Introduction
Colorectal cancer (CRC) is the third most common
cancer and the second leading cause of cancer-related mortality
worldwide (1). Surgery,
chemotherapy and radiotherapy are three primary modalities in CRC
treatment; however, several alternative agents, including vitexin,
nitric oxide and sodium selenite, have been reported to inhibit
tumor progression and increase sensitivity to chemoradiotherapy in
CRC (2–4). This indicates that alternative
therapies could be employed to augment the efficacy of primary
treatments and even achieve tumor suppressive purposes
independently.
Hydrogen is the most abundant element in the
universe. Deuterium and protium are two stable isotopes of
hydrogen, which exhibit differences not only in physical and
chemical properties but also in biological functions (5). The concentration of deuterium in
natural water is ~0.015% [150 parts per million (ppm)] (6). Deuterium-depleted water (DDW) is
defined as water with a deuterium concentration of <150 ppm, and
it was reported to have an inhibitory effect on xenotransplanted
tumor growth in mice for the first time in the 1990s (7). Subsequently, studies reported the
effects of DDW in several areas, including in cancer suppression
(8), anti-senescence (9), insulin sensitization (10) and neuroprotection (11). However, the functions of DDW in CRC
treatment remain unclear.
Oxidative stress has been reported to serve a major
role in CRC progression (12).
Furthermore, the inhibition of reactive oxygen species (ROS)
production is considered a critical signaling mechanism of the DDW
anticancer function (13,14). Forkhead box (Fox)M1, a member of the
FOX transcription factor family, is recognized as a master
regulator of cancer growth, stemness and metastasis in CRC
(15). Previous studies have
reported crosstalk between ROS production and FoxM1 expression
(16,17); however, whether the FoxM1 signaling
pathway can be blocked by DDW, and the role of ROS in this process,
remain unknown.
Therefore, the objective of this study was to
systematically investigate the impact of DDW on malignant
biological behaviors and potential molecular mechanisms in CRC
cells cultured in vitro, specifically focusing on its
effects on cell proliferation, stemness, migration, invasion and
regulation of the ROS/FoxM1 signaling pathway. The results of the
present study may facilitate the clinical application of DDW for
CRC adjuvant therapy in the future.
Materials and methods
Cell culture and experimental
groups
The DDW (deuterium %=25 ppm) used in the present
study was provided by HYD LLC. Regular ddH2O or DDW was
used to dissolve RPMI-1640 medium powder (Gibco; Thermo Fisher
Scientific, Inc.). A total of two CRC cell lines, HT-29 (cat. no.
STCC10801G) and DLD-1 (cat. no. STCC10810G), were purchased from
Wuhan Servicebio Technology Co., Ltd. and authenticated by STR
profiling. Cells were cultured in conditioned RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin solution
(Sigma-Aldrich; Merck KGaA) at 37°C in a humidified atmosphere
containing 5% CO2. The control group contained cells
that were cultured in medium prepared with regular
ddH2O, whereas the cells in the DDW group were cultured
in medium prepared with DDW.
EdU incorporation assay
The EdU assay was performed using a commercial kit
(cat. no. C10310-1; Guangzhou RiboBio Co., Ltd.). HT-29 and DLD-1
cells were seeded into 6-well plates at a density of
1×105 cells per well. After culturing in serum-free
medium for 72 h, cells were incubated with 0.1% EdU solution for 3
h at 37°C, then fixed with 4% paraformaldehyde (PFA) for 30 min at
room temperature. Cells were then incubated with appollo staining
complex for 30 min at room temperature to react with EdU, followed
by incubation with Hoechst 33342 staining reagent for 30 min at
room temperature to label the cell nuclei. Fluorescent staining was
recorded using the Operetta CLS high-content analysis system
(PerkinElmer, Inc.). A total of five visual fields were randomly
selected and the number of EdU positive cells was assessed using
ImageJ software (version 1.53 K; National Institutes of Health).
The following formula was used to determine the percentage of EdU
positive cells: EdU positive cells (%)=(EdU positive cells/total
cells) ×100%.
Colony formation assay
HT-29 and DLD-1 cells were seeded into 6-well plates
at a density of 1×103 cells per well and cultured with conditioned
medium. The medium was changed every 3 days. After 10 days, the
cells were fixed with 4% PFA for 30 min at room temperature and
then stained with 0.1% crystal violet for 20 min at room
temperature. The colonies consisting of at least 40 cells were
counted under an inverted light microscope (ECLIPSE Ts2; Nikon
Corporation) using ImageJ software.
Tumor-sphere formation assay
DMEM powder and F-12 medium powder (both from Gibco;
Thermo Fisher Scientific, Inc.) were dissolved with regular ddH2O
or DDW. Both media were mixed at a ratio of 1:1 to prepare the
DMEM/F-12 medium. HT-29 and DLD-1 cells were seeded into ultra-low
attachment 24-well plates (Corning, Inc.) at a density of
1×103 cells per well and cultured in DMEM/F12 medium
supplemented with 1% B27, 20 ng/ml human FGF and 20 ng/ml human EGF
(Invitrogen™; Thermo Fisher Scientific, Inc). Fresh medium was
added every 3 days. After 2 weeks, cell spheres were counted under
an inverted light microscope (ECLIPSE Ts2; Nikon Corporation), and
the sphere formation efficiency (SFE) was calculated using the
following formula: SFE (%)=number of spheres/1,000 ×100%.
Wound healing assay
HT-29 and DLD-1 cells were cultured in 6-well plates
until they formed confluent monolayers. A gap was created across
the middle of the well using a 200 µl sterile tip. The cells were
then cultured in serum-free medium for an additional 48 h. An
inverted light microscope (ECLIPSE Ts2; Nikon Corporation) was used
to capture images both before and after the 48-h incubation period
to evaluate the migration rate. Experiments were repeated in
quintuplicate. A visual field was randomly selected, and the same
field was imaged for every time point. The area of blank space was
measured using ImageJ software. The following formula was used to
assess the wound healed ratio: Wound healed ratio
(%)=[1-(area48 h/area0 h)] ×100%.
Transwell assay
HT-29 and DLD-1 cells were suspended in serum-free
medium. The inner membranes of Transwell inserts (cat. no. 3464;
Corning, Inc.) were coated with 10% Matrigel (cat. no. 354234;
Corning, Inc.), followed by the seeding of 2×104 cells
with 200 µl serum-free DMEM into each upper chamber. A total of 750
µl DMEM supplemented with 10% FBS was added to each lower chamber
of the 24-well plate. After incubating for 24 h at 37°C, the cells
were fixed with 4% PFA for 30 min at room temperature and then
stained with 0.1% crystal violet for 20 min at room temperature.
Cells that successfully invaded the bottom side of membrane were
visualized using an inverted light microscope (ECLIPSE Ts2; Nikon
Corporation).
Intracellular ROS detection
Intracellular ROS levels were assessed using the
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) method. HT-29
and DLD-1 cells were incubated with the DCFH-DA probes
(Sigma-Aldrich; Merck KGaA) for 30 min at 37°C and then treated
with H2O2 (50 µM) for 30 min at 37°C. Cells were then fixed with 4%
PFA for 30 min at room temperature. The fluorescence intensity of
DCF was recorded using the Operetta CLS High-Content Analysis
System (PerkinElmer, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated using TRIzol™ reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. RT-qPCR was performed using the
SuperScript™ III Platinum™ One-Step qRT-PCR Kit (cat. no. 11732020;
Invitrogen; Thermo Fisher Scientific, Inc.). The sequences of the
primers used for qPCR were as follows: FoxM1 forward,
5′-ACGTCCCCAAGCCAGGCTC-3′ and reverse, 5′-CTACTGTAGCTCAGGAATAA-3′;
cyclin D1 (CCND1) forward, 5′-CGCTTCCTGTCGCTGGAGCC-3′ and
reverse, 5′-CTTCTCGGCCGTCAGGGGGA-3′; matrix metalloproteinase 9
(MMP9) forward, 5′-TTCTGCCCGGACCAAGGATA-3′ and reverse,
5′-ACATAGGGTACATGAGCGCC-3′; and β-actin (reference gene) forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′. The thermocycling conditions were as
follows: 50°C for 15 min and 95°C for 2 min, and then 40 cycles of
95°C for 15 sec and 60°C for 30 sec. The relative gene expression
was calculated using the 2−∆∆Cq method (18).
Lentiviral transduction
FoxM1-overexpressing (OE-FoxM1) and negative control
(OE-NC) lentiviral particles (1×108 TU/ml) were
purchased from Shanghai GeneChem Co., Ltd., both originating from
the same plasmid backbone (Ubi-MCS-3FLAG-SV40-puromycin). HT-29 and
DLD-1 cells were seeded into 6-well plates at a density of
1×105 cells per well and incubated overnight. HT-29 and
DLD-1 cells were transduced with the specific lentivirus at a
multiplicity of infection of 10. After a 24-h incubation, fresh
medium was changed to terminate the transduction. Stable infected
cells were selected by puromycin (5 µg/ml) incubation for 48 h, and
the surviving cells were continuously maintained in fresh DMEM with
10% FBS and 1.25 µg/ml puromycin for passaging. FoxM1 expression in
the recombinant cells was validated by RT-qPCR and western blotting
before subsequent experiments.
Western blotting
Total proteins from HT-29 and DLD-1 cells were
extracted using RIPA lysis buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology) and quantified using the BCA Protein
Assay Kit (cat. no. P0012; Beyotime Institute of Biotechnology).
Proteins (40 µg per lane) were separated by 4–20% SDS-PAGE (cat.
no. P0468S; Beyotime Institute of Biotechnology) and subsequently
transferred onto PVDF membranes (MilliporeSigma). The membranes
were blocked with 5% fat-free milk (Bio-Rad Laboratories, Inc.) for
1 h at room temperature and incubated with primary antibodies
(1:1,000 dilution by PBS) against β-actin (cat. no. 66009-1-Ig),
octamer-binding transcription factor-4 (OCT-4; cat. no.
11263-1-AP), Nanog (cat. no. 14295-1-AP), MMP-9 (cat. no.
10375-2-AP), CCND1 (cat. no. 26939-1-AP) and FoxM1 (cat. no.
13147-1-AP) (all Proteintech Group, Inc.) at 4°C overnight. Protein
signals were labeled with HRP-conjugated secondary antibodies
(1:5,000 dilution by PBS; cat. nos. SA00001-1 or SA00001-2;
Proteintech Group, Inc.) for 1 h at room temperature and visualized
using an ECL kit (cat. no. WBKLS0500; MilliporeSigma). The
densitometry was measured using ImageJ software.
Statistical analysis
Continuous data are expressed as mean ± standard
deviation. Differences between two groups were analyzed using the
unpaired Student's t-test or Mann-Whitney U test as appropriate.
One-way ANOVA was used to compare the mean differences among three
or more groups, and the least significant difference test was used
as a post-hoc test. The data analysis for this paper was generated
using GraphPad Prism 10 software (Dotmatics). P<0.05 was
considered to indicate a statistically significant difference.
Results
DDW inhibits the proliferation and
stemness of CRC cells
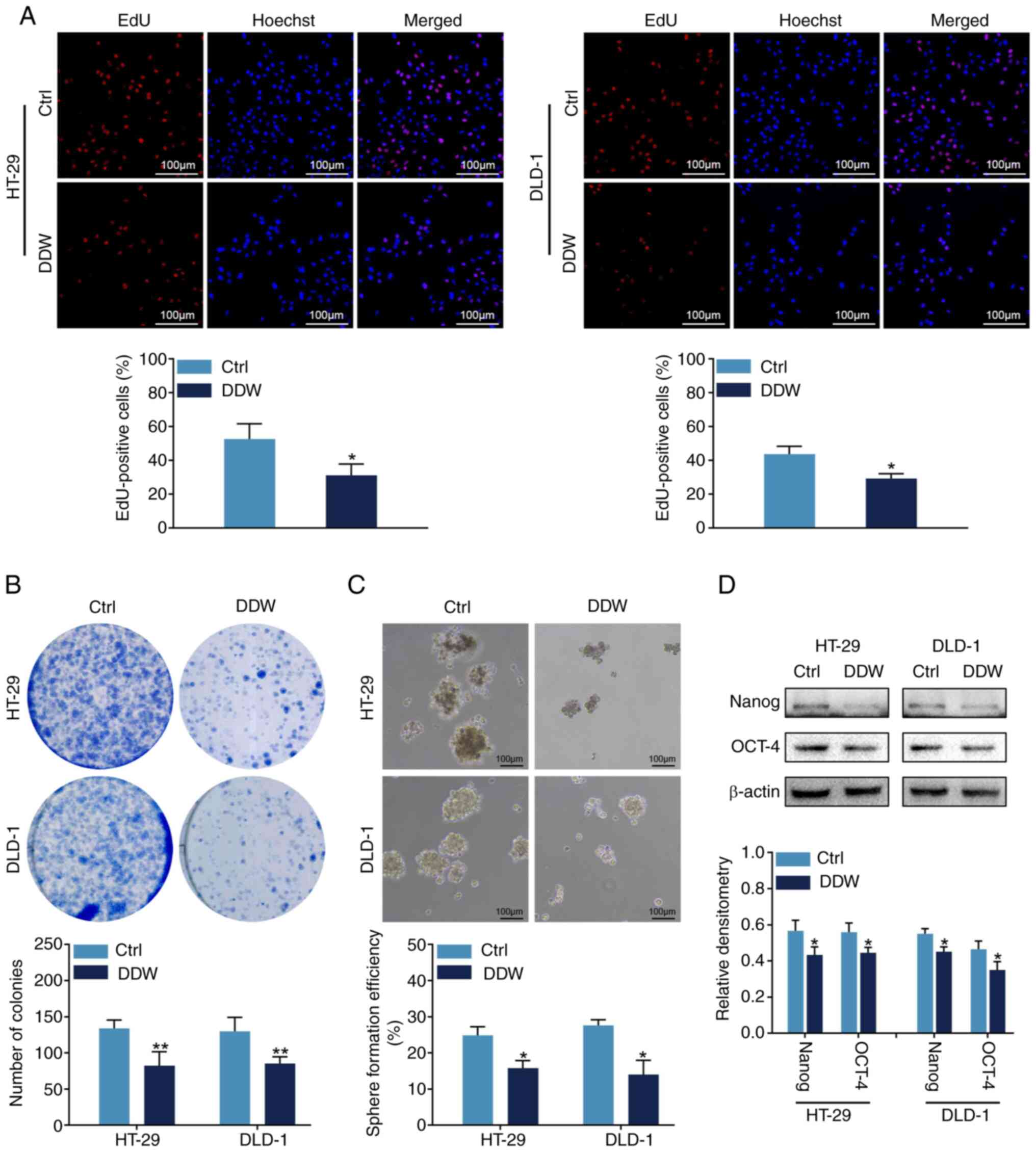
To assess the biological functions of DDW in CRC
malignancy, HT-29 and DLD-1 cells were cultured in conditioned
medium prepared with regular ddH2O or DDW. It was
demonstrated that, compared with the control (regular
ddH2O) treatment, DDW treatment reduced the proportion
of EdU-positive cells (Fig. 1A) and
the number of colonies formed (Fig.
1B). These results indicated that DDW could inhibit the
proliferation of CRC cells in vitro. Notably, the results
also revealed that DDW treatment impaired the sphere formation
efficiency of HT-29 and DLD-1 cells (Fig. 1C). This finding implied that DDW
might interfere with the stem cell-like phenotype of CRC cells.
Therefore, the expression levels of two typical stemness markers
were analyzed using western blotting. The findings demonstrated
that, compared with the control group, the protein levels of Nanog
and OCT-4 were significantly downregulated in the DDW treatment
group (Fig. 1D). These results
indicated that DDW treatment impaired the self-renewal ability of
HT-29 and DLD-1 cells.
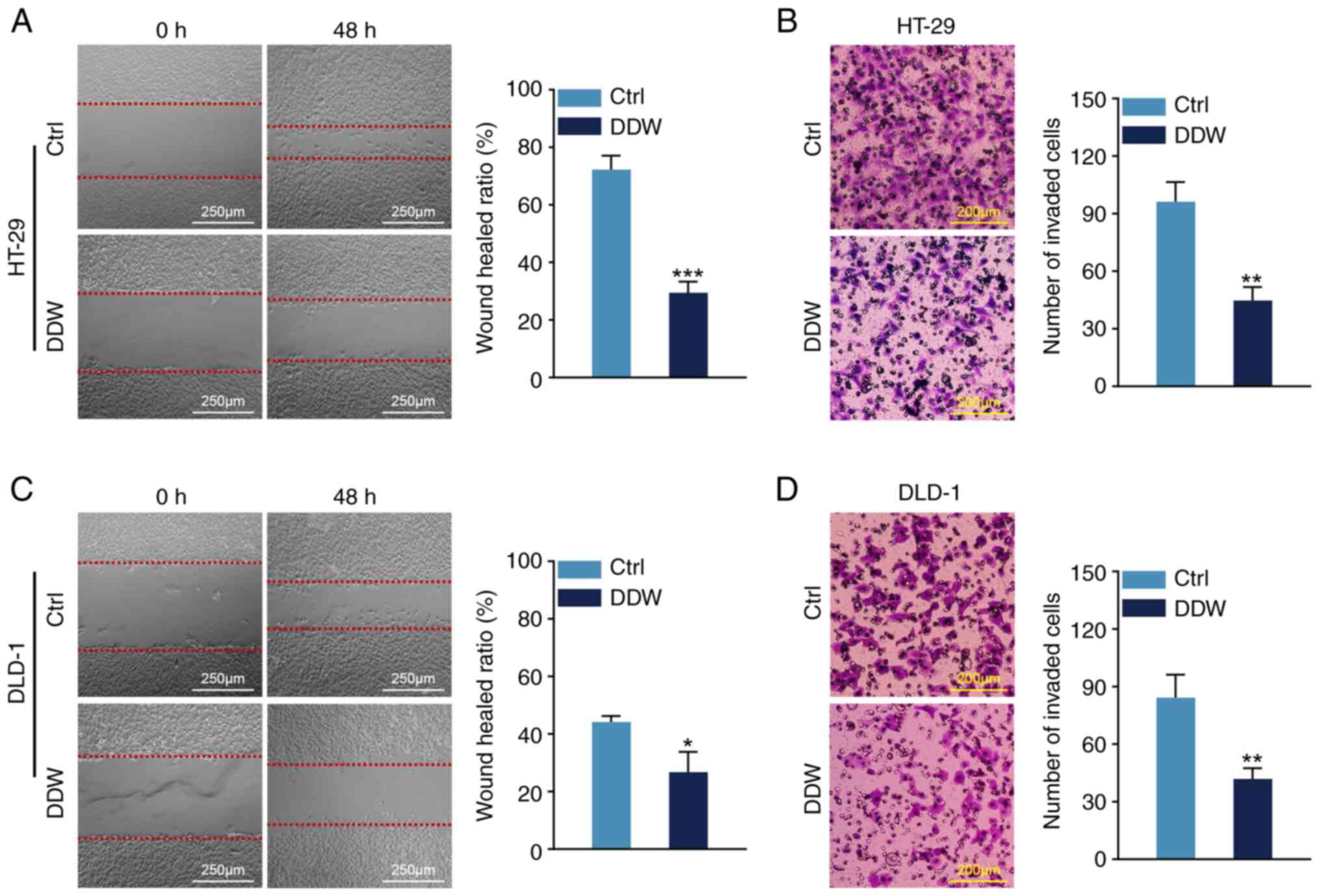
DDW restrains the migration and
invasion of CRC cells
Subsequently, wound healing and Transwell assays
were performed to evaluate the effect of DDW on cancer cell
mobility. Compared with the control treatment, the migrated
distances of HT-29 and DLD-1 cells were significantly reduced when
treated with DDW (Fig. 2A and C).
Additionally, there was a notable reduction in the number of
invaded cells for both the HT-29 and DLD-1 cells treated by DDW in
the Transwell assays (Fig. 2B and
D). These findings suggested that DDW treatment is associated
with a significant reduction in CRC cell migration and
invasion.
DDW downregulates FoxM1 signaling
through suppressing ROS production in CRC cells
To determine whether the ROS/FoxM1 axis was involved
in the aforementioned findings, ROS production in response to DDW
treatment was evaluated in the CRC cells. The findings revealed
that, compared with the control treatment, DDW treatment
significantly reduced the ROS levels in the HT-29 and DLD-1 cell
lines (Fig. 3A). Subsequently,
RT-qPCR and western blotting were used to evaluate FoxM1
expression. Compared with the control treatment, DDW treatment
significantly reduced the mRNA and protein levels of FoxM1
(Fig. 3B and C). Moreover, the
expression levels of CCND1 and MMP9, two typical downstream targets
of FoxM1 (19,20), were lower in HT-29 and DLD-1 cells
treated with DDW, compared with the control treatment (Fig. 3B and C). Furthermore,
H2O2 was utilized to induce intracellular ROS
production. Compared with the cells treated by DDW alone,
additional treatment with H2O2 not only
blocked the antioxidant effect of DDW (Fig. 3A) but also rescued the expression of
FoxM1 (Fig. 3D). These results
indicated that DDW treatment downregulated FoxM1 expression
possibly through inhibiting ROS production.
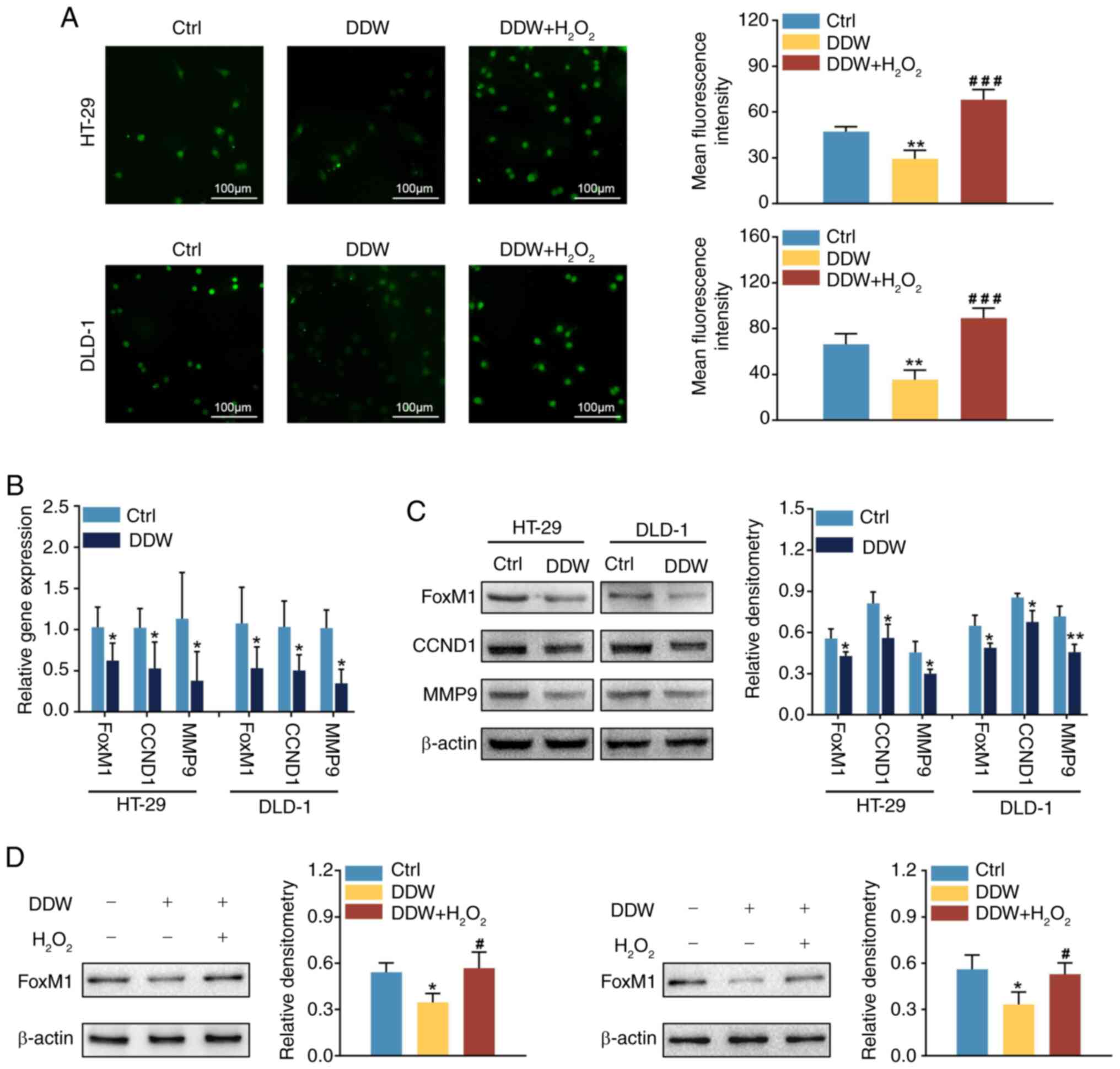 | Figure 3.DDW downregulates FoxM1 signaling
through suppressing ROS production in colorectal cancer cells. (A)
Levels of ROS in HT-29 and DLD-1 cells treated with DDW or DDW +
H2O2 were assessed using
2′,7′-dichlorodihydrofluorescein diacetate fluorescent analysis.
The mRNA and protein levels of FoxM1, CCND1 and MMP9 in HT-29 and
DLD-1 cells treated with DDW were analyzed using (B) reverse
transcription-quantitative PCR and (C) western blotting. (D)
Protein levels of FoxM1 in HT-29 and DLD-1 cells treated with DDW
or DDW + H2O2 were analyzed using western
blotting. *P<0.05 vs. Ctrl group, **P<0.01 vs. Ctrl group,
#P<0.05 vs. DDW group, ###P<0.001 vs.
DDW group. DDW, deuterium-depleted water; FoxM1, forkhead box
protein M1; ROS, reactive oxygen species; CCND1, cyclin D1; MMP9,
matrix metalloproteinase 9; Ctrl, control. |
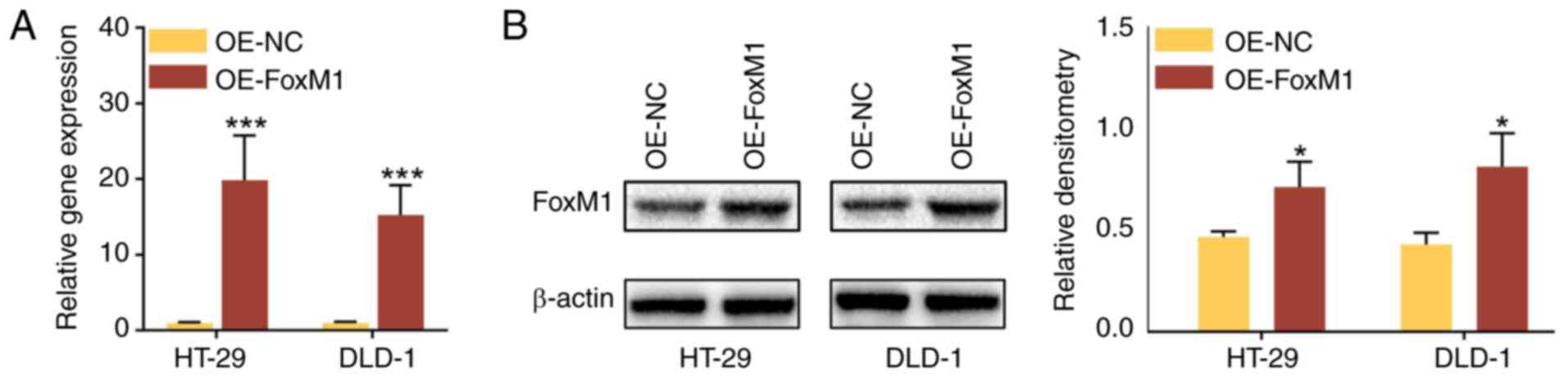
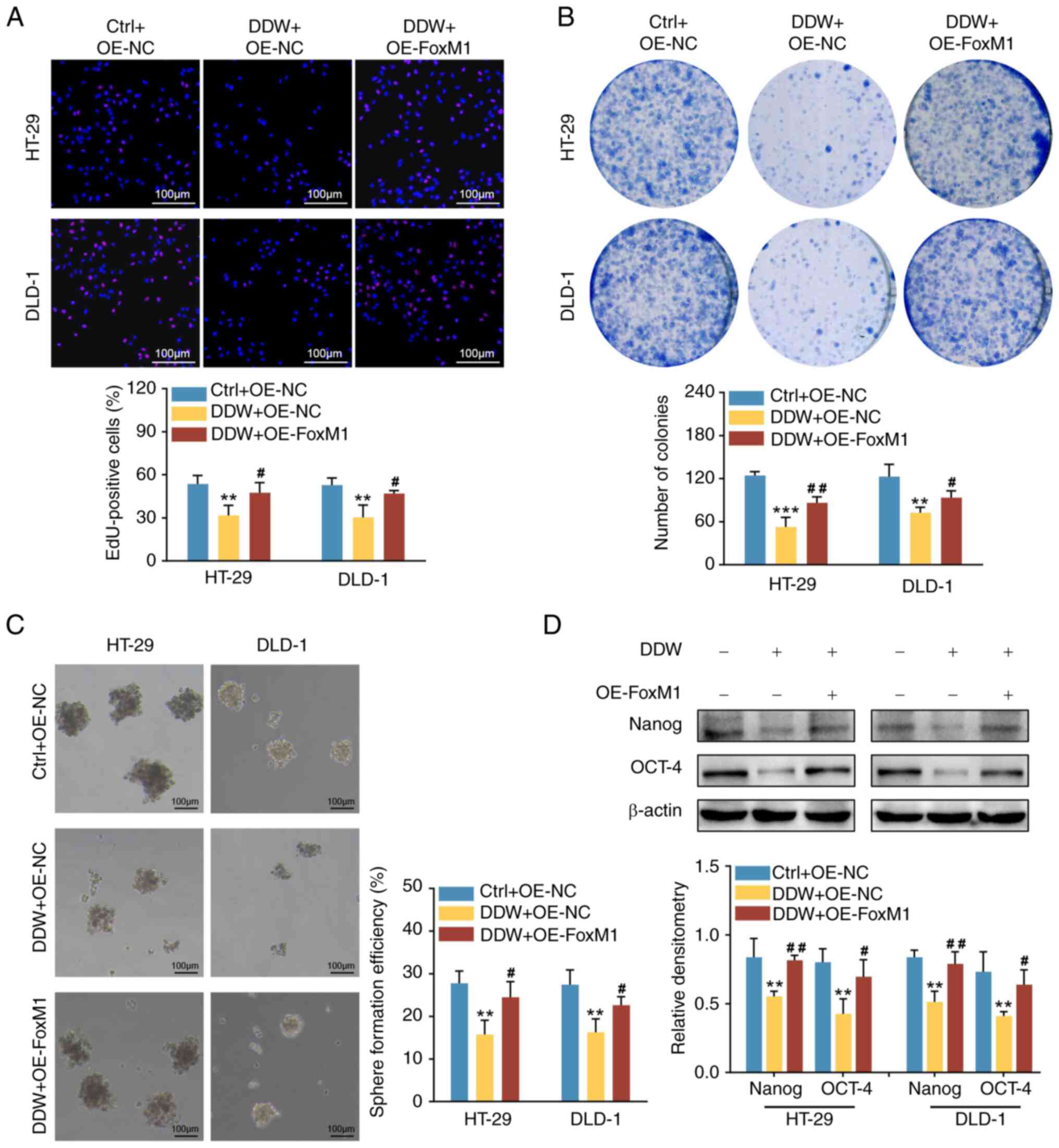
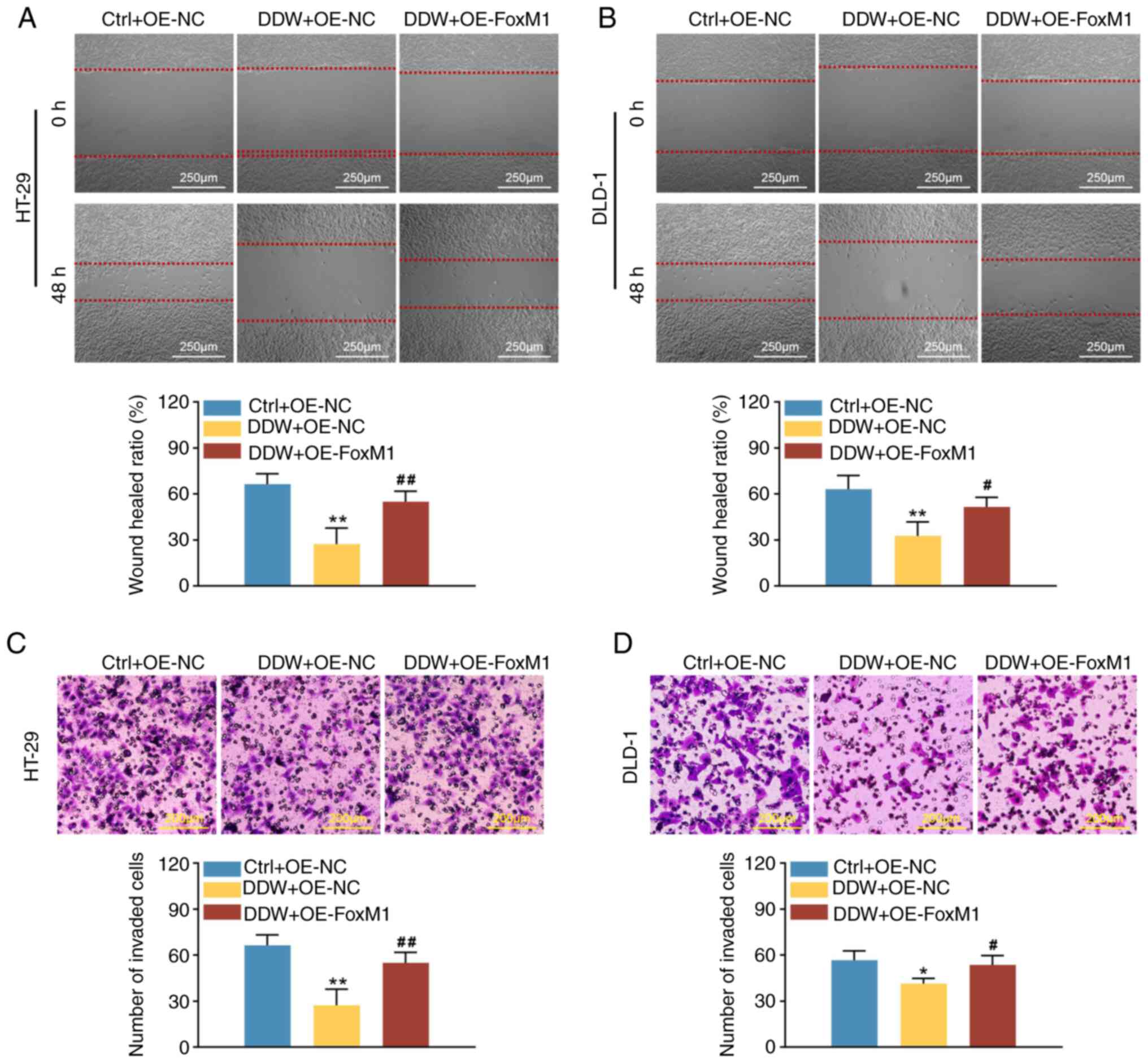
Overexpression of FoxM1 abolishes the
anticancer effects of DDW in CRC cells
To assess whether the anticancer effects of DDW were
dependent on the inhibition of FoxM1, FoxM1 was overexpressed in
HT-29 and DLD-1 cells using lentiviral transduction. The mRNA and
protein expression levels were significantly upregulated in
OE-FoxM1 cells compared with the OE-NC cells (Fig. 4). Furthermore, compared with the
OE-NC lentiviral transduction, the inhibitory effects of DDW on the
proliferation (Fig. 5A and B),
stemness (Fig. 5C and D), migration
(Fig. 6A) and invasion (Fig. 6B) of HT-29 and DLD-1 cells were
significantly abrogated by OE-FoxM1 lentiviral transduction. In
addition, overexpression of FoxM1 also rescued the expression
levels of CCND1 and MMP-9 in CRC cells treated with DDW, as
demonstrated using RT-qPCR (Fig. 7A and
C) and western blotting (Fig. 7B
and D). These results validated that DDW may exert its tumor
suppressive effects through inhibiting FoxM1 expression.
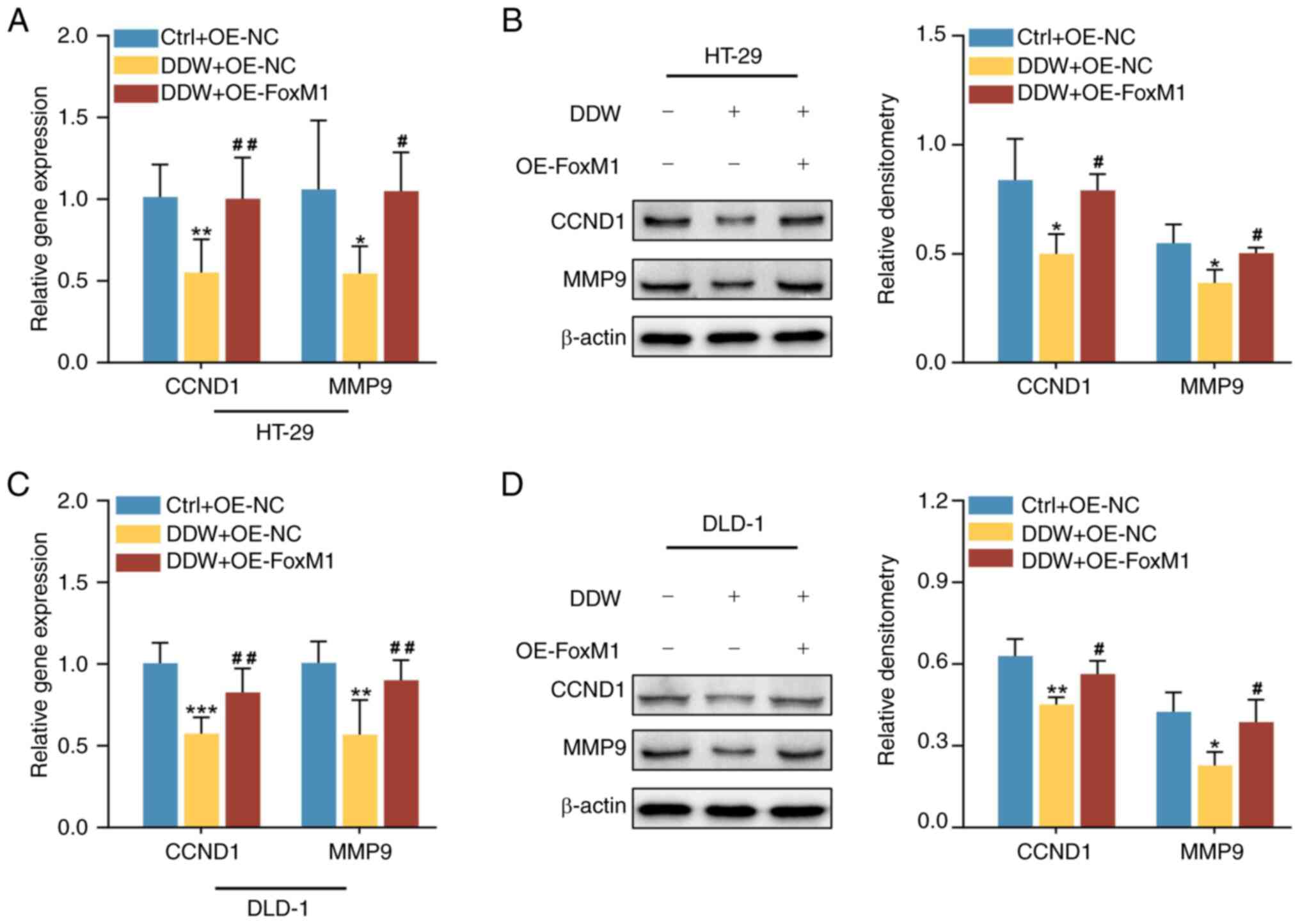 | Figure 7.Overexpression of FoxM1 rescues the
DDW-mediated inhibition of CCND1 and MMP9 in colorectal cancer
cells. The (A) mRNA and (B) protein expression levels of CCND1 and
MMP9 in HT-29 cells were assessed using RT-qPCR and western
blotting, respectively. The (C) mRNA and (D) protein expression
levels of CCND1 and MMP9 in DLD-1 cells were assessed using RT-qPCR
and western blotting, respectively. *P<0.05 vs. Ctrl + OE-NC
group, **P<0.01 vs. Ctrl + OE-NC group, ***P<0.001 vs. Ctrl +
OE-NC group, #P<0.05 vs. DDW + OE-NC group,
##P<0.01 vs. DDW + OE-NC group. FoxM1, forkhead box
protein M1; DDW, deuterium-depleted water; CCND1, cyclin D1; MMP9,
matrix metalloproteinase 9; OE, overexpression; Ctrl, control; NC,
negative control; RT-qPCR, reverse transcription-quantitative
PCR. |
Discussion
Water is the most common compound on Earth, but its
isotopic composition is heterogeneous due to kinetic isotope
fractionation (21). Given that the
variation of the levels of deuterium in different terrestrial water
sources is minimal, it is difficult to observe significant
differences in biological effects among populations consuming
natural water with varying deuterium concentrations. Artificial DDW
can be prepared by desalination, distillation and catalytic
exchange (22). The
deuterium-content of DDW used in translational and clinical studies
ranges from 25 to 125 ppm; however, Yavari et al (23) reported that the synergistic
anticancer effects of DDW combined with 5-fluorouracil gradually
faded with the increase of deuterium content from 30 to 150 ppm.
This indicated that a dose-dependent inhibition of cancer
progression may exist in DDW-treated cells. Therefore, the present
study used 25 ppm DDW to treat HT-29 and DLD-1 cells.
To the best of our knowledge, the present study is
the first to demonstrate that DDW exerts anticancer effects through
inhibiting the ROS/FoxM1 signaling pathway in CRC cells. This
finding was demonstrated by the following results: i) DDW treatment
inhibited cell proliferation, stemness, migration and invasion
in vitro; ii) DDW treatment decreased ROS production as well
as the expression of FoxM1 and its downstream targets, CCND1 and
MMP-9; iii) the induction of ROS partly rescued FoxM1 expression in
DDW-treated CRC cells; and iv) overexpression of FoxM1 abrogated
DDW-mediated tumor suppressive effects.
DDW has been shown to suppress the proliferation of
breast cancer cells by arresting them at the G0 or G1 phases
(23). Notably, the results of the
present study revealed that DDW treatment decreased the expression
of CCND1 in CRC cells. As a critical mediator of cell cycle
progression, CCND1 is responsible for transiting cells from the G1
to S phase (24). The results of
the present study also demonstrated that DDW inhibited cell
migration, cell invasion and MMP-9 expression, which is in
accordance with the results in nasopharyngeal carcinoma cells
reported by Wang et al (25). Moreover, a growing body of research
has reported promising outcomes regarding the clinical application
of DDW. Concomitant administration of DDW with conventional
therapies has been demonstrated to achieve improved overall
survival rates compared with the use of chemoradiotherapy alone in
patients with advanced-stage glioblastoma multiforme (26) and lung cancer (27). Furthermore, ~30% of patients with
CRC who receive initial curative treatment typically develop tumor
relapse (28). However, a
retrospective analysis reported that consumption of DDW markedly
reduced this rate to 18.5% (8),
supporting the strong tumor-prevention effect of DDW.
Although overproduction of ROS induced by
chemoradiotherapy leads to the death of cancer cells, slightly
elevated ROS levels resulting from intracellular hypermetabolism
facilitate tumor progression by activating pro-survival
transcription factors including hypoxia-inducible factor 1α
(HIF-1α), activator protein-1 and NF-κB (29). The present study demonstrated that
manipulation of the degree of ROS production could disrupt the
inhibitory efficacy of DDW toward the expression of FoxM1, and the
overexpression of FoxM1 counteracted the tumor-inhibiting effects
of DDW. These results indicate that ROS-induced FoxM1 could be the
target of DDW in CRC treatment. However, the molecular mechanisms
by which ROS upregulates FOXM1 are complex. Park et al
(30) reported that endogenous ROS
were constantly generated when cancer cells were cultured in
favorable conditions with sufficient energy metabolism and oxygen
supply, causing the upregulation of FoxM1 to enhance the rates of
proliferation and metastasis. This viewpoint was corroborated by
the finding that ROS-induced upregulation of FoxM1 promotes aerobic
glycolysis in glioblastoma (31).
Moreover, ROS have also been demonstrated to activate the FoxM1
promoter and induce its expression in a HIF-1α-dependent manner
(32).
However, some limitations of the present study
should be noted. First, the detailed mechanisms by which DDW
inhibits ROS production remain unclear. Future research will focus
on mitochondrial-related biological changes to explore whether DDW
inhibits the production of ROS by disrupting mitochondrial
function. Second, the in vivo anticancer effects of DDW were
not evaluated in the present study. Animal experiments and
population-based randomized controlled clinical trials will be
introduced to further demonstrate the therapeutic effects of DDW in
colorectal cancer.
In summary, the findings of the present study
suggest that DDW has multiple anticancer effects in CRC cells, and
DDW-mediated ROS/FoxM1 signaling inactivation could be a possible
pathway in these effects. Future studies should focus on the
clinical application of DDW as a promising alternative therapeutic
agent in CRC.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Beijing Hospitals
Authority Youth Programme (grant no. QML20210906) and the Natural
Science Basic Research Program of Shaanxi (grant no.
2021JQ-397).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CL and YJ conceptualized the present study. CL wrote
the original draft of the manuscript. CL and XC contributed to the
experiments. CL, XC and YJ analyzed the data. All authors read and
approved the final version of the manuscript. CL and YJ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Y, Liang J, Chen S, Lin N, Xu S, Miao
J, Zhang J, Chen C, Yuan X, Xie Z, et al: Discovery of vitexin as a
novel VDR agonist that mitigates the transition from chronic
intestinal inflammation to colorectal cancer. Mol Cancer.
23:1962024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zou Y, He Y, Tan L, Xu X, Qi C and Zhang
Y: Discovery of cytotoxic nitric oxide-releasing piperlongumine
derivatives targeting Wnt/β-catenin in colon cancer cells. J Nat
Prod. 87:1893–1902. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang H, Yang X, Zhang Z and Xu H: Both
calcium and ROS as common signals mediate Na(2)SeO(3)-induced
apoptosis in SW480 human colonic carcinoma cells. J Inorg Biochem.
97:221–230. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dunlap MK, Kringle L, Kay BD and Kimmel
GA: Proton diffusion and hydrogen/deuterium exchange in amorphous
solid water at temperatures from 114 to 134 K. J Chem Phys.
161:2445042024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Korchinsky N, Davis AM and Boros LG:
Nutritional deuterium depletion and health: A scoping review.
Metabolomics. 20:1172024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Somlyai G, Jancsó G, Jákli G, Vass K,
Barna B, Lakics V and Gaál T: Naturally occurring deuterium is
essential for the normal growth rate of cells. FEBS Lett. 317:1–4.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kovács BZ, Puskás LG, Nagy LI, Papp A,
Gyöngyi Z, Fórizs I, Czuppon G, Somlyai I and Somlyai G: Blocking
the increase of intracellular deuterium concentration prevents the
expression of cancer-related genes, tumor development, and tumor
recurrence in cancer patients. Cancer Control.
29:107327482110689632022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Avila DS, Somlyai G, Somlyai I and Aschner
M: Anti-aging effects of deuterium depletion on Mn-induced toxicity
in a C. elegans model. Toxicol Lett. 211:319–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kondo M, Sawada K, Matsuda Y, Abe M,
Sanechika N, Takanashi Y, Mori Y, Kimura M and Toyoda M: Study of
the effects of deuterium-depleted water on the expression of GLUT4
and insulin resistance in the muscle cell line C2C12. Biomedicines.
12:17712024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu Y, Qin D, Yang H, Wang W, Xiao J, Zhou
L and Fu H: Neuroprotective effects of deuterium-depleted water
(DDW) against H2O2-induced oxidative stress in differentiated pc12
cells through the PI3K/Akt signaling pathway. Neurochem Res.
45:1034–1044. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin S, Li Y, Zamyatnin AA, Werner J and
Bazhin AV: Reactive oxygen species and colorectal cancer. J Cell
Physiol. 233:5119–5132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bayrak BB, Kulak GY, Yanardag R and Yarat
A: Short term deuterium depletion in drinking water reduced tumor
induced oxidative stress in mice liver. Pathol Res Pract.
240:1541862022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fatemi F, Golbodagh A, Hojihosseini R,
Dadkhah A, Akbarzadeh K, Dini S and Malayeri MRM: Anti-inflammatory
effects of deuterium-depleted water plus Rosa Damascena Mill.
essential oil via cyclooxygenase-2 pathway in rats. Turk J Pharm
Sci. 17:99–107. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laissue P: The forkhead-box family of
transcription factors: Key molecular players in colorectal cancer
pathogenesis. Mol Cancer. 18:52019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu G, Yang L, Xu Y, Jiang X, Jiang X,
Huang L, Mao L and Cai S: FABP4 induces asthmatic airway epithelial
barrier dysfunction via ROS-activated FoxM1. Biochem Biophys Res
Commun. 495:1432–1439. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun L, Wang Y, Wang L, Yao B, Chen T, Li
Q, Liu Z, Liu R, Niu Y, Song T, et al: Resolvin D1 prevents
epithelial-mesenchymal transition and reduces the stemness features
of hepatocellular carcinoma by inhibiting paracrine of
cancer-associated fibroblast-derived COMP. J Exp Clin Cancer Res.
38:1702019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao L, Liu L, Dong Z and Xiong J: miR-149
suppresses human non-small cell lung cancer growth and metastasis
by inhibiting the FOXM1/cyclin D1/MMP2 axis. Oncol Rep.
38:3522–3530. 2017.PubMed/NCBI
|
|
20
|
Jin H, Li XJ, Park MH and Kim SM:
FOXM1-mediated downregulation of uPA and MMP9 by
3,3′-diindolylmethane inhibits migration and invasion of human
colorectal cancer cells. Oncol Rep. 33:3171–3177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Szoeke SP, Sarkar M, Quiñones Meléndez
E, Blossey PN and Noone D: A simple model for the evaporation of
hydrometeors and their isotopes. J Geophys Res Atmos.
129:e2024JD0411262024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang F and Meng C: Method for the
production of deuterium-depleted potable water. Ind Eng Chem Res.
50:378–381. 2011. View Article : Google Scholar
|
|
23
|
Yavari K and Kooshesh L: Deuterium
depleted water inhibits the proliferation of human MCF7 breast
cancer cell lines by inducing cell cycle arrest. Nutr Cancer.
71:1019–1029. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang W and Medeiros LJ: Utility of cyclin
D1 in the diagnostic workup of hematopoietic neoplasms: What can
cyclin D1 Do for Us? Adv Anat Pathol. 26:281–291. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H, Zhu B, He Z, Fu H, Dai Z, Huang G,
Li B, Qin D, Zhang X, Tian L, et al: Deuterium-depleted water (DDW)
inhibits the proliferation and migration of nasopharyngeal
carcinoma cells in vitro. Biomed Pharmacother. 67:489–496. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Somlyai G, Kovács BZ, Papp A and Somlyai
I: A preliminary study indicating improvement in the median
survival time of glioblastoma multiforme patients by the
application of deuterium depletion in combination with conventional
therapy. Biomedicines. 11:19892023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gyöngyi Z, Budán F, Szabó I, Ember I, Kiss
I, Krempels K, Somlyai I and Somlyai G: Deuterium depleted water
effects on survival of lung cancer patients and expression of Kras,
Bcl2, and Myc genes in mouse lung. Nutr Cancer. 65:240–246. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nikolouzakis TK, Vassilopoulou L,
Fragkiadaki P, Mariolis Sapsakos T, Papadakis GZ, Spandidos DA,
Tsatsakis AM and Tsiaoussis J: Improving diagnosis, prognosis and
prediction by using biomarkers in CRC patients (Review). Oncol Rep.
39:2455–2472. 2018.PubMed/NCBI
|
|
29
|
Averill-Bates D: Reactive oxygen species
and cell signaling. Review. Biochim Biophys Acta Mol Cell Res.
1871:1195732024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park HJ, Carr JR, Wang Z, Nogueira V, Hay
N, Tyner AL, Lau LF, Costa RH and Raychaudhuri P: FoxM1, a critical
regulator of oxidative stress during oncogenesis. EMBO J.
28:2908–2918. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su X, Yang Y, Yang Q, Pang B, Sun S, Wang
Y, Qiao Q, Guo C, Liu H and Pang Q: NOX4-derived ROS-induced
overexpression of FOXM1 regulates aerobic glycolysis in
glioblastoma. BMC Cancer. 21:11812021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xia L, Mo P, Huang W, Zhang L, Wang Y, Zhu
H, Tian D, Liu J, Chen Z, Zhang Y, et al: The
TNF-α/ROS/HIF-1-induced upregulation of FoxMI expression promotes
HCC proliferation and resistance to apoptosis. Carcinogenesis.
33:2250–2259. 2012. View Article : Google Scholar : PubMed/NCBI
|