Introduction
A non-reversible imbalance in myocardial blood
supply and demand results in myocardial ischemia in patients with
coronary artery disease (CAD), which can further lead to heart
failure and myocardial infarction. CAD is currently a major cause
of death worldwide, with 31% of all deaths resulting from it
(1). During invasive coronary
radiography, fractional flow reserve (FFR) is measured, and this is
used as a gold standard for determining myocardial ischemia caused
by coronary stenosis. However, both its invasive nature and the
risk of complications serve to limit its clinical applicability
(2). Coronary computed tomography
(CT) angiography (CCTA) is recognized as the most accurate means
for excluding CAD (3).
Nevertheless, a purely anatomical assessment of hemodynamics is
hardly able to provide sufficient guidance for clinical
treatment.
Currently, the application of non-invasive
techniques to assess myocardial ischemia due to abnormal coronary
hemodynamics reduces the occurrence of adverse cardiac events. FFR
obtained by CCTA (FFRCT), based on deep machine learning
algorithms, has been shown to be an effective assessment method for
detecting ischemia, demonstrating a high diagnostic performance
compared with invasive FFR (4-6).
Additionally, when CCTA-derived plaque characteristics and
composition have been obtained using a semi-automated program, this
has been shown to improve the ability of CCTA to predict
hemodynamic abnormalities by obtaining more information about the
lesion (7,8). Previous studies have also suggested
that low-density non-calcified plaque (LD-NCP) acts as a substitute
for the necrotic lipid core, and the larger its volume, the higher
the possibility of ischemia (9,10).
However, the potential of quantitative CCTA-derived plaque combined
with FFRCT for lesion-specific ischemia detection needs
to be explored further. To meet this end, the present study aimed
to evaluate the predictive performance of deep learning-based
FFRCT in combination with CCTA-derived plaque
characteristics for identifying lesion-specific ischemia according
to the gold standard of invasive FFR.
Materials and methods
Study population
The present study was a retrospective study
conducted at a single center. A total of 144 patients with coronary
heart disease admitted to The First Affiliated Hospital of Hebei
North University (Zhangjiakou, China) between February 2019 and
March 2022 were included in the current study. Invasive coronary
angiography (ICA) and CCTA were both performed on all patients, and
the interval between the two examinations was ≤30 days. The
inclusion criteria were as follows: i) Complete clinical data and
CCTA images were available for the patient; and ii) these were of
sufficient quality for FFRCT and plaque analysis. The
exclusion criteria were as follows: i) Poor coronary CTA image
quality; ii) patients who had previously undergone
revascularization procedures (for example, cardiac bypass graft
and/or percutaneous coronary intervention); iii) the patient had
contraindications to adenosine, nitrates or β-blockers; and iv) the
patient had been diagnosed with a combination of severe
cardiovascular disease (for example, severe arrhythmias and/or
severe heart failure). The current study was approved by the Ethics
Committee of The First Affiliated Hospital of Hebei North
University (Zhangjiakou, China; approval no. K2020237), and written
informed consent was obtained from all of the participants.
ICA and FFR techniques
ICA and FFR were performed in accordance with
standard practices (11). The FFR
pressure-wire was placed at least 20 mm distal to the ≥2 mm vessel
stenosis after ICA. Adenosine (140-180 µg/kg/min; Pfizer, Inc.) was
used to induce hyperemia, and both the distal coronary pressure
(Pd) and the aortic pressure (Pa) were measured simultaneously at
baseline and during maximal hyperemia. Based on a beat-to-beat
calculation, the FFR was determined as the mean Pd divided by the
mean Pa at maximal hyperemia. Lesion-specific ischemia was defined
upon calculating a FFR value of ≤0.80.
CCTA acquisition
CCTA was performed using an Aquilion ONE ViSION CT
scanner (320-MDCT; Canon Medical Systems Corporation).
Nitroglycerin (0.8 mg; Xinyi Pharmaceutical Co., Ltd.) was given
sublingually to all patients prior to the CT scan, and patients
whose heart rate pre-scan was >60 beats/min were administered
metoprolol (AstraZeneca Pharmaceutical Co., Ltd.) orally (20-40
mg), with the heart rate held at ≤60 beats/min. The isotonic
contrast agent, iodixanol (320 mg iodine/ml; Jiangsu Hengrui
Medicine Co., Ltd.), was injected at a rate of 5.5 ml/sec using a
double-barrel hyperbaric syringe, followed immediately by injection
of 30 ml of 0.9% sodium chloride solution at the same rate.
Regarding the scan parameters, the tube voltage was set at 100 kV,
and the tube current was automatically modulated. Monitoring was
set in the descending aorta at the level of 1 cm below the tracheal
bifurcation. The scan was automatically triggered using SUREStart™
software (version 1.0; Canon Medical Systems Corporation) when 200
Hounsfield units (HU) were reached, ranging from below the tracheal
ridge to the diaphragm surface, and the clearest coronary image was
selected for reconstruction. The CCTA images were analyzed and
processed by two physicians with professional diagnostic imaging
qualifications in the Department of Medical Imaging, The First
Affiliated Hospital of Hebei North University. The two physicians
were blinded to the clinical information and CCTA results of the
patients, and differences in the assessment results were
re-evaluated by a third experienced physician, before subsequently
being discussed to obtain the final results. The branches of the
stenotic coronary arteries [i.e., the left anterior descending
artery (LAD), the left circumflex artery (LCX) and the right
coronary artery (RCA)] were observed.
Coronary plaque analysis
The scan-specific algorithm, Vitrea FX version 4.0
(Vital Images; Canon Medical Systems Corporation), was used to
assess the plaque characteristics for each coronary lesion segment
≥2 mm. By using this automated method, CCTA was able to rapidly
measure plaque features (12).
Vitrea FX utilizes multiplanar CCTA images to identify the proximal
and distal center points of each lesion, and then the vessel
borders and plaque are automatedly segmented. For each lesion,
plaque volumes were measured for the following subtypes of plaque:
Total plaque, fibrous plaque, calcified plaque (CP), NCP and LD-NCP
(the latter was defined as attenuation <30 HU). NCP was further
classified by plaque HU into two components: Necrotic core (-30 to
30 HU) and fibrous plaque (131 to 350 HU) (13,14).
Semi-quantitative measurements were conducted at the region of
maximal stenosis degree to determine the diameter of the minimal
lumen. To calculate the remodeling index (RI), the cross-sectional
vessel area at the site of greatest stenosis was divided by the
mean cross-sectional vessel area at the proximal point of the
reference. The plaque burden was calculated according to the plaque
volume divided by the vessel volume of the analyzed coronary lesion
(i.e., plaque volume/vessel volume x100). The presence of the
napkin-ring sign, defined as a low-density plaque core surrounded
by high-density areas (15), was
investigated. Spotty calcifications were defined as calcified
plaque of length <3 mm within a lesion (16). The volumes and characteristics of
coronary plaques were assessed for each patient and for each
vessel.
Deep learning-based
FFRCT
FFRCT measurements were conducted using
deep learning-based DEEPVESSEL® FFR software (version
1.0; Beijing Keya Medical Technology Co., Ltd.), and the CCTA
images of the patients were uploaded to its image-computing
platform using Digital Imaging and Communications in Medicine
(DICOM), the standard for the communication and management of
medical imaging information and associated data. The system
automatically calculated the FFRCT value of each
coronary artery via a hydrodynamic model (17), which was presented as a color
coronary tree, with different colors indicating different
FFRCT values. FFRCT values ≤0.80 were
considered to be indicative of lesion-specific ischemia (18).
Statistical analysis
The continuous variables are expressed as the mean ±
standard deviation (SD) or median (interquartile range), whereas
categorical variables are expressed as numbers (percentages). As
required, unpaired Student's t-test, Pearson's χ2 test
or Mann-Whitney U-test were used for data comparisons. To determine
the predictors of ischemia, logistic regression analysis was
conducted (for FFRCT values ≤0.80). Receiver operating
characteristic (ROC) curves and the area under the curve (AUC) were
used to evaluate the predictive values of FFRCT, LD-NCP,
and the combination of FFRCT and LD-NCP for
lesion-specific ischemia, and pairwise comparisons of AUC were made
using the DeLong test (19).
P<0.05 with a 95% confidence interval (CI) was considered to
indicate a statistically significant difference. All of the
statistical analyses were conducted using SPSS software, version
26.0 (IBM Corp.) and MedCalc software, version 20 (MedCalc Software
bvba).
Results
Patient characteristics
In the study population, 144 patients with 243
vessels were investigated by FFR (Fig.
1). The mean age (± SD) of the patients was 62.0±5.4 years, the
mean body mass index (± SD) was 25.0±1.5 kg/m2 and
96/144 (66.7%) of the participants were male. Regarding risk
factors for coronary heart disease, 67/144 (46.5%) of the patients
had hypertension, 43/144 (29.9%) had diabetes, 76/144 (52.8%) had
hyperlipidemia and 47/144 (32.6%) had a history of smoking. All
patients underwent ICA within 30 days of CCTA, with a mean interval
(± SD) of 19.1±5.8 days. Of the 243 vessels, 136/243 (56.0%) were
the LAD, 57/243 (23.5%) were the RCA and 50/243 (20.6%) were the
LCX. The basic characteristics of the included study population are
shown in Table I.
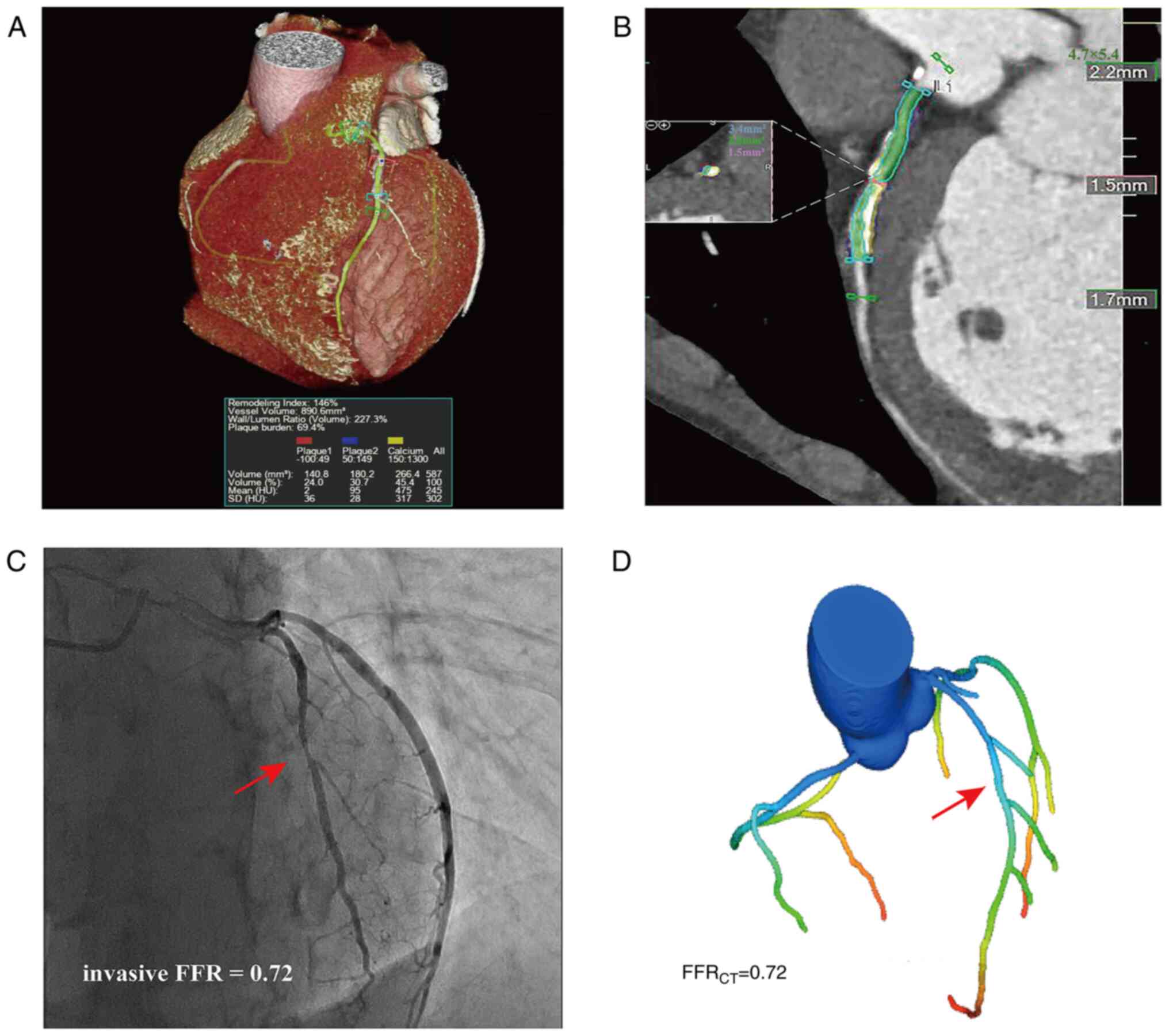 | Figure 1Case example of a 61-year-old male.
(A) Coronary computed tomography angiography demonstrating a
stenotic plaque of the LAD in a 3D imaging scan, revealing the
mixed plaque composition of the proximal portion of the LAD:
Calcified plaque, 266.4 mm3 (yellow ); non-calcified
plaque, 180.2 mm3 (blue ); and low-density non-calcified
plaque, 140.8 mm3 (red). (B) Color-coded semi-automatic
plaque quantification of the lesion, as determined by (C) invasive
coronary angiography, showing 50% stenosis of the LAD (red arrow),
with the measured invasive FFR being 0.72. (D) Three-dimensional
color-coding, revealing that FFRCT in the distal LAD was
0.72. LAD, left anterior descending artery; FFR, fractional flow
reserve; FFRCT, computed tomography angiography-derived
FFR; SD, standard deviation; HU, Hounsfield units. |
 | Table IBasic characteristics of all study
patients. |
Table I
Basic characteristics of all study
patients.
| Characteristic | FFR ≤0.80
(n=82) | FFR >0.8
(n=62) | P-value | Total (n=144) |
|---|
| Age,
yearsa | 61±5.8 | 63±4.7 | 0.065b | 62±5.4 |
| Male sex, n
(%) | 60 (73.2) | 36 (58.1) | 0.057c | 96 (66.7) |
| Body mass index,
kg/m2a | 24±2.8 | 25±1.4 | 0.877b | 25±1.5 |
| Presence of
hypertension, n (%) | 37 (45.1) | 30 (48.4) | 0.679c | 67 (46.5) |
| Presence of
diabetes mellitus, n (%) | 24 (29.3) | 19 (30.6) | 0.858c | 43 (29.9) |
| Presence of
dyslipidemia, n (%) | 46 (56.1) | 30 (48.4) | 0.359c | 76 (52.8) |
| History of smoking,
n (%) | 24 (29.3) | 20 (32.3) | 0.700c | 47 (32.6) |
| Period from CCTA to
invasive coronary angiography, daysa | 19.5±5.7 | 18.7±5.9 | 0.447b | 19.1±5.8 |
| Vascular
leveld | | | | |
|
Left
anterior descending artery, n (%) | 79 (55.2) | 67 (67.0) | 0.066c | 136 (56.0) |
|
Left
circumflex artery, n (%) | 25 (17.5) | 25 (25.0) | 0.154c | 50 (20.6) |
|
Right
coronary artery, n (%) | 39 (27.3) | 18 (18.0) | 0.093c | 57 (23.5) |
Association of plaque characteristics
and lesion-specific ischemia
Associations between plaque characteristics and
lesion-specific ischemia were evaluated. The plaque characteristics
RI, CP, fibrous plaque volume, plaque burden, napkin-ring sign,
spotty calcifications and stenosis >50% showed no significant
differences when comparing between lesion-specific ischemia and
non-significant lesions (P>0.05). By contrast, NCP, LD-NCP,
total plaque volume and plaque length were significantly different
in lesion-specific ischemia compared with non-significant lesions
(P<0.05). Table II summarizes
the different quantitative and qualitative plaque characteristics
and their association with FFRCT ≤0.80. Univariate
logistic regression analysis demonstrated that NCP [odds ratio
(OR), 1.158; 95% CI, 1.103-1.215; P<0.0001], LD-NCP (OR, 1.128;
95% CI, 1.094-1.164; P<0.0001) and plaque length (OR, 1.147; 95%
CI, 1.043-1.261; P=0.005) were significantly associated with
lesion-specific ischemia. Finally, multivariate logistic regression
revealed that LD-NCP (OR, 1.18; 95% CI, 1.105-1.256; P<0.001)
was an independent predictor of lesion-specific ischemia (Table III).
 | Table IIPlaque characteristics and
FFRCT according to lesion-specific ischemia (FFR
≤0.80). |
Table II
Plaque characteristics and
FFRCT according to lesion-specific ischemia (FFR
≤0.80).
| Characteristic | Overall
(n=243) | FFR ≤0.80
(n=143) | FFR >0.80
(n=100) | P-value |
|---|
|
FFRCT | 0.81±0.08 | 0.76±0.07 | 0.86±0.04 |
<0.0001a |
| Remodeling
index | 1.08 (0.74) | 1.1 (0.46) | 1.07 (0.74) | 0.293c |
| Calcified plaque
(mm3) | 72.9±12.6 | 74.0±11 | 71.0±14.7 | 0.074b |
| Non-calcified
plaque (mm3) | 255±44 | 286±23 | 210±22 |
<0.0001a |
| Low-density
non-calcified plaque (mm3) | 46±14 | 53±13 | 35±11 |
<0.0001a |
| Total plaque volume
(mm3) | 268±71 | 275±82 | 257±50 | 0.031a |
| Fibrous plaque
volume (mm3) | 58.4±11.5 | 59.2±11.0 | 57.0±12.0 | 0.167b |
| Plaque length
(mm) | 16.5±2.9 | 17.0±3.2 | 15.9±2.3 | 0.002a |
| Plaque burden, n
(%) | 104(66) | 104(65) | 101(67) | 0.051c |
| Napkin ring sign, n
(%) | 58 (23.9) | 37 (25.9) | 21 (21.0) | 0.38b |
| Spotty
calcification, n (%) | 54 (22.2) | 34 (23.8) | 20 (20.0) | 0.486b |
| Stenosis >50%, n
(%) | 144 (59.3) | 91 (63.6) | 53 (53.0) | 0.097b |
 | Table IIIUnivariate and multivariate logistic
regression analysis of coronary CT angiography-derived plaque
markers and FFRCT. |
Table III
Univariate and multivariate logistic
regression analysis of coronary CT angiography-derived plaque
markers and FFRCT.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variable | P-value | OR (95% CI) | P-value | OR (95% CI) |
|---|
| FFRCT
<0.80 |
<0.0001a | 23.197
(11.171-48.084) |
<0.0001a | 23.276
(3.505-54.561) |
| Non-calcified
plaque, mm3 |
<0.0001a | 1.158
(1.103-1.215) | 0.0630 | 1.130
(0.994-1.286) |
| Low-density
non-calcified plaque, mm3 |
<0.0001a | 1.128
(1.094-1.164) | 0.0030a | 1.178
(1.105-1.256) |
| Plaque length,
mm | 0.0050a | 1.147
(1.043-1.261) | 0.6180 | 0.866
(0.492-1.525) |
| Total plaque
volume, mm3 | 0.0610 | 1.004
(1.000-1.007) | - | - |
Association of FFRCT and
lesion-specific ischemia
FFRCT was found to be significantly
associated with the presence of ischemia (P<0.0001) (Table II). According to the univariate
regression analysis, FFRCT was a significant predictor
of lesion-specific ischemia (OR, 23.20; 95% CI, 11.171-48.084;
P<0.0001), and was a strong independent predictor of ischemia
(OR, 23.28; 95% CI, 3.505-54.561; P<0.0001) (Table III).
Combined assessment of LD-NCP and
FFRCT for identifying ischemia
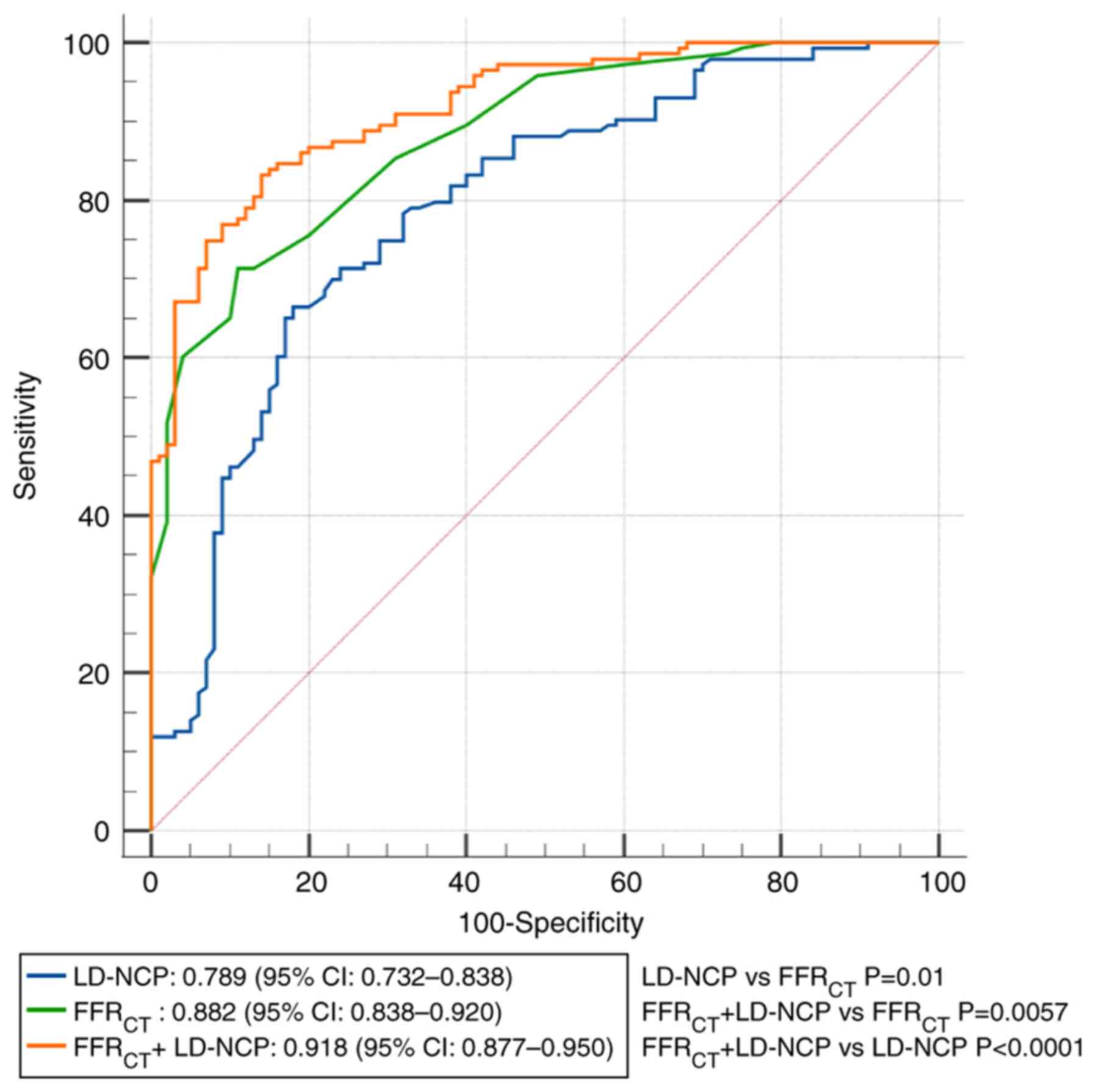
The AUC values for the identification of
FFRCT ≤0.80 were 0.79 (95% CI, 0.732-0.838) for LD-NCP,
0.88 (95% CI, 0.835-0.920) for FFRCT and 0.92 (95% CI,
0.877-0.950) for LD-NCP + FFRCT. FFRCT showed
a better predictive performance relative to LD-NCP for
lesion-specific ischemia (0.88 vs. 0.79; P=0.01). The addition of
FFRCT to LD-NCP further enhanced the predictive
performance, albeit with incremental discriminatory power, compared
with LD-NCP alone (0.92 vs. 0.79; P<0.0001) or FFRCT
alone (0.92 vs. 0.88; P=0.0057). In Fig. 2 and Table IV, analyses of the ROC curves for
optimal thresholds for identifying lesion-specific ischemia are
shown, as well as the results of the sensitivity, specificity,
positive predictive value, negative predictive value and cut-off
value calculations.
 | Table IVDiagnostic performance of coronary CT
angiography-derived plaque markers and FFRCT for the
identification of lesion-specific ischemia. |
Table IV
Diagnostic performance of coronary CT
angiography-derived plaque markers and FFRCT for the
identification of lesion-specific ischemia.
| Variable | LD-NCP |
FFRCT | FFRCT +
LD-NCP |
|---|
| Area under the
curve | 0.789
(0.732-0.838) | 0.882
(0.835-0.920) | 0.918
(0.877-0.950) |
| Sensitivity, % (95%
CI) | 66.43
(58.1-74.1) | 71.33
(63.2-78.6) | 83.22
(76.1-88.9) |
| Specificity, % (95%
CI) | 82 (73.1-89.0) | 89 (81.2-94.4) | 86 (77.6-92.1) |
| Positive predictive
value, % (95% CI) | 84.1
(77.4-89.1) | 90.3
(84.0-94.2) | 89.5
(83.9-93.3) |
| Negative predictive
value, % (95% CI) | 63.1
(57.1-68.8) | 68.5
(62.4-73.9) | 78.2
(71.2-83.9) |
| Cut-off value | 46.3
mm3 | 0.80 | 0.41 |
Discussion
The results of the present study have demonstrated
that CCTA-based FFRCT and plaque characteristics,
especially LD-NCP, are predictors of lesion-specific ischemia.
Importantly, FFRCT and LD-NCP have been shown to be
significant predictors of specific ischemia, and in combination,
they synergistically increase the predictive value for ischemia
compared with FFRCT or LD-NCP alone.
Several studies have found a correlation between
CCTA-derived plaque characteristics and ischemia, as well as
significant differences between ischemia and non-significant
lesions based on multiple quantitative and qualitative plaque
characteristics (20-22).
In the present study, it was found that plaque length, NCP volume
and LD-NCP volume were not only significantly different when
comparing between ischemia-causing lesions and non-significant
lesions, but they were also useful in terms of predicting
lesion-specific ischemia. These findings are similar to those
reported by Diaz-Zamudio et al (23) and Iguchi et al (24), showing the predictive value of
plaque length, NCP volume and LD-NCP volume. However, contrary to
the findings of the present study, Gaur et al (25) reported a significant association
between CCTA-derived RI and the presence of ischemia. By contrast,
a different study indicated no significant correlation between
plaque length or RI derived from CCTA and ischemia (26). It is likely that the determinants
of study outcomes will differ significantly, which could explain
the differences in results seen among studies.
LD-NCP is considered a surrogate for necrotic core
plaques, and it has been shown to be useful in assessing the
hemodynamic significance of coronary arteries (27). Notably, the results of the present
study revealed that LD-NCP volume was an independent predictor for
lesion-specific ischemia; this is a similar result to that found in
a previous total-vessel study, which revealed that a higher
probability of ischemia was associated with a higher LD-NCP volume
(28). A retrospective study also
suggested that LD-NCP volume predicts acute coronary syndromes,
both on a per-patient and a per-vessel basis (29). Notably, the volume of LD-NCP was
shown to be associated with the endothelial dysfunction caused by
local inflammation and oxidative stress, and an increase in the
LD-NCP volume led to reduced bioavailability of the vasodilator,
nitric oxide, which made it difficult for the blood vessels to
dilate under conditions of stress, thereby leading to ischemia
(9). This mechanism may account
for the ability of LD-NCP to act as a significant predictor of
ischemia. However, in the presence of high-grade stenosis, plaque
analysis, such as that of NCP and LD-NCP, may be less useful in
terms of diagnosing ischemia (28).
Over the course of the last few decades, researchers
have pursued an ideal non-invasive imaging diagnostic for ischemia.
Numerous studies have shown a higher diagnostic accuracy of
FFRCT for lesion-specific ischemia compared with
invasive FFR (30-33).
In addition, FFRCT based on deep-learning algorithms has
been used to evaluate the hemodynamics of coronary arteries
(34). A combined multicenter
meta-analysis study revealed a high predictive value of
FFRCT for lesion-specific ischemia, with an AUC value of
0.86(35). A similar AUC value was
derived in the present study (AUC, 0.88), and deep-learning
FFRCT showed excellent predictive performance (OR,
23.19; P<0.0001) in terms of identifying ischemia. Additionally,
FFRCT provided superior discriminatory performance over
LD-NCP (AUC, 0.88 vs. 0.79; P=0.006), and the addition of
FFRCT to LD-NCP demonstrated an incremental increase in
predictive value (AUC, 0.92 vs. 0.79; P<0.0001), which is
consistent with the findings of a previous study by von Knebel
Doeberitz et al (36).
However, in contrast with the present study results, a previous
study found that the combination of FFR and LD-NCP was unable to
increase the predictive value of FFR alone for lesion-specific
ischemia (25). However, this
previous study added stenosis >50% as a predictive index, and
the presence of a difference in FFRCT when accompanied
by markedly stenotic coronary arteries may explain why the addition
of FFRCT had no incremental value for ischemia.
The present study had certain limitations. Firstly,
the design protocol for retrospective studies and the relatively
small sample size of the included study cases may have led to the
existence of selection bias. Therefore, more prospective,
multicenter studies are needed in the future to validate the
findings. Secondly, FFRCT values may vary, depending on
factors such as fluid dynamics models, blood viscosity and
individual differences (37),
which require continuous optimization of image quality and
algorithms. Combining FFRCT and plaque features based on
deep machine learning models may improve the identification of
ischemia. Thirdly, the analysis of plaque characteristics may be
limited by the resolution of CT, and the volume measurement of
LD-NCP will also be affected to a certain extent (38). Therefore, in addition to improving
the CT resolution, it is necessary to verify different CT scanners
and different tube voltages prior to their clinical application.
Fourthly, patients with severe cardiovascular disease or previous
revascularization were excluded from the present study, and the
predictive performance of FFRCT and LD-NCP for ischemia
in this group of patients requires further study. Lastly, since the
automated software only calculated the total plaque length and
burden, but could not measure the length of different types of
plaques or the volume of blood vessels where they were located
(39), the algorithm needs to be
improved in the future to explore the predictive value of plaque
length, volume and burden for lesion-specific ischemia.
In conclusion, CCTA-derived plaque characteristics
and FFRCT have been demonstrated to have predictive
value in terms of identifying lesion-specific ischemia.
Furthermore, the addition of FFRCT to LD-NCP showed
incremental discriminatory power for ischemia compared with
FFRCT or LD-NCP alone.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Zhangjiakou Key
Research and Development Program Projects (grant no. 2021030D).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SC and LT conceived the study and wrote the
manuscript. LT, FY and TD performed the experiments. LT and FL
carried out the data collection and data analysis. SC and FY
assessed the quality of the studies. LT, SC and FL confirm the
authenticity of all the raw data. SC, LT, TD, FL and FY reviewed
the results. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The Ethics Review Board of The First Affiliated
Hospital, Hebei North University (Zhangjiakou, China) examined and
approved the study protocol. All patients included in the study
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Basha MAA, Aly SA, Ismail AAA, Bahaaeldin
HA and Shehata SM: The validity and applicability of CAD-RADS in
the management of patients with coronary artery disease. Insights
Imaging. 10(117)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Balfour PC Jr, Gonzalez JA and Kramer CM:
Non-invasive assessment of low- and intermediate-risk patients with
chest pain. Trends Cardiovasc Med. 27:182–189. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
von Ballmoos MW, Haring B, Juillerat P and
Alkadhi H: Meta-analysis: Diagnostic performance of
low-radiation-dose coronary computed tomography angiography. Ann
Intern Med. 154:413–420. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li Y, Qiu H, Hou Z, Zheng J, Li J, Yin Y
and Gao R: Additional value of deep learning computed tomographic
angiography-based fractional flow reserve in detecting coronary
stenosis and predicting outcomes. Acta Radiol. 63:133–140.
2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wardziak Ł, Kruk M, Pleban W, Demkow M,
Rużyłło W, Dzielińska Z and Kępka C: Coronary CTA enhanced with CTA
based FFR analysis provides higher diagnostic value than invasive
coronary angiography in patients with intermediate coronary
stenosis. J Cardiovasc Comput Tomogr. 13:62–67. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gohmann RF, Pawelka K, Seitz P, Majunke N,
Heiser L, Renatus K, Desch S, Lauten P, Holzhey D, Noack T, et al:
Combined cCTA and TAVR planning for ruling out significant CAD:
Added value of ML-based CT-FFR. JACC Cardiovasc Imaging.
15:476–486. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dwivedi G, Liu Y, Tewari S, Inacio J,
Pelletier-Galarneau M and Chow BJ: Incremental prognostic value of
quantified vulnerable plaque by cardiac computed tomography: A
pilot study. J Thorac Imaging. 31:373–379. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dey D, Schepis T, Marwan M, Slomka PJ,
Berman DS and Achenbach S: Automated three-dimensional
quantification of noncalcified coronary plaque from coronary CT
angiography: Comparison with intravascular US. Radiology.
257:516–522. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ahmadi A, Kini A and Narula J: Discordance
between ischemia and stenosis, or PINSS and NIPSS: Are we ready for
new vocabulary? JACC Cardiovasc Imaging. 8:111–114. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ahmadi N, Ruiz-Garcia J, Hajsadeghi F,
Azen S, Mack W, Hodis H and Lerman A: Impaired coronary artery
distensibility is an endothelium-dependent process and is
associated with vulnerable plaque composition. Clin Physiol Funct
Imaging. 36:261–268. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Naidu SS, Rao SV, Blankenship J, Cavendish
JJ, Farah T, Moussa I, Rihal CS, Srinivas VS and Yakubov SJ:
Society for Cardiovascular Angiography and Interventions. Clinical
expert consensus statement on best practices in the cardiac
catheterization laboratory: Society for cardiovascular angiography
and interventions. Catheter Cardiovasc Interv. 80:456–464.
2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
de Jonge GJ, van Ooijen PM, Overbosch J,
Gueorguieva AL, Janssen-van der Weide MC and Oudkerk M: Comparison
of (semi-)automatic and manually adjusted measurements of left
ventricular function in dual source computed tomography using three
different software tools. Int J Cardiovasc Imaging. 27:787–794.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chang HJ, Lin FY, Lee SE, Andreini D, Bax
J, Cademartiri F, Chinnaiyan K, Chow BJW, Conte E, Cury RC, et al:
Coronary atherosclerotic precursors of acute coronary syndromes. J
Am Coll Cardiol. 71:2511–2522. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
de Graaf MA, Broersen A, Kitslaar PH, Roos
CJ, Dijkstra J, Lelieveldt BP, Jukema JW, Schalij MJ, Delgado V,
Bax JJ, et al: Automatic quantification and characterization of
coronary atherosclerosis with computed tomography coronary
angiography: Cross-correlation with intravascular ultrasound
virtual histology. Int J Cardiovasc Imaging. 29:1177–1190.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Maurovich-Horvat P, Schlett CL, Alkadhi H,
Nakano M, Otsuka F, Stolzmann P, Scheffel H, Ferencik M, Kriegel
MF, Seifarth H, et al: The napkin-ring sign indicates advanced
atherosclerotic lesions in coronary CT angiography. JACC Cardiovasc
Imaging. 5:1243–1252. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mori H, Torii S, Kutyna M, Sakamoto A,
Finn AV and Virmani R: Coronary artery calcification and its
progression: What does it really mean? JACC Cardiovasc Imaging.
11:127–142. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang H, Tang ZR, Li W, Fan T, Zhao J, Kang
M, Dong R and Qu Y: Prediction of the risk of C5 palsy after
posterior laminectomy and fusion with cervical myelopathy using a
support vector machine: an analysis of 184 consecutive patients. J
Orthop Surg Res. 16(332)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Coenen A, Lubbers MM, Kurata A, Kono A,
Dedic A, Chelu RG, Dijkshoorn ML, Gijsen FJ, Ouhlous M, van Geuns
RJ and Nieman K: Fractional flow reserve computed from noninvasive
CT angiography data: Diagnostic performance of an on-site
clinician-operated computational fluid dynamics algorithm.
Radiology. 274:674–683. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
DeLong ER, DeLong DM and Clarke-Pearson
DL: Comparing the areas under two or more correlated receiver
operating characteristic curves: A nonparametric approach.
Biometrics. 44:837–845. 1988.PubMed/NCBI
|
|
20
|
Tesche C, De Cecco CN, Caruso D, Baumann
S, Renker M, Mangold S, Dyer KT, Varga-Szemes A, Baquet M, Jochheim
D, et al: Coronary CT angiography derived morphological and
functional quantitative plaque markers correlated with invasive
fractional flow reserve for detecting hemodynamically significant
stenosis. J Cardiovasc Comput Tomogr. 10:199–206. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hell MM, Dey D, Marwan M, Achenbach S,
Schmid J and Schuhbaeck A: Non-invasive prediction of
hemodynamically significant coronary artery stenoses by contrast
density difference in coronary CT angiography. Eur J Radiol.
84:1502–1508. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Park HB, Heo R, Ó Hartaigh B, Cho I,
Gransar H, Nakazato R, Leipsic J, Mancini GBJ, Koo BK, Otake H, et
al: Atherosclerotic plaque characteristics by CT angiography
identify coronary lesions that cause ischemia: a direct comparison
to fractional flow reserve. JACC Cardiovasc Imaging. 8:1–10.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Diaz-Zamudio M, Dey D, Schuhbaeck A,
Nakazato R, Gransar H, Slomka PJ, Narula J, Berman DS, Achenbach S,
Min JK, et al: Automated quantitative plaque burden from coronary
CT angiography noninvasively predicts hemodynamic significance by
using fractional flow reserve in intermediate coronary lesions.
Radiology. 276:408–415. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Iguchi T, Hasegawa T, Nishimura S, Nakata
S, Kataoka T, Ehara S, Hanatani A, Shimada K and Yoshiyama M:
Impact of lesion length on functional significance in intermediate
coronary lesions. Clin Cardiol. 36:172–177. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gaur S, Øvrehus KA, Dey D, Leipsic J,
Bøtker HE, Jensen JM, Narula J, Ahmadi A, Achenbach S, Ko BS, et
al: Coronary plaque quantification and fractional flow reserve by
coronary computed tomography angiography identify ischaemia-causing
lesions. Eur Heart J. 37:1220–1227. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Doris MK, Otaki Y, Arnson Y, Tamarappoo B,
Goeller M, Gransar H, Wang F, Hayes S, Friedman J, Thomson L, et
al: Non-invasive fractional flow reserve in vessels without severe
obstructive stenosis is associated with coronary plaque burden. J
Cardiovasc Comput Tomogr. 12:379–384. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shmilovich H, Cheng VY, Tamarappoo BK, Dey
D, Nakazato R, Gransar H, Thomson LE, Hayes SW, Friedman JD,
Germano G, et al: Vulnerable plaque features on coronary CT
angiography as markers of inducible regional myocardial
hypoperfusion from severe coronary artery stenoses.
Atherosclerosis. 219:588–595. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Øvrehus KA, Gaur S, Leipsic J, Jensen JM,
Dey D, Bøtker HE, Ahmadi A, Achenbach S, Ko B and Nørgaard BL:
CT-based total vessel plaque analyses improves prediction of
hemodynamic significance lesions as assessed by fractional flow
reserve in patients with stable angina pectoris. J Cardiovasc
Comput Tomogr. 12:344–349. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dey D, Achenbach S, Schuhbaeck A,
Pflederer T, Nakazato R, Slomka PJ, Berman DS and Marwan M:
Comparison of quantitative atherosclerotic plaque burden from
coronary CT angiography in patients with first acute coronary
syndrome and stable coronary artery disease. J Cardiovasc Comput
Tomogr. 8:368–374. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nakazato R, Park HB, Berman DS, Gransar H,
Koo BK, Erglis A, Lin FY, Dunning AM, Budoff MJ, Malpeso J, et al:
Noninvasive fractional flow reserve derived from computed
tomography angiography for coronary lesions of intermediate
stenosis severity: Results from the DeFACTO study. Circ Cardiovasc
Imaging. 6:881–889. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nørgaard BL, Leipsic J, Gaur S,
Seneviratne S, Ko BS, Ito H, Jensen JM, Mauri L, De Bruyne B,
Bezerra H, et al: Diagnostic performance of noninvasive fractional
flow reserve derived from coronary computed tomography angiography
in suspected coronary artery disease: The NXT trial (analysis of
coronary blood flow using CT angiography: Next steps). J Am Coll
Cardiol. 63:1145–1155. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gaur S, Bezerra HG, Lassen JF,
Christiansen EH, Tanaka K, Jensen JM, Oldroyd KG, Leipsic J,
Achenbach S, Kaltoft AK, et al: Fractional flow reserve derived
from coronary CT angiography: Variation of repeated analyses. J
Cardiovasc Comput Tomogr. 8:307–314. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li W, Wang H, Dong S, Tang ZR, Chen L, Cai
X, Hu Z and Yin C: Establishment and validation of a nomogram and
web calculator for the risk of new vertebral compression fractures
and cement leakage after percutaneous vertebroplasty in patients
with osteoporotic vertebral compression fractures. Eur Spine J.
31:1108–1121. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tesche C, De Cecco CN, Baumann S, Renker
M, McLaurin TW, Duguay TM, Bayer RR II, Steinberg DH, Grant KL,
Canstein C, et al: Coronary CT angiography-derived fractional flow
reserve: Machine learning algorithm versus computational fluid
dynamics modeling. Radiology. 288:64–72. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tang CX, Wang YN, Zhou F, Schoepf UJ,
Assen MV, Stroud RE, Li JH, Zhang XL, Lu MJ, Zhou CS, et al:
Diagnostic performance of fractional flow reserve derived from
coronary CT angiography for detection of lesion-specific ischemia:
A multi-center study and meta-analysis. Eur J Radiol. 116:90–97.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
von Knebel Doeberitz PL, De Cecco CN,
Schoepf UJ, Duguay TM, Albrecht MH, van Assen M, Bauer MJ, Savage
RH, Pannell JT, De Santis D, et al: Coronary CT angiography-derived
plaque quantification with artificial intelligence CT fractional
flow reserve for the identification of lesion-specific ischemia.
Eur Radiol. 29:2378–2387. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nørgaard BL, Terkelsen CJ, Mathiassen ON,
Grove EL, Bøtker HE, Parner E, Leipsic J, Steffensen FH, Riis AH,
Pedersen K, et al: Coronary CT angiographic and flow reserve-guided
management of patients with stable ischemic heart disease. J Am
Coll Cardiol. 72:2123–2134. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Goeller M, Rahman Ihdayhid A, Cadet S, Lin
A, Adams D, Thakur U, Yap G, Marwan M, Achenbach S, Dey D and Ko B:
Pericoronary adipose tissue and quantitative global non-calcified
plaque characteristics from CT angiography do not differ in matched
South Asian, East Asian and European-origin Caucasian patients with
stable chest pain. Eur J Radiol. 125(108874)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
van Assen M, Varga-Szemes A, Schoepf UJ,
Duguay TM, Hudson HT, Egorova S, Johnson K, St Pierre S, Zaki B,
Oudkerk M, et al: Automated plaque analysis for the prognostication
of major adverse cardiac events. Eur J Radiol. 116:76–83.
2019.PubMed/NCBI View Article : Google Scholar
|