Introduction
Extracorporeal shock wave therapy (ESWT) was first
used for kidney stones, as a method to disintegrate them (1). At the beginning of the 1990s, it was
used as a non-invasive procedure to successfully treat calcific
tendinitis. From then on, further tendon disorders were effectively
addressed, with subsequent research work on their biological and
clinical effects.
Basically, there are two types of ESWT: Focused and
radial shockwave therapy. Focused ESWT is extensively used in
clinical practice; it comprises high-energy pressure pulses that
converge to a focal point, where maximal pressure is reached. They
have an initial high positive pressure wave (up to 80 MPa) with a
rapid raise time (30-120 nsec), followed by a negative wave (5-10
MPa). The pulse duration is short, 5 µsec (2). Wave energy is released at tissue
interfaces that have different acoustic impedances, causing
compressive and shear loads. Microscopic gas bubbles develop and
collapse in the interstitial fluid of tissues, a phenomenon called
cavitation. It produces high localized stresses, a mechanical
stimulation (3). Radial ESWT is
characterized by a diverging pressure field, with a maximal
pressure at the source (4). Certain
researchers agree that radial ESWT cannot be described as real
extracorporeal shockwaves as they lack their physical features and
proposed to name them radial pressure wave therapy, as a distinct
form of therapy (5).
Energy flux density (EFD) determines the energy flow
through an area perpendicular to the direction of wave propagation
and its units are mJ/mm2. The classification of ESWT
includes low (<0.08 mJ/mm2), medium (<0.28
mJ/mm2) and high (<0.60 mJ/mm2) EFD
(6).
Microscopically, tendons are composed of cells,
tenocytes and extracellular matrix (ECM) that contains collagen,
elastin and ground substance. Tenocytes, spindle-shaped cells, are
responsible for matrix maintenance and repair and occupy 5% of the
tissue volume. ECM contains mainly type I collagen, while type III
collagen is the next-most abundant and critical in pathologic
tendons and tendon-healing processes (7).
Tenocytes may convert mechanical stimulation into a
biochemical response, leading to the release of growth factors and
cellular adaptation (8). The
process is known as mechanotransduction and may lead to
maintenance, remodeling or degeneration of the tendon through
regulation of anabolic and catabolic genes. The mechanisms of
action remain to be fully elucidated.
The therapeutic field of ESWT is continuously
expanding, as research adds new opportunities. Post-stroke
spasticity was addressed with comparable efficacity with botulinum
toxin and both types of ESWT, with the radial form providing the
best short- and medium-term results (9).
Materials and methods
A comprehensive literature search was performed in
the online databases PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Cochrane Library
(https://www.cochrane.org/Both) using
combinations of the following key words: ‘extracorporeal shockwave
therapy’, ‘biological effect’ and ‘tendon’. through. The search
included papers available as an abstract since 1988 up to December
2021. The inclusion criteria were as follows: i) Studies on human
or animal tendons, either in vivo or in vitro, with
ESWT application; and ii) full-text available at the authors’
institution. Studies with quantitative assessment of pain and
function were excluded.
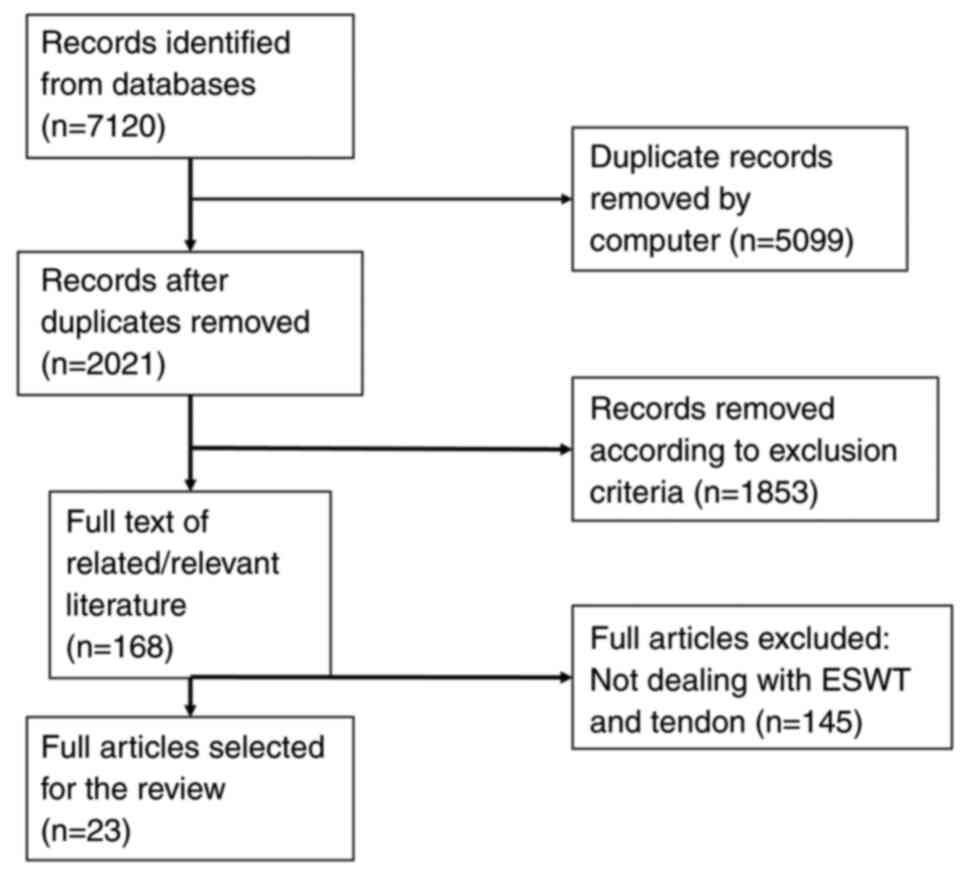
A total of two authors (DP, DC) independently
identified 7,120 titles that were manually checked to exclude
reviews and clinical trials and to retain only in vivo or
in vitro studies on human and animal tendon tissue. After
exclusion of duplicates, the full-text of 23 records was examined
(Fig. 1) and the following data
were extracted and entered in an Excel document: i) First author
name and year; ii) type of tendon, human or type of animal, in
vivo or in vitro; iii) ESWT doses; and iv) moments of
the analysis. Further data on outcomes were extracted: v)
Definition of the optimal dose; vi) neoangiogenesis; vii)
histopathologic changes; viii) biochemical changes; and ix)
mechanical properties.
Data regarding the optimal dose were analyzed in an
attempt to obtain information regarding the following points:
Optimal dose for achieving the maximum biologic effect, possible
consequences of failing to apply the optimal dose and factors that
influence the optimal dose. Furthermore, the investigation was
focused on biological effects of ESWT on angiogenesis, cellularity,
ECM and biomechanical properties of the tendons.
Results and Discussion
Studies retrieved
A summary of the studies (n=23) and their key
features/findings is provided in Table
I. According to the subjects, studies in the literature were
able to be classified as in vivo or in vitro studies.
In vivo studies had been performed on rats [6 papers
(10-15)],
rabbits [5 papers (6,16-19)],
horses [one paper (20)], ponies [2
papers (21,22)] and dogs [one paper (23)], an on normal tendons [9 papers
(6,12,14,16,17,19,21-23)]
and diseased tendons [6 papers (10,11,13,18,20,24)].
The experimental model for diseased tendons was either a ruptured
tendon, in the form of a complete and sutured rupture or a partial
rupture [2 papers (10,13)] or a collagenase-induced tendinopathy
[4 papers (11,15,18,20)].
Two studies were performed on human tissue, namely one on human
Achilles tendon, using microdialysis of the peritendinous
environment, and one on rotator cuff tendinopathy with surgical
cure. In general, one limb was subjected to ESWT application and
the contralateral limb was used as a control.
 | Table IStudies on biological effects of ESWT
on human and animal tendons. |
Table I
Studies on biological effects of ESWT
on human and animal tendons.
| Author, year | Studied
tissue/organism | ESWT doses,
sessions | Study
time-points | Optimal dose |
Neoangiogenesis | Cellularity | ECM | Mechanical
properties | (Refs.) |
|---|
| Rompe, 1998 | 42 rabbits, normal
Achilles tendon, in vivo | One session, 1,000
pulses, 0.08, 0.28 and 0.60 mJ/mm2 | 1, 7, 14 and 28
days after | 0.08 and 0.28
mJ/mm2 caused no damage; with 0.60 mJ/mm2,
fibrinoid necrosis, inflammation and reparative peritendinous
reactions occurred | N/A | N/A | N/A | N/A | (6) |
| Orhan, 2001 | 48 rats, cut and
sutured Achilles tendon, in vivo | One session 500
shocks, 0.12 mJ/mm2 | 2 and 3 weeks | N/A | N/A | Week 2: Intense
inflammatory reaction;week 3: Improved, organized healing | Increased
hydroxyproline formation (days 3 and 9) | N/A | (10) |
| Wang, 2002 | 8 dogs, normal
Achilles tendon-bone junction, in vivo | One session, 0.18
mJ/mm2, 1,000 pulses | Before treatment
and at 4 and 8 weeks after | N/A | New capillaries at
4 weeks (17-fold increase) and at 8 weeks (16-fold); muscularized
vessels at 4 and 8 weeks | N/A | N/A | N/A | (23) |
| Maier, 2002 | 36 rabbits, normal
quadriceps tendon, in vivo | 1,500 pulses at
0.35, 0.5, 0.9 or 1.2 mJ/mm2 | 10 and 28 days | 0.35
mJ/mm2: No histologic alteration at 10 days 0.5 and 0.9
mJ/mm2: Minimal edema in the paratenon that disappeared
at 28 days; 1.2 mJ/mm2: Modified tendon structure at 10
days | N/A | N/A | N/A | N/A | (16) |
| Wang, 2003 | 50 rabbits, normal
Achilles tendon, in vivo | One session 500
shocks, 0.12 mJ/mm2 | 24 h; 1, 4, 8 and
12 weeks | N/A | Neovascularization
increased at 4 weeks, persisting at 12 weeks (eNOS, VEGF,
PCNA) | N/A | N/A | N/A | (17) |
| Chen, 2004 | 48 rats,
collagenase-induced Achilles tendinosis, in vivo | One session, 200
shocks, 0.16 mJ/mm2 | 1, 2, 4, 6 and 12
weeks after session | N/A | N/A | Tenocyte
proliferation (PCNA); tenocyte stimulation (TGF-β1, IGF-1) | N/A | Restauration of
mechanical load-to-failure and stiffness of healing tendons | (11) |
| Hsu, 2004 | 18 rabbits,
Collagenase-induced patellar tendinopathy, in vivo | Two weekly
sessions, 1,500 pulses, 0.29 mJ/mm2 | 4 and 16 weeks
after treatment | N/A | 4 weeks: New
capillaries in the peritendinous tissue, larger blood vessels,
differentiated arterioles and venules | 4 weeks: Tenocyte
stimulation; 16 weeks: Increased healing process | Higher HP levels at
weeks 4 and 16; higher pyridinoline levels (up to 10 times at 4
weeks) | 4 and 16 weeks:
Higher ultimate tensile load than control that increased from 4 to
16 weeks | (18) |
| Orhan, 2004 | 32 rats, normal
Achilles tendon, in vivo | One session of
1,000 pulses at 0.15 mJ/mm2, 1,500 pulses at 0.15
mJ/mm2, 2,000 pulses at 0.20 mJ/mm2 | 3 weeks | 0.15
mJ/mm2, at 1,000 and 1,500 pulses, no alteration of
structure 0.20 mJ/mm2 and 2,000 pulses: Alteration of
tendon structure | N/A | N/A | N/A | N/A | (12) |
| Orhan, 2004 | 48 rats, ruptured
Achilles tendon, in vivo | One session 500
pulses, 0.19 mJ/mm2 | 20 days | N/A | Increased number of
capillaries | N/A | Absent or minimal
adhesions that did not distort the configuration of the tendon | The mean force
required to rupture the tendon was higher | (13) |
| Kersh, 2006 | 6 horses,
collagenase-induced tendinosis of superficial digital flexor
tendon, in vivo | 3 sessions, 1,500
shocks, 0.14 mJ/mm2 (3 weeks interval between
sessions) | 5 weeks after
completion | N/A | Neovascularization
(significantly more capillaries); increased metachromasia within
the intima and subintima of larger arteries | Increased number of
fibroblasts (not significant); increase in the number of tenocyte
nuclei; tenocytes were actively producing ECM | N/A | N/A | (20) |
| Bosch, 2007 | 6 ponies, normal
superficial flexor tendon; normal common digital extensor tendon,
in vivo | Focused session
ESWT, 600 shocks, 0.14 mJ/mm2 | After 3 h and 6
weeks | N/A | N/A | At 3 h: Higher
synthesis rate of GAG and collagen; at 6 weeks: Decreased GAG and
total collagen | N/A | N/A | (21) |
| Chao, 2008 | Rats, cultured
Achilles tendon normal cells, in vitro | One session of
focused ESWT 0.36 or 0.68 mJ/mm2 50, 100, 250 or 500
pulses | 24, 48 and 96
h | Optimal dose: 100
pulses at 0.36 mJ/mm2 that maintained cell
viability | N/A | Tenocyte
proliferation (PCNA); increased tenocyte synthesis (TGF-β1,
IGF-1) | N/A | N/A | (25) |
| Han, 2009 | Human, cultured
Achilles tendinopathy and normal FHL tendon, in vitro | One session 0.17
mJ/mm2, 250, 500, 1,000 or 2,000 pulses | 72 h | Optimal dose for
cell viability: 500 pulses; optimal dose for cell proliferation:
500 pulses | N/A | N/A | Normal tenocytes:
Increase of IL-1; diseased tenocytes: Decrease of MMP-1, MMP-13 and
IL-6 | N/A | (28) |
| Bosch, 2009 | 6 ponies, normal
superficial digital flexor tendon; normal common digital extensor
tendon, in vivo | Focused session
ESWT, 600 shocks, 0.14 mJ/mm2 | After 3 h and 6
weeks | N/A | N/A | 3 h: Tenocyte
activation, structural disorganization | 3 h: Increased
collagen cleavage; 6 weeks: No collagen damage | N/A | (22) |
| Zhang, 2011 | 12 Rats, normal
knee tendon, in vivo | 2 different doses:
3,000 pulses of 0.15 or 0.4 mJ/mm2 | 4 days | N/A | N/A | N/A | Increased lubricin
in tendons, dose-dependent | N/A | (14) |
| Vetrano, 2011 | Human, cultured
normal semitendinosus cells, in vitro | One session 0.08,
0.14 and 0.17 mJ/mm2, 500 and 1,000 pulses | 1, 4, 8 and 12
days | None of the
regimens affected viability of cells | N/A | None of the
regimens affected viability of cells. 1,000 pulses, 0.14
mJ/mm2 promoted tenocyte proliferation, prevented
phenotype drift | Increased collagen
synthesis, particularly type I | N/A | (26) |
| Penteado, 2011 | 30 rabbits, normal
patellar tendon, tibial insertion, in vivo | One session, 6
different regimens (0.18, 0.27 or 0.36 mJ/mm2, 1,000 and
2,000 pulses) | 6 weeks | N/A | No difference in
blood vessels | N/A | N/A | N/A | (19) |
| Leone, 2012 | Human, cultured
tendinopathic cells from Achilles tendon. Normal semitendinosus
tendon, in vitro | One session, 0.14
mJ/mm2, 1,000 pulses | 1 and 4 days | N/A | N/A | ESWT induced no
morphological variations (normal and diseased); ESWT induced
proliferation of tenocytes (normal and diseased), more in diseased;
ESWT induced cell migration in both cultures after trauma, more in
diseased (wound repair) | ESWT reduced the
increased concentrations of Col I and Scx in diseased cultures | N/A | (29) |
| Branes, 2012 | Human, 31 rotator
cuff tendinopathy (10 treated and 21 controls), in vivo | One session, 0.3
mJ/mm2, 4,000 pulses | After session | N/A | ESWT increased VVA,
nodular neo-angiogenesis for grade III in deeper layers, no
response for grade IV | N/A | N/A | N/A | (24) |
| Yoo, 2012 | 45 rats,
collagenase-induced Achilles tendinopathy, in vivo | 4 sessions, 1,000
pulses, 0.085 mJ/mm2, days 5, 8, 12 and 15 | Days 7, 12, 19, 26
and 33 after baseline | N/A | Day 33: No
inflammatory cells or fibrotic tissue | Increase in
fibrillary diameter and fibrillary adhesion force | N/A | N/A | (15) |
| de Girolamo,
2014 | Human, cultured
normal tendons, in vitro | One session, 1,000
pulses, 0.17 mJ/mm2 | Days 1, 2, 4 and 7
after treatment | Increased cell
viability | N/A | N/A | Increased
expression of Scx and type I collagen, increased IL-β1, IL-6 and
IL-10 | N/A | (27) |
| Waugh, 2015 | Human, normal
(n=19) and tendinopathic (n=10) tendons, in vivo | One session, 2,500
pulses, 0.064 mJ/mm2 | 1, 2, 3 and 4
h | N/A | N/A | Elevated levels of
IL-6 and IL-8 post ESWT remained higher at 4 h; elevated levels of
pro-MMP-9, without active form increase | N/A | N/A | (34) |
| Leone, 2016 | Human, cultured
normal semitendinosus and ruptured Achilles, in vitro | One session, 1,000
pulses, 0.14 mJ/mm2 | 21 days | N/A | Increased tenocyte
proliferation, migration and collagen synthesis, more intense in
ruptured tendons | N/A | N/A | N/A | (33) |
In vitro studies had been performed on
cultured tendon cells from either normal or diseased tendons. A
total of three papers reported results on cultured normal tendon
cells and three papers compared cultured normal cells with
tendinopathic cells. It is noteworthy that in vitro studies
were not able to be directly translated to in vivo
conditions, although they shed light on important mechanisms of
action.
Finding the optimal dose. Information was
structured according to whether the experimental model was in
vivo or in vitro, and the focus was on cell viability
and ECM alterations.
In vivo, for normal rabbit Achilles tendon,
Rompe et al (6) determined
three main EFD levels with different effects. Doses of up to 0.28
mJ/mm2 did not damage the tendon and adjacent
structures, or the alterations were transitory and reversible
within four weeks. However, a dose of 0.60 mJ/mm2
produced marked damage to the tendon and paratenon, with fibrinoid
necrosis within the tendon and inflammatory and reparative
peritendinous reactions that were still present at 4 weeks
(6). In the normal rabbit
quadriceps tendon, a lower EFD (0.35 mJ/mm2) left no
structural alteration at 10 days, a medium EFD (0.5 and 0.9
mJ/mm2) produced edema in the paratenon at 10 days that
resolved at 28 days, and a higher EFD (1.2 mJ/mm2)
induced marked modifications in cells and ECM at 10 days.
Researchers opined that the quadriceps tendon is more ‘resistant’
to ESWT than the Achilles tendon (16). In rats, normal Achilles tendon
exposed to 0.15 mJ/mm2 and 1,000 or 1,500 pulses
exhibited no structural alteration at 3 weeks. A higher dose of
0.20 mJ/mm2 and 2,000 pulses produced collagen
disorganization, inflammatory mononuclear reaction and an increased
number of capillaries (12).
In vitro, in normal Achilles rat tenocyte
cultures, doses of 0.36 mJ/mm2 and 50, 100 and 250
pulses did not alter normal cell viability, whereas the same EFD
with a higher number of pulses and an EFD of 0.68
mJ/mm2, irrespective of the number of pulses, reduced
the proportion of viable cells. The cell viability suppression was
found to be dose-dependent (25).
Normal human tendon cell cultures exhibited no modification when
exposed to 0.08, 0.14 and 0.17 mJ/mm2, either at 500 or
1,000 pulses. Furthermore, certain energy levels were found to
augment cell viability: 0.14 mJ/mm2 and 1,000 pulses was
found to increase the number of viable cells at 24 and 48 h
(26), and a dose of 0.17
mJ/mm2 and 1,000 pulses promoted cell viability at 7 and
10 days after treatment, as measured by the increased content of
DNA (27). For both tendinopathic
and normal human cell cultures, 0.17 mJ/mm2 and 250 or
500 pulses did not alter cell viability, whereas a higher number of
pulses (1,000 and 2,000) led to cell death. Furthermore, a regimen
with 500 pulses was indicated to promote cell proliferation
(28). The variation among the
reported doses may be explained by the variability of the model on
which they were applied, as cultures were harvested from different
human tendons.
As for the ECM, in an in vivo study on normal
ponies' tendons, 0.14 mJ/mm2 and 600 pulses produced a
rapid stimulation of matrix turnover (3 h) and an increase of
enzymatic cleavage of collagen. In the long term (6 weeks), damaged
collagen was decreased, presumably replaced by mature collagen
(22).
Corroborating the results of the published papers,
it may be concluded that there is a variability of optimal doses
between in vivo and in vitro studies, among different
tendons in the same species and among the same tendons in various
species. There is a clear dose-related effect of ESWT, both in
terms of cell viability and ECM.
Neovascularization. It is widely accepted
that neovascularization is important for healing, as it removes
by-products and increases the oxygen content and the number of
inflammatory and reparatory cells.
In vivo, in normal rabbit tendons,
significantly increased neo-vessels were observed at 4 weeks after
one session of 0.12 mJ/mm2, 500 pulses, which persisted
up to 12 weeks. Angiogenesis-related markers [endothelial nitric
oxide synthase (eNOS), vascular endothelial growth factor (VEGF)
and proliferating cell nuclear antigen (PCNA)] were significantly
increased at one week. eNOS and VEGF displayed higher levels up to
8 weeks and decreased up to 12 weeks, whereas PCNA continued to
have increased levels at 12 weeks (17). In normal dog Achilles tendon-bone
junction, application of one session of 0.18 mJ/mm2,
1,000 pulses, was followed by an increase of new capillaries at 4
weeks (17-fold) and 8 weeks (16-fold), most of them located
adjacent to the peritendineum. New capillaries were also noted in
the adjacent tendon tissues. Muscularized vessels, in a
proportionate number to capillaries, were found at 4 weeks and
persisted at 8 weeks, mostly in the peritendineum and the adjacent
tendon. Both capillaries and muscularized vessels are indicative of
neovascularization. No alteration of normal cancellous bone
architecture was reported (23). By
contrast, a study on normal insertional patellar tendon (rabbit)
indicated no alteration in the number of capillaries at 6 weeks
after one session of different ESWT regimens (1,000 or 2,000
pulses, 0.18, 0.27 or 0.36 mJ/mm2) (19).
Achilles tendinopathy (rabbit) displayed new
capillaries in the peritendinous tissue together with larger blood
vessels, including differentiated arterioles and venules, at 4
weeks after 2-weekly sessions of 0.29 mJ/mm2, 1,500
pulses (18). A similar result was
reported for the tendinosis of superficial digital flexor tendon
(horse) after 3 ESWT sessions, each one 3 weeks apart, with 0.14
mJ/mm2, 1,500 pulses. At five weeks after treatment
completion, there were significantly more capillaries in the
treated tendons (20). In the study
model of a cut Achillean tendon (rat), one session of 500 pulses at
0.19 mJ/mm2 produced a significant increase in the
number of capillaries after 20 days (13). In humans with grade III rotator cuff
tendinopathy (Riley classification), one session of 4,000 pulses at
0.3 mJ/mm2 increased the vascular volume area with
evidence of nodular neo-angiogenesis in deeper tissue layers. For
grade IV tendinopathy, evidence of new vessel formation was not
significant, mostly due to the small number of cases (24).
In normal human cell cultures, ESWT induced a strong
and significant release of TGF-β and VEGF, with a maximum at day 2
and persistence of higher levels at day 7. VEGF is stimulated by
the release of certain cytokines (IL-6 and IL-10) and is an
important promotor of neo-angiogenesis, contributing to the healing
process (27).
Cellularity. In the regeneration process, the
cornerstone becomes the activated tenocyte that behaves like a
modified form of fibroblast or fibrocyte.
In the in vivo tendinopathic model, ESWT
induced acceleration of the healing process, increasing the number
of blast-like forms at 4 weeks. At 16 weeks, the healing process
was complete, with mature tenocytes in a parallel array (18). Increased expression of PCNA, a
protein associated with the S-phase of a dividing cell and a marker
of tenocyte proliferation, was documented up to 12 weeks. The
presence of immature forms, blast-like, recruited from the
peritendon into the lesion, was evident at one week; at 4 weeks,
the mature forms (spindle-shaped tenocytes) were arranged into
tendon bundles (11).
Tenocyte metabolism is accelerated with intense
production of growth factors (TGF-β1 and IGF-1) involved in ECM
biosynthesis. Tenocytes displayed an increased number of nuclei and
were actively producing ECM (20).
Within 6 weeks after ESWT application, TGF-β1 and IGF-1 were
significantly increased compared to the control, contributing to
tendon healing (11).
In vitro, in normal cell cultures (rats),
ESWT at an optimal dose promoted tenocyte proliferation as
indicated by upregulation of PCNA as early as 6 h. At 48 h, there
was no more stimulatory effect. At 24 h, there was increased
expression of TGF-β1 as a mark of increased ECM production and
increased production of NO by fibroblasts. NO production was
increased at 24 h by certain values of EFD (50 and 100 pulses and
0.36 mJ/mm2), while a higher number of pulses (250 and
500) did not affect NO production (12). Normal human cell cultures did not
display any alteration of NO levels after ESWT. Thus, the role of
NO in promoting tendon healing remains to be fully elucidated
(27).
One session of ESWT increased the proliferation of
either healthy or ruptured tendon-derived human cultured cells,
with a more prominent effect on the ruptured tendon-derived cells
(a ratio of 1.75). ESWT induced a migratory phenotype in both types
of culture, particularly evident for cells derived from ruptured
tendons, triggering cell mobility (29).
Tissue regeneration requires maintenance of the cell
phenotype. Phenotyping drift, a natural tendency in cultured rabbit
and avian tenocytes, has also been demonstrated in human Achilles
tendon cultures, as a tendency to shift from an elongated shape
toward an ovoid shape. The phenomenon may be responsible for
altered protein synthesis in vivo, increasing the ratio of
type III:I collagen and inducing tendon pathologies (30). In normal human tendon cultures, one
session of ESWT was proved to prevent the phenotyping drift,
maintaining the normal tenocyte secretory profile of type I
collagen (26).
The cellular compartment of the human tendon
includes, besides tenocytes, a population of stem cells, human
tendon-derived stem/progenitor cells (hTSPCs), characterized by
several phenotypes and a potential for multilineage differentiation
(osteoblasts, adipocytes, chondrocytes, tenocytes) (31,32).
In cultures of both human normal and diseased tendon cells, ESWT
was able to enhance hTSPC differentiation toward a tenocytic-like
lineage, visible at day 21(33).
ECM. On normal tendons (ponies), ESWT
application was followed by a rapid increase of degraded collagen
at 3 h and a decrease at 6 weeks. The levels of glycosaminoglycans
(GAG) at 3 h were unchanged and decreased at 6 weeks. However, no
changes in hyaluronic acid, DNA and total collagen levels were
noted either at 3 h or at 6 weeks. The results pointed to an
anabolic response. The degraded collagen increase at 3 h may
reflect an accelerated collagen turnover (slightly improbable for
such a short duration) or direct physical damage. Overall, the
anabolic response decreased at 6 weeks (21). On normal cultured human cells, ESWT
increased the collagen content, predominantly type I (26).
Tendon regeneration is based on collagen synthesis,
initially in the form of type III, followed by a maturation process
and transformation in type I. Type I collagen appears like well
organized, banded fibrils and provides high tensile strength. Type
III collagen takes a woven pattern. Hydroxyproline is an aminoacid
component of collagens accounting for ~13% and represents a
reliable index for newly formed collagen. Pyridinoline is a
crosslink residue of collagen and reflects its rate of
degradation.
In the cut and sutured tendon model (rats), ESWT
increased the hydroxyproline content, i.e., collagen synthesis,
from 2- to 3-fold at days 3 and 9 compared to the control (10). The healing process under ESWT was
followed by minimal or absent adhesions that did not disturb the
configuration of the tendon.
In the model of collagenase-induced tendinopathy
(rats), the optimal dose of ESWT reversed the increased
concentrations of DNA, GAG and hydroxyproline to normal. Fiber
bundles displayed a regular arrangement at 12 weeks. Higher doses
elicited inhibitory effects on biochemical characteristics and an
irregular array of fiber bundles (11). In the same model of tendinopathy
(rabbits), the ESWT-treated tendons displayed higher levels of
hydroxyproline and pyridinoline at 4 and 16 weeks, as an indication
of intensive collagen synthesis and maturation (18).
At the nanostructural level, ESWT increased the
collagen fibril diameter and the fibrillary adhesion force,
accelerating healing (15).
In vitro, normal human cultured tissue
displayed increased expression of scleraxis at 24 h, which
decreased later, and an increased expression of type I collagen at
day 4 that returned to normal levels at day 7(27). Human tendinopathic cultures were
characterized by higher gene expression of type I collagen and
scleraxis, a possible indication of the repair process; ESWT
induced a reduction of their levels toward normal. The process may
be interpreted as an impairment effect of ESWT on cell
differentiation in favor of cell proliferation and migration to
ensure regeneration (29).
Metalloproteases and cytokines. Cultures from
normal tendon subjected to one session of ESWT exhibited increased
levels of specific interleukins (IL-β1, IL-6 and IL-10), with a
maximum at day one for IL-β1 and IL-6 and at day 2 for IL-10, and
persistence of higher values at day 7. In this context, IL-β1
induced IL-6 release, which, in turn, promoted IL-10 increase, an
anti-inflammatory cytokine to limit the inflammation process
(27).
It was postulated that excreted MMPs and cytokines,
particularly interleukins, from diseased tenocytes are breaking the
ECM and induce tendon damage. Decreasing levels of these substances
may reflect healing. Increased excretion of IL-6 and MMP-1, MMP-2
and MMP-13 was found in diseased cultured tenocytes; ESWT
application reduced the higher levels of MMP-1, MMP-13 and IL-6 at
72 h, possibly contributing to a metabolic normalization. In normal
cultured human tenocytes, only IL-1 levels were increased after
ESWT, an event that necessitates further research (28).
Analysis of the microdialysate from the
peritendinous space of human normal and tendinopathic patellar and
Achilles tendons revealed that tendinopathy was associated with
high levels of IL-6 and IL-8. ESWT application was followed by an
increase of these two interleukins, in both normal and diseased
tendons within the interval from 1 to 4 h. IL-β1 and IL-2
variations were not significant pre- and post-treatment. Pro-MMP-9,
a latent form, increased after ESWT, without any change in the
active form levels. The lack of significance of MMP activity
changes raised the possibility of grouping individuals as
responders and non-responders, with a proportion of responders of
60% in the general population (defined as exhibiting a minimum
5-fold increase in any MMP level at any point post-ESWT) (34).
The large spectrum of results of the two papers on
IL and MMP levels deserves further investigation.
Lubricin production. Lubricin, a lubricating
molecule, found in diarthrodial joints, is produced also by
tenocytes enhancing the gliding of the tendons. ESWT increased
lubricin expression and production in a dose-dependent manner in
normal rat knee tendons and the effect was visible in the short
term (4 days) (14).
Biomechanical properties of tissue after ESWT
application. In the model of collagenase-induced tendinopathy,
ESWT was followed by restoration of mechanical load-to-failure and
stiffness of healing tendons, with a higher ultimate tensile load
that increased from 4 to 16 weeks (11,18).
The mean force required to rupture the tendon was higher 20 days
after one session of ESWT (13).
ESWT, a method derived from urinary lithotripsy,
represents a new therapeutic approach for tendon pathology, with
encouraging results on pain and function. The biological mechanisms
that sustain its clinical results have been extensively studied
both in vitro and in vivo.
Research from the last 20 years indicated that there
is a certain optimal dose for the maximum therapeutic effect for
each studied species and for each studied tendon. Tendon- and
species-specificity is a trait of the therapy. Energies that exceed
the optimal value reduce cell viability and disorganize the ECM in
a dose-dependent manner, with deleterious consequences on tendon
structure.
Scientific papers agreed on several structural
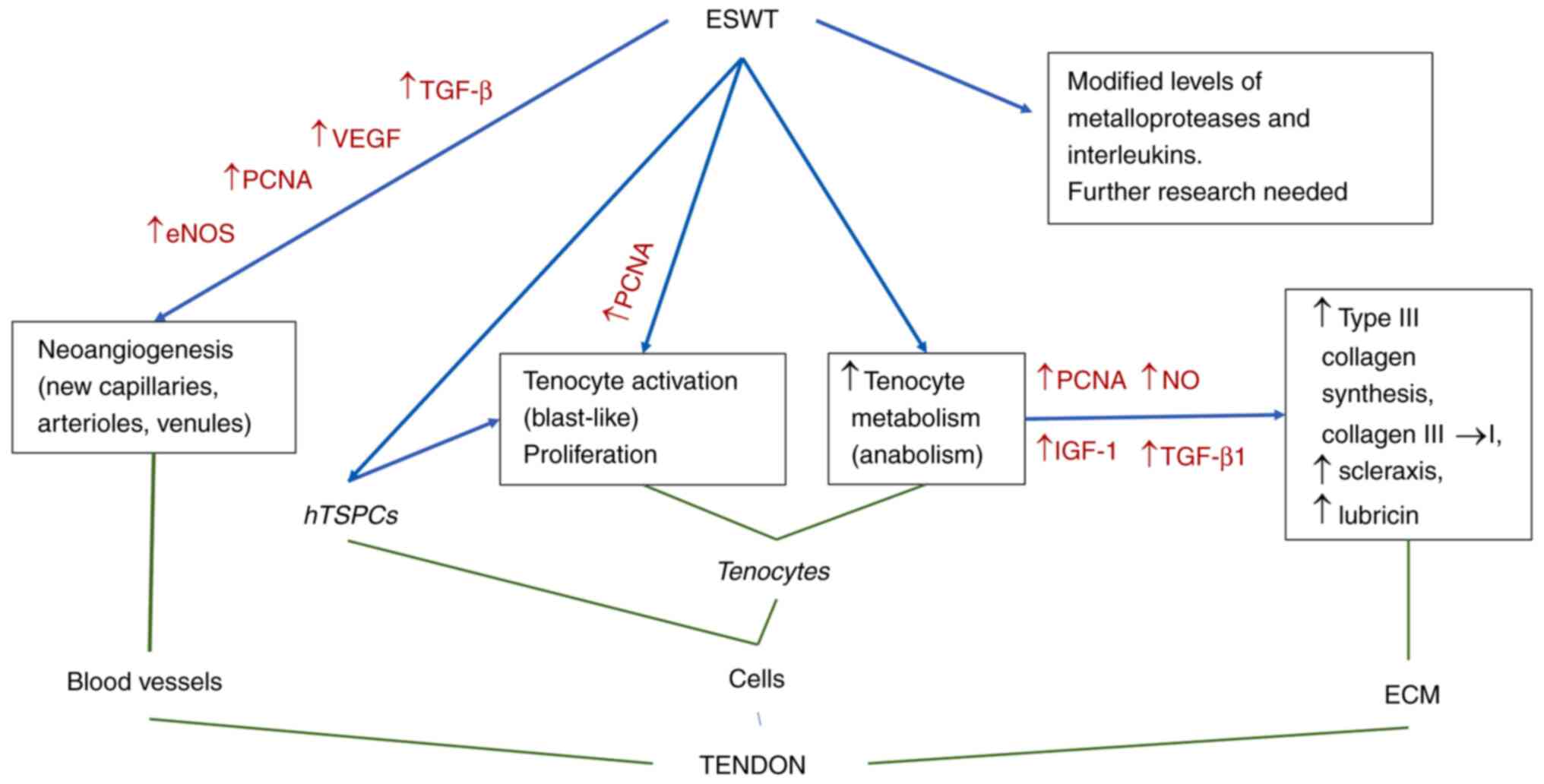
alterations that promote tendon healing. ESWT induces
neovascularization in the tendinopathic tissue, with extensive
capillary formation from the peritendinous structures. The
inflammatory reaction that followed the collagenase-induced
tendinopathy was accelerated by ESWT.
The therapy acts on both cellular and extracellular
compartments of the tendon structure. Tenocyte proliferation and
activation as blast-like forms with enhanced protein synthesis
together with accelerated mobility reconstruct the normal
structure. Accelerated collagen turnover, with type III collagen
synthesis and subsequent type I collagen maturation, was documented
in the ECM. As a result, biomechanical properties of the treated
tendons improved significantly in comparison with non-treated
tendinopathic structures. The mechanisms are outlined in Fig. 2.
An important clinical effect on plantar fasciitis, a
structure that may be assimilated to a tendon, is worth mentioning.
Biological studies on plantar fascia are currently lacking; the
analgesic effect of ESWT is comparable to that of autologous
blood-derived products in the medium-term (6 months). Among the
forms of ESWT, the success rate is higher with medium- and
high-intensity ESWT (35,36).
Future research will focus on the role of cytokines
and metalloproteases that mediate, at a cellular level, the
degenerative process and its evolution under ESWT application.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
Conceptualization: DP; methodology: DP and DC;
resources: MIS and DC; writing and draft preparation: DP, MIS and
DC. All authors have read and agreed to the published version of
the manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Notarnicola A and Moretti B: The
biological effects of extracorporeal shock wave therapy (eswt) on
tendon tissue. Muscles Ligaments Tendons J. 2:33–37.
2012.PubMed/NCBI
|
|
2
|
Sturtevant B: Shock wave physics of
lithotriptors. In: Smith's Textbook of Endourology. Smith A,
Badlani GH, Bagley DH et al (eds): Quality Medical
Publishing Inc, St Louis, 529-552,1996.
|
|
3
|
Brümmer F..Bräuner T and Hülser DF:
Biological effects of shock waves. World J Urol. 8:224–232.
1990.
|
|
4
|
Rosso F, Bonasia DE, Marmotti A, Cottino U
and Rossi R: Mechanical STIMULATION (Pulsed Electromagnetic Fields
‘PEMF’ and Extracorporeal Shock Wave Therapy ‘ESWT’) and tendon
regeneration: A possible alternative. Front Aging Neurosci.
7(211)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
van der Worp H, van den Akker-Scheek I,
van Schie H and Zwerver J: ESWT for tendinopathy: Technology and
clinical implications. Knee Surg Sports Traumatol Arthrosc.
21:1451–1458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rompe JD, Kirkpatrick CJ, Küllmer K,
Schwitalle M and Krischek O: Dose-related effects of shock waves on
rabbit tendo Achillis. A sonographic and histological study. J Bone
Joint Surg Br. 80:546–552. 1998.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lipman K, Wang C, Ting K, Soo C and Zheng
Z: Tendinopathy: Injury, repair, and current exploration. Drug Des
Devel Ther. 12:591–603. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Skutek M, van Griensven M, Zeichen J,
Brauer N and Bosch U: Cyclic mechanical stretching modulates
secretion pattern of growth factors in human tendon fibroblasts.
Eur J Appl Physiol. 86:48–52. 2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hsu PC, Chang KV, Chiu YH, Wu WT and
Özçakar L: Comparative effectiveness of botulinum toxin injections
and extracorporeal shockwave therapy for post-stroke spasticity: A
systematic review and network meta-analysis. EClinicalMedicine.
43(101222)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Orhan Z, Alper M, Akman Y, Yavuz O and
Yalçiner A: An experimental study on the application of
extracorporeal shock waves in the treatment of tendon injuries:
Preliminary report. J Orthop Sci. 6:566–570. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen YJ, Wang CJ, Yang KD, Kuo YR, Huang
HC, Huang YT, Sun YC and Wang FS: Extracorporeal shock waves
promote healing of collagenase-induced Achilles tendinitis and
increase TGF-beta1 and IGF-I expression. J Orthop Res. 22:854–861.
2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Orhan Z, Cam K, Alper M and Ozturan K: The
effects of extracorporeal shock waves on the rat Achilles tendon:
Is there a critical dose for tissue injury? Arch Orthop Trauma
Surg. 124:631–635. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Orhan Z, Ozturan K, Guven A and Cam K: The
effect of extracorporeal shock waves on a rat model of injury to
tendo Achillis. A histological and biomechanical study. J Bone
Joint Surg Br. 86:613–618. 2004.PubMed/NCBI
|
|
14
|
Zhang D, Kearney CJ, Cheriyan T, Schmid TM
and Spector M: Extracorporeal shockwave-induced expression of
lubricin in tendons and septa. Cell Tissue Res. 346:255–262.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yoo SD, Choi S, Lee GJ, Chon J, Jeong YS,
Park HK and Kim HS: Effects of extracorporeal shockwave therapy on
nanostructural and biomechanical responses in the
collagenase-induced Achilles tendinitis animal model. Lasers Med
Sci. 27:1195–1204. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Maier M, Tischer T, Milz S, Weiler C,
Nerlich A, Pellengahr C, Schmitz C and Refior HJ: Dose-related
effects of extracorporeal shock waves on rabbit quadriceps tendon
integrity. Arch Orthop Trauma Surg. 122:436–441. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang CJ, Wang FS, Yang KD, Weng LH, Hsu
CC, Huang CS and Yang LC: Shock wave therapy induces
neovascularization at the tendon-bone junction. A study in rabbits.
J Orthop Res. 21:984–989. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hsu RW, Hsu WH, Tai CL and Lee KF: Effect
of shock-wave therapy on patellar tendinopathy in a rabbit model. J
Orthop Res. 22:221–227. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Penteado FT, Faloppa F, Giusti G, Moraes
VY, Belloti JC and Santos JB: High-energy extracorporeal shockwave
therapy in a patellar tendon animal model: A vascularization
focused study. Clinics (Sao Paulo). 66:1611–1614. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kersh KD, McClure SR, Van Sickle D and
Evans RB: The evaluation of extracorporeal shock wave therapy on
collagenase induced superficial digital flexor tendonitis. Vet Comp
Orthop Traumatol. 19:99–105. 2006.PubMed/NCBI
|
|
21
|
Bosch G, Lin YL, van Schie HT, van De Lest
CH, Barneveld A and van Weeren PR: Effect of extracorporeal shock
wave therapy on the biochemical composition and metabolic activity
of tenocytes in normal tendinous structures in ponies. Equine Vet
J. 39:226–231. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bosch G, de Mos M, van Binsbergen R, van
Schie HT, van de Lest CH and van Weeren PR: The effect of focused
extracorporeal shock wave therapy on collagen matrix and gene
expression in normal tendons and ligaments. Equine Vet J.
41:335–341. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang CJ, Huang HY and Pai CH: Shock
wave-enhanced neovascularization at the tendon-bone junction: An
experiment in dogs. J Foot Ankle Surg. 41:16–22. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
BrañEs J, Contreras HR, Cabello P, Antonic
V, Guiloff LJ and Brañes M: Shoulder rotator cuff responses to
extracorporeal shockwave therapy: Morphological and
immunohistochemical analysis. Shoulder Elbow. 4:163–168. 2012.
|
|
25
|
Chao YH, Tsuang YH, Sun JS, Chen LT,
Chiang YF, Wang CC and Chen MH: Effects of shock waves on tenocyte
proliferation and extracellular matrix metabolism. Ultrasound Med
Biol. 34:841–852. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vetrano M, d'Alessandro F, Torrisi MR,
Ferretti A, Vulpiani MC and Visco V: Extracorporeal shock wave
therapy promotes cell proliferation and collagen synthesis of
primary cultured human tenocytes. Knee Surg Sports Traumatol
Arthrosc. 19:2159–2168. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
de Girolamo L, Stanco D, Galliera E,
Viganò M, Lovati AB, Marazzi MG, Romeo P and Sansone V:
Soft-focused extracorporeal shock waves increase the expression of
tendon-specific markers and the release of anti-inflammatory
cytokines in an adherent culture model of primary human tendon
cells. Ultrasound Med Biol. 40:1204–1215. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Han SH, Lee JW, Guyton GP, Parks BG,
Courneya JP and Schon LC: J.Leonard Goldner award 2008. Effect of
extracorporeal shock wave therapy on cultured tenocytes. Foot Ankle
Int. 30:93–98. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Leone L, Vetrano M, Ranieri D, Raffa S,
Vulpiani MC, Ferretti A, Torrisi MR and Visco V: Extracorporeal
shock wave treatment (ESWT) improves in vitro functional
activities of ruptured human tendon-derived tenocytes. PLoS One.
7(e49759)2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yao L, Bestwick CS, Bestwick LA, Maffulli
N and Aspden RM: Phenotypic drift in human tenocyte culture. Tissue
Eng. 12:1843–1849. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lui PP and Chan KM: Tendon-derived stem
cells (TDSCs): From basic science to potential roles in tendon
pathology and tissue engineering applications. Stem Cell Rev Rep.
7:883–897. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Qin S, Wang W, Liu Z, Hua X, Fu S, Dong F,
Li A, Liu Z, Wang P, Dai L, et al: Fibrochondrogenic
differentiation potential of tendon-derived stem/progenitor cells
from human patellar tendon. J Orthop Translat. 22:101–108.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Leone L, Raffa S, Vetrano M, Ranieri D,
Malisan F, Scrofani C, Vulpiani MC, Ferretti A, Torrisi MR and
Visco V: Extracorporeal shock wave treatment (ESWT) enhances the
in vitro-induced differentiation of human tendon-derived
stem/progenitor cells (hTSPCs). Oncotarget. 7:6410–6423.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Waugh CM, Morrissey D, Jones E, Riley GP,
Langberg H and Screen HR: In vivo biological response to
extracorporeal shockwave therapy in human tendinopathy. Eur Cell
Mater. 29:268–280. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hsiao MY, Hung CY, Chang KV, Chien KL, Tu
YK and Wang TG: Comparative effectiveness of autologous
blood-derived products, shock-wave therapy and corticosteroids for
treatment of plantar fasciitis: A network meta-analysis.
Rheumatology (Oxford). 54:1735–1743. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chang KV, Chen SY, Chen WS, Tu YK and
Chien KL: Comparative effectiveness of focused shock wave therapy
of different intensity levels and radial shock wave therapy for
treating plantar fasciitis: A systematic review and network
meta-analysis. Arch Phys Med Rehabil. 93:1259–1268. 2012.PubMed/NCBI View Article : Google Scholar
|