling regulator. Cell Signal.
25:190–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brabletz T, Kalluri R, Nieto MA and
Weinberg RA: EMT in cancer. Nat Rev Cancer. 18:128–134. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
George JT, Jolly MK, Xu S, Somarelli JA
and Levine H: Survival outcomes in cancer patients predicted by a
partial EMT Gene expression scoring metric. Cancer Res.
77:6415–6428. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duhamel S, Goyette MA, Thibault MP, Filion
D, Gaboury L and Côté JF: The E3 ubiquitin ligase HectD1 suppresses
EMT and metastasis by targeting the +TIP ACF7 for degradation. Cell
Rep. 22:1016–1030. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singh M, Yelle N, Venugopal C and Singh
SK: EMT: Mechanisms and therapeutic implications. Pharmacol Ther.
182:80–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Feng J, Zhang X, Zhu H, Wang X, Ni S and
Huang J: FoxQ1 overexpression influences poor prognosis in
non-small cell lung cancer, associates with the phenomenon of EMT.
PLoS One. 7:e399372012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lamprecht S, Kaller M, Schmidt EM, Blaj C,
Schiergens TS, Engel J, Jung A, Hermeking H, Grünewald TGP,
Kirchner T, et al: PBX3 is part of an EMT regulatory network and
indicates poor outcome in colorectal cancer. Clin Cancer Res.
24:1974–1986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim S, Kim Y, Lee J and Chung J:
Regulation of FOXO1 by TAK1-Nemo-like kinase pathway. J Biol Chem.
285:8122–8129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ishikawa T, Shimizu D, Kito A, Ota I,
Sasaki T, Tanabe M, Yamada A, Arioka H, Shimizu S, Wakasugi J, et
al: Breast cancer manifested by hematologic disorders. J Thorac
Dis. 4:650–654. 2012.PubMed/NCBI
|
|
40
|
Yasuda J, Tsuchiya A, Yamada T, Sakamoto
M, Sekiya T and Hirohashi S: Nemo-like kinase induces apoptosis in
DLD-1 human colon cancer cells. Biochem Biophys Res Commun.
308:227–233. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie SL, Fan S, Zhang SY, Chen WX, Li QX,
Pan GK, Zhang HQ, Wang WW, Weng B, Zhang Z, et al: SOX8 regulates
cancer stem-like properties and cisplatin-induced EMT in tongue
squamous cell carcinoma by acting on the Wnt/β-catenin pathway. Int
J Cancer. 142:1252–1265. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ma F, Li W, Liu C, Li W, Yu H, Lei B, Ren
Y, Li Z, Pang D and Qian C: MiR-23a promotes TGF-β1-induced EMT and
tumor metastasis in breast cancer cells by directly targeting CDH1
and activating Wnt/β-catenin signaling. Oncotarget. 8:69538–69550.
2017.PubMed/NCBI
|
|
43
|
Hu Y, Qi MF, Xu QL, Kong XY, Cai R, Chen
QQ, Tang HY and Jiang W: Candidate tumor suppressor ZNF154
suppresses invasion and metastasis in NPC by inhibiting the EMT via
Wnt/β-catenin signalling. Oncotarget. 8:85749–85758.
2017.PubMed/NCBI
|
|
44
|
Guo YH, Wang LQ, Li B, Xu H, Yang JH,
Zheng LS, Yu P, Zhou AD, Zhang Y, Xie SJ, et al: Wnt/β-catenin
pathway transactivates microRNA-150 that promotes EMT of colorectal
cancer cells by suppressing CREB signaling. Oncotarget.
7:42513–42526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu X, Li Z, Song Y, Wang R, Han L, Wang
Q, Jiang K, Kang C and Zhang Q: AURKA induces EMT by regulating
histone modification through Wnt/β-catenin and PI3K/Akt signaling
pathway in gastric cancer. Oncotarget. 7:33152–33164.
2016.PubMed/NCBI
|
|
46
|
Gu Y, Wang Q, Guo K, Qin W, Liao W, Wang
S, Ding Y and Lin J: TUSC3 promotes colorectal cancer progression
and epithelial-mesenchymal transition (EMT) through WNT/β-catenin
and MAPK signalling. J Pathol. 239:60–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Duan H, Yan Z, Chen W, Wu Y, Han J, Guo H
and Qiao J: TET1 inhibits EMT of ovarian cancer cells through
activating Wnt/β-catenin signaling inhibitors DKK1 and SFRP2.
Gynecol Oncol. 147:408–417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liang Z, Lu L, Mao J, Li X, Qian H and Xu
W: Curcumin reversed chronic tobacco smoke exposure induced
urocystic EMT and acquisition of cancer stem cells properties via
Wnt/β-catenin. Cell Death Dis. 8:e30662017. View Article : Google Scholar : PubMed/NCBI
|