Introduction
Irritable bowel syndrome (IBS) is a functional and
often debilitating disorder of the gastrointestinal tract. It can
be characterised by IBS with diarrhoea (IBS-D), IBS with
constipation (IBS-C) or mixed constipation and diarrhoea (IBS-M)
(1). A diagnosis of IBS is made
based on the patient's symptoms of abdominal pain and altered bowel
habits, using the 2016 Rome criteria guidelines, ROME IV (2). IBS affects ~11% of the population
globally (1).
The underlying causes of IBS are likely
multifactorial, including dysbiosis, abnormal gut motility, stress,
an altered gut-brain axis, increased mucosal permeability,
inflammation or impaired immune function and heightened visceral
sensitivity (3). A major factor in
the pathogenesis of this condition may be the presence of small
intestinal bacterial overgrowth (SIBO). The prevalence of SIBO in
patients with IBS symptoms has been reported to be as high as
43-78% in certain studies (4-6).
In healthy individuals, bacteria colonise the entire
length of the gastrointestinal tract, ranging from
101-103 bacteria/g in the stomach to
1011-1012 bacteria/g in the colon (7). Bacteria in the small intestine are
usually Gram-positive aerobes, where the colon is normally
colonised by anaerobes. In some cases, conditions within the
gastrointestinal tract prohibit natural defence mechanisms to
prevent bacteria from overgrowing within the small intestine. This
may include, but is not limited to, elevated pH levels within the
stomach or decreased gastric secretions, dysrhythmic activity
altering the intestinal motility and an impaired cellular or
humoral immune defence or structural issues with the ileocaecal
valve (8). These conditions may
arise following medications which alter these conditions, such as
proton pump inhibitors, antibiotics and anticholinergics, amongst
others (7). SIBO involves
bacterial overgrowth within the small intestine, usually defined as
the presence of >105 colony forming units (CFU)/ml
(9,10).
When SIBO is present, carbon dioxide, hydrogen,
methane and short-chain fatty acids are produced as a result of
bacterial metabolism. These by-products can cause unpleasant
abdominal and gastrointestinal symptoms, such as bloating,
excessive belching, flatulence, epigastric and abdominal pain,
nausea, early satiety, fatigue and altered bowel habits. SIBO has
also been associated with increased inflammation and intestinal
permeability, where it is linked to the pathogenesis of other
conditions, such as non-alcoholic fatty liver disease (11,12).
SIBO may lead to an impaired nutrient status by reducing the
absorptive capacity of the intestinal villi and the deconjugation
of bile salts, leading to decreased fat and fat-soluble nutrient
absorption, and competing with the host for vitamin B12 absorption
(13).
The diagnostic test for SIBO is a breath test for
the detection of hydrogen and methane gas. These gases are not
produced by humans, but are produced by microbial fermentation.
These gases diffuse through the gut wall into the circulatory
system and are excreted in the breath and therefore can be used as
a direct measurement of SIBO, bacterial fermentation and
carbohydrate malabsorption. This is a simple and non-invasive
procedure although there are limitations (14). Two major groups have convened to
standardise the methods and interpretation of breath testing. A
number of testing facilities have followed the 2017 North American
Consensus recommendations (15)
which have been updated in 2019 by the Association of
Gastrointestinal Physiology (AGIP) committee of the British Society
of Gastroenterology for the UK (16).
The present study describes a case of IBS with a
diagnosis of SIBO using a lactulose hydrogen and methane breath
test in accordance with the AGIP protocols and guidelines (16). In this patient, SIBO was eradicated
using a diet low in fermentable oligo-, di-, mono-saccharides and
polyols (FODMAP) and a herbal formulation, which resolved the
symptoms of IBS and improved the quality of life of the patient,
even following the re-introduction of high FODMAP foods.
Case report
The patient discussed herein was female at the age
of 48 with IBS symptoms described by the patient as severe for at
least 10 years. Symptoms included bloating, cramps, flatulence and
constipation along with fatigue. Intolerance to garlic was severe,
causing vomiting and thus the patient completely avoided its
consumption. She also identified other foods as potential triggers,
such as cabbage, wheat, lactose and foods high in sugar. The
patient had a history of frequent antibiotic use and stress.
Upon her first visit to the Glenville Nutrition
Ireland clinic, the patient received a patient code which was used
to anonymise all identifying personal data and test results. The
patient's personal data and records are securely stored on a clinic
system (Clinic Office™) in accordance with the General
Data Protection Regulation 2018(17). In accordance with the regulations,
the clinic privacy policy is available online at www.glenvillenutrition.ie. When the case was
considered for publication, the patient signed a consent form
agreeing for anonymous data, including the test results to be
used.
Prior to the initial consultation and at the 6-week
follow-up, the patient completed a quality of life questionnaire
validated for IBS and functional digestive disorders, which yields
a score ranging from 0 (poor quality of life) to 100 (good quality
of life) (18). In the initial
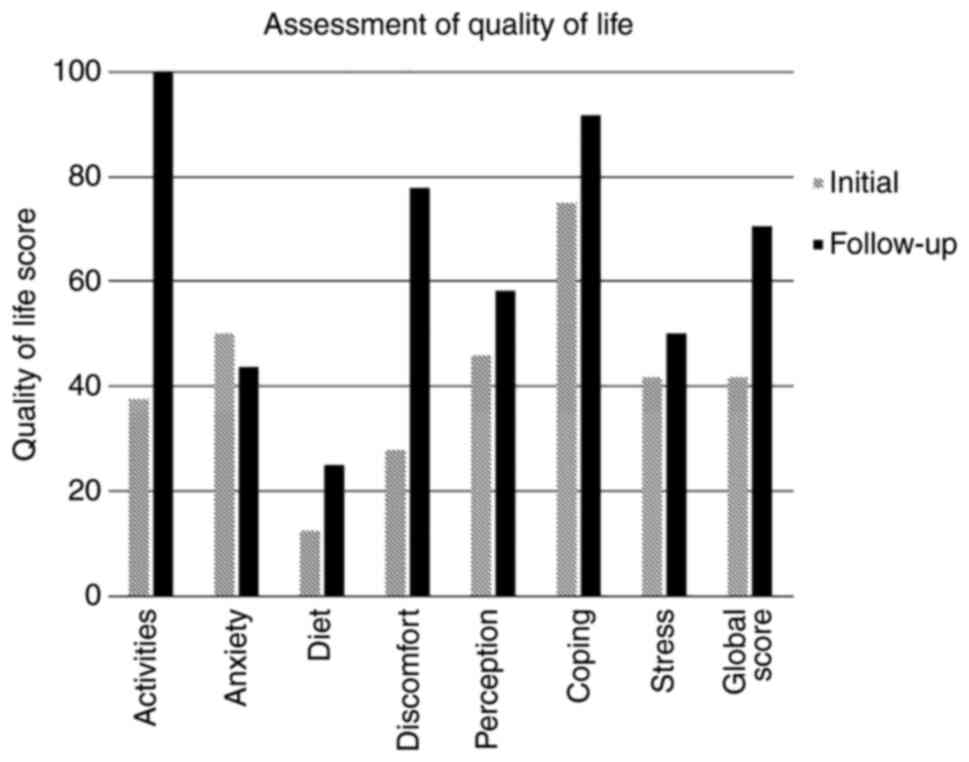
assessment, the patient's handicap of daily living was deemed to be
extreme in the categories of activity, diet and discomfort. Her
global score was 42 (Fig. 1).
A strict 24-h preparatory diet of only boiled
chicken or poached fish, white rice, eggs and water was followed,
including 12 h of fasting (16).
The patient had not taken any antibiotics, laxatives, antacids,
proton pump inhibitors or other medications or supplements relating
to digestion, such as enzymes or probiotics for at least 1 month.
She had begun following a low FODMAP diet at 1 week prior to her
initial test due to her own research. At the time of testing,
constipation was reduced due to the avoidance of ‘trigger’ foods
and delayed bowel transit time was not apparent.
SIBO breath testing was carried out at the
GastroLife Clinic in Dublin, Ireland using a lactulose substrate in
accordance with the AGIP guidelines (16) and was performed using the Bedfont
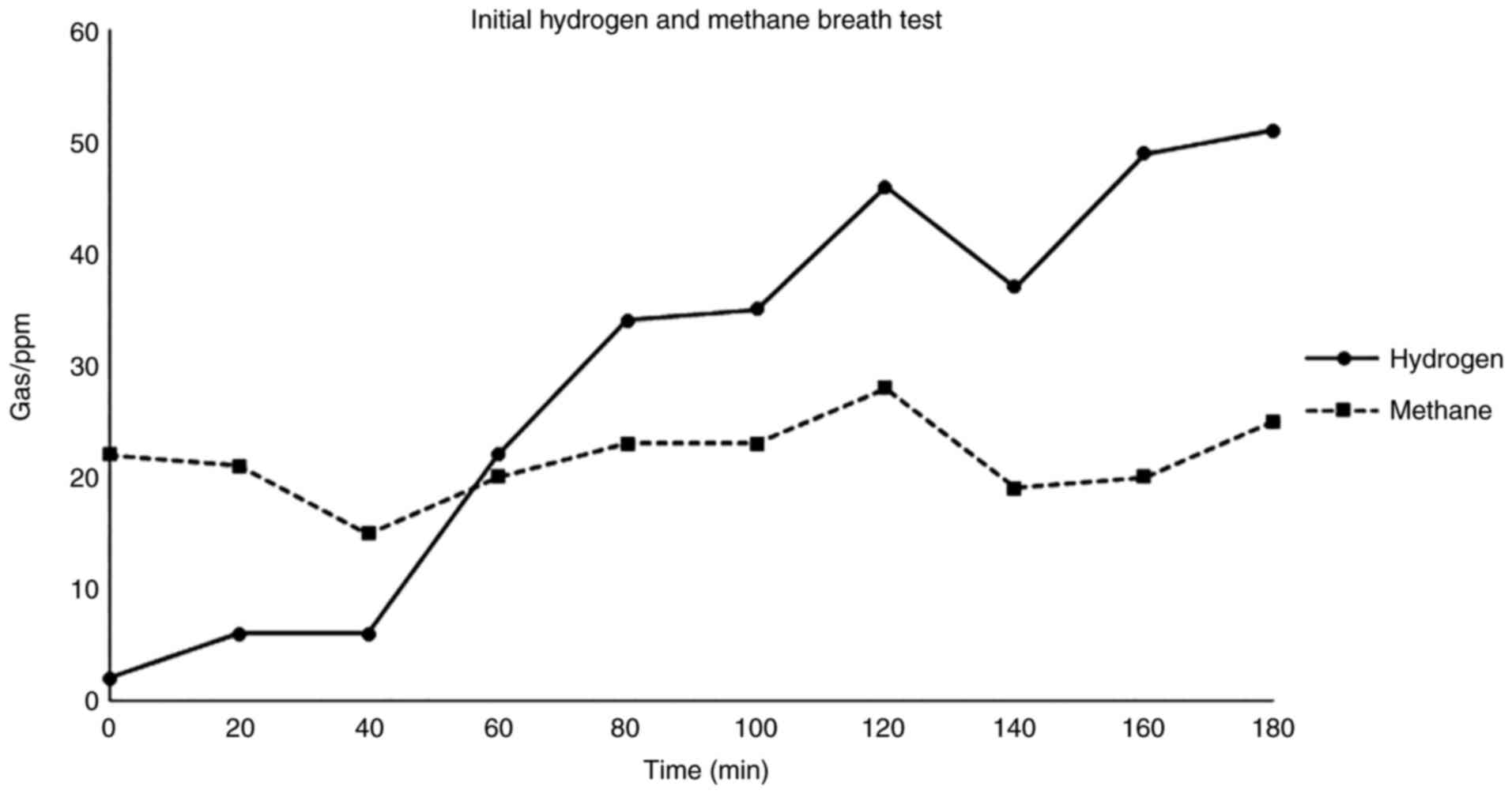
Scientific GastroCHECK™ gastrolyzer. A positive SIBO
test was indicated by a rise in hydrogen gas of >20 ppm over the
lowest preceding value within 100 min and methanogenesis was
indicated by a rise in methane gas of >12 ppm (Fig. 2).
A strict low FODMAP diet was recommended under the
supervision of a nutritional therapist using the Monash University
FODMAP University App for the most up-to-date food list (19). A herbal supplement termed Candex
SIBO (Nutri Advanced; Table I) was
used to support the eradication of small intestinal bacteria; the
dose of this was slowly increased to two capsules three times per
day for 28 days. A mild digestive enzyme was also used before meals
to support digestion and motility (Nutri Advanced Marshmallow and
Gamma Oryzanol). This contains 120 mg marshmallow root, 65 mg gamma
oryzanol and 165 mg mixed digestive enzyme blend per capsule,
including amylase, lipase, cellulase, lactase, invertase, peptidase
and maltase. It should be noted that the herbal supplements were
provided to the patient free of charge by Nutri Advanced.
 | Table IIngredients of the herbal formulation,
Nutri Advanced Candex SIBO. |
Table I
Ingredients of the herbal formulation,
Nutri Advanced Candex SIBO.
| Constituents of Nutri
Advanced Candex SIBO | Amount per capsule
(mg) |
|---|
| Caprylic acid | 167 |
| Grapefruit seed
extract | 150 |
| Berberine | 75 |
| Garlic extract | 70 |
| Oregano extract | 50 |
The patient was extremely compliant with the dietary
restrictions and supplements. Upon the completion of the 28-day
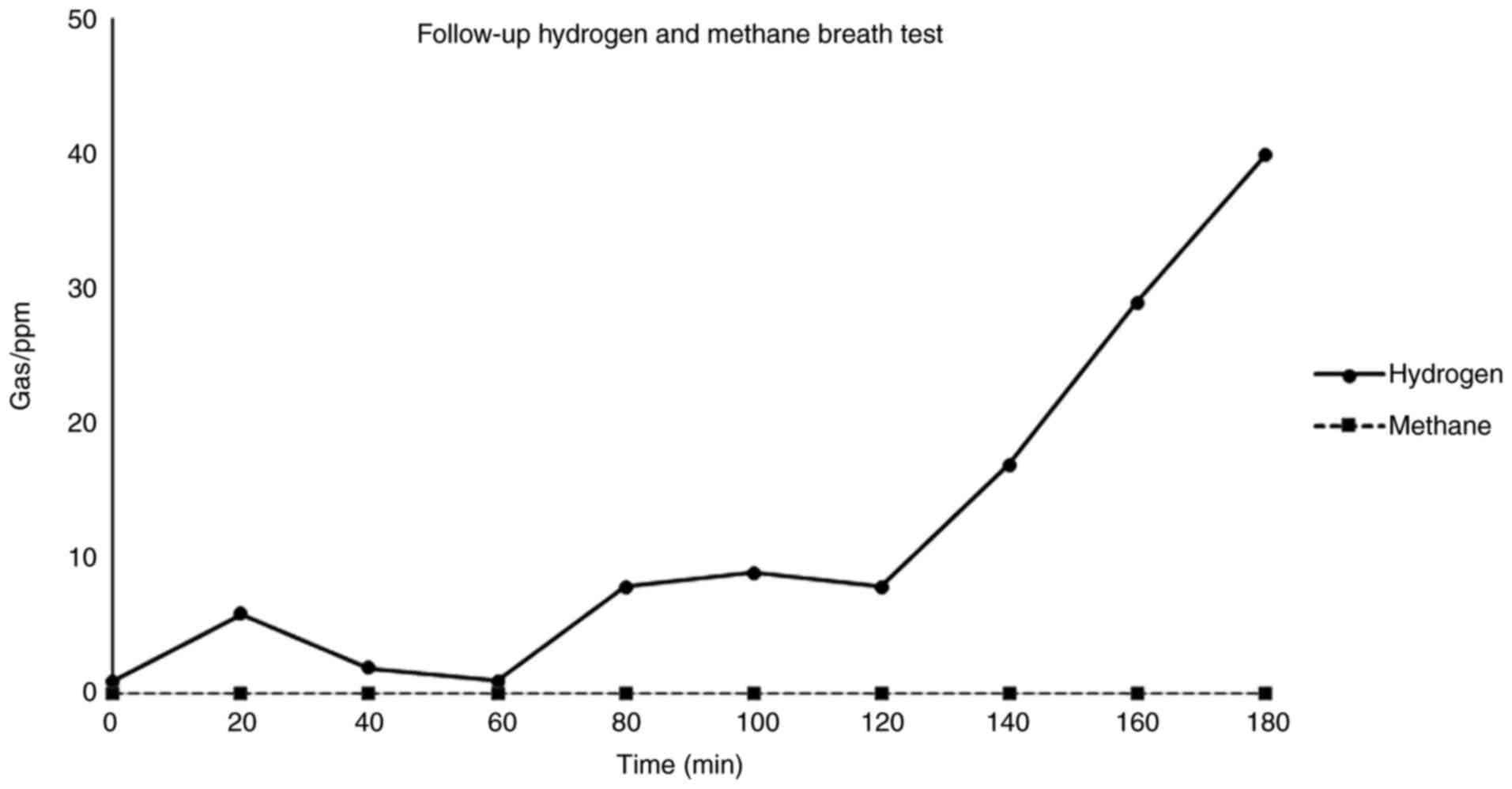
protocol, the patient repeated the breath test within 3 days. The
follow-up breath test revealed a marked improvement (Fig. 3). The result was classified as
negative for SIBO. Hydrogen gas output indicated normal colonic
bacteria and methane was completely absent from the sample
throughout. The patient reported feeling considerably better and
her quality of life global score increased from 42 to 71 (Fig. 1). Specifically, in the categories
of activity and discomfort where she was previously categorised as
extreme, there was a marked improvement, where her digestive
symptoms no longer had an impact on her quality of life. The score
for diet improved to a lesser degree and the score for anxiety did
not improve, as the patient was still apprehensive about
introducing foods to her diet at the time of testing. The patient
had re-introduced wheat, cabbage and had enjoyed a glass of wine.
She was counselled to support re-introducing further high FODMAP
foods slowly and continued to feel well at the 3-month verbal
follow up.
Discussion
Rifaximin is the most commonly used antibiotic in
the treatment of IBS and for the eradication of SIBO. Rifaximin is
poorly absorbed and thus has a low systemic activity; however, it
is effective against Gram-negative and -positive aerobic and
anaerobic bacteria in the digestive tract (20). Clinical studies on SIBO have
reported an improvement in IBS symptoms and the normalisation of
breath tests in 33-86% of participants, although these studies
differ in diagnostic criteria and methods (21-24).
A number of herbal and food-based nutraceuticals have also been
used for a number of years for their antimicrobial effects, such as
garlic, rosemary and cloves (25).
There is an increasing awareness of these herbs and nutraceuticals
amongst the public, and thus there is an increased demand for
complementary approaches to healthcare. In a previous study
comparing a combined herbal therapy and rifaximin in patients with
a positive lactulose breath test, the response rate to herbal
therapy was 46% compared with one of 34% to rifaximin (n=104)
(26). The herbal formula used in
the present case report study, outlined in Table I, was similar to the herbal therapy
used in that study (26).
The low FOMDAP diet is the most widely researched
diet in the management of IBS. Developed by Monash University, it
is low in specific carbohydrates that are categorised as
fermentable oligo-, di-, monosaccharides and polyols (19). It can be used to manage the
symptoms of IBS; however, its long-term use may be associated with
a reduction in dietary quality (27). Only a limited number of studies to
date have clearly demonstrated the effect of a low FODMAP diet
alone on SIBO. In a previous randomised controlled trial, a 21-day
low FODMAP diet did not reduce the area under the curve for
hydrogen on the lactulose breath test (28). In another small, yet well-designed
study, Ong et al (29)
demonstrated that participants (both healthy subjects and patients
with IBS) while on a low FODMAP diet produced less hydrogen than
those on a high FODMAP diet. There was no lactulose administration
during breath testing, which would be a FODMAP challenge. In this
crossover design study, participants who had previously reduced
their hydrogen output on a low FODMAP diet, had an increased
hydrogen output again once they changed to eating a high FODMAP
diet. Their digestive symptoms also returned when they ate a high
FODMAP diet (29).
Another crossover study analysing colonic bacteria
demonstrated that the low FODMAP diet reduced the faecal bacterial
growth of Actinobacteria, Bifidobacterium and
Faecalibacterium prausnitzii (30). Bacterial growth increased again
after a high-fructo-oligosaccharides diet was re-introduced after 3
weeks compared with a placebo. There are thus concerns over the
effects of a low FODMAP diet on colonic bacteria and this is one of
the reasons why a low FODMAP diet is not a suitable long-term
solution for IBS. It is not yet well understood whether a low
FOMDAP diet actually reduces small intestinal bacterial overgrowth,
although the current available evidence in humans would suggest
that it may not, or at least that the effect may be transient.
In the present case report, the low FODMAP diet may
have reduced the symptoms of SIBO in the patient; it was suggested
that the herbal supplement led to the eradication of the bacterial
overgrowth. The improvement in symptoms persisted at 3 months,
following the re-introduction of high FODMAP foods, which was
confirmed verbally at the follow-up consultation. There is no
evidence to suggest that this is a long-term solution and it is
widely understood that IBS is a complex condition which may relapse
or re-occur. SIBO may also not be the only component of the
digestive issues in the patient described herein, although the
improvement in symptoms coincided with the normalisation of the
lactulose breath test. Further studies with larger patient cohorts
are required to investigate the role of SIBO in IBS, to compare the
treatment options, such as commonly available herbal options vs.
antibiotics with and without a low FODMAP diet, and to determine
the long-term outcomes of SIBO treatment.
SIBO is currently under-investigated in patients
with IBS despite possibly being a contributing factor to the
pathology and aetiology. It is important to bear in mind that
testing has limitations; it is recommended that testing be
performed in laboratories that strictly adhere to the best
available guidelines. It also worth noting that SIBO is not
exclusive to IBS, and it is not a factor in all cases of IBS. The
present case report may provide valuable insight into SIBO, as it
demonstrates that the eradication of this condition using a herbal
therapy and a low FODMAP diet reduced the symptoms of IBS and
improved the quality of life of the patient, concurrent with the
normalisation of the lactulose breath test. However, further
studies are warranted to develop an effective treatment
protocol.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CPW assessed the patient, recommended the breath
test and designed the protocol. CPW collected the data and drafted
the manuscript. MTD performed the breath testing and contributed to
the design of the manuscript. HL contributed to the study design
and the final revision of the manuscript. All authors confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The patient signed a consent form for her anonymous
data to be used for the purposes of the present study.
Patient consent for publication
The patient signed a consent form for her anonymous
data to be used in this publication.
Competing interests
MTD is the Director and owner of the GastroLife
clinic. CPW and HL are the directors and owners of Glenville
Nutrition Clinic Ireland.
References
|
1
|
Canavan C, West J and Card T: The
epidemiology of irritable bowel syndrome. Clin Epidemiol. 6:71–80.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Drossman DA and Hasler WL: Rome
IV-Functional GI disorders: Disorders of gut-brain interaction.
Gastroenterology. 150:1257–1261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Camilleri M: Peripheral mechanisms in
irritable bowel syndrome. N Engl J Med. 367:1626–1635.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pimentel M, Chow EJ and Lin HC:
Eradication of small intestinal bacterial overgrowth reduces
symptoms of irritable bowel syndrome. Am J Gastroenterol.
95:3503–3506. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pyleris E, Giamarellos-Bourboulis EJ,
Tzivras D, Koussoulas V, Barbatzas C and Pimentel M: The prevalence
of overgrowth by aerobic bacteria in the small intestine by small
bowel culture: Relationship with irritable bowel syndrome. Dig Dis
Sci. 57:1321–1329. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Carrara M, Desideri S, Azzurro M, Bulighin
GM, Di Piramo D, Lomonaco L and Adamo S: Small intestine bacterial
overgrowth in patients with irritable bowel syndrome. Eur Rev Med
Pharmacol Sci. 12:197–202. 2008.PubMed/NCBI
|
|
7
|
Ghoshal UC, Shukla R and Ghoshal U: Small
intestinal bacterial overgrowth and irritable bowel syndrome: A
bridge between functional organic dichotomy. Gut Liver. 11:196–208.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Pimentel M, Saad RJ, Long MD and Rao SSC:
ACG clinical guideline: Small intestinal bacterial overgrowth. Am J
Gastroenterol. 115:165–178. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Corazza GR, Menozzi MG, Strocchi A,
Rasciti L, Vaira D, Lecchini R, Avanzini P, Chezzi C and Gasbarrini
G: The diagnosis of small bowel bacterial overgrowth. Reliability
of jejunal culture and inadequacy of breath hydrogen testing.
Gastroenterology. 98:302–309. 1990.PubMed/NCBI View Article : Google Scholar
|
|
10
|
American Gastroenterological Association
medical position statement: Guidelines for the evaluation and
management of chronic diarrhea. Gastroenterology. 116:1461–1463.
1999.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Miele L, Valenza V, La Torre G, Montalto
M, Cammarota G, Ricci R, Masciana R, Forgione A, Gabrieli ML,
Perotti G, et al: Increased intestinal permeability and tight
junction alterations in nonalcoholic fatty liver disease.
Hepatology. 49:1877–1887. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kapil S, Duseja A, Sharma BK, Singla B,
Chakraborti A, Das A, Ray P, Dhiman RK and Chawla Y: Small
intestinal bacterial overgrowth and toll-like receptor signaling in
patients with non-alcoholic fatty liver disease. J Gastroenterol
Hepatol. 31:213–221. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dukowicz AC, Lacy BE and Levine GM: Small
intestinal bacterial overgrowth: A comprehensive review.
Gastroenterol Hepatol (NY). 3:112–122. 2007.PubMed/NCBI
|
|
14
|
Saad RJ and Chey WD: Breath testing for
small intestinal bacterial overgrowth: Maximizing test accuracy.
Clin Gastroenterol Hepatol. 12:1964–1972. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rezaie A, Buresi M, Lembo A, Lin H,
McCallum R, Rao S, Schmulson M, Valdovinos M, Zakko S and Pimentel
M: Hydrogen and methane-based breath testing in gastrointestinal
disorders: The north American consensus. Am J Gastroenterol.
112:775–784. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
The Association of Gastrointestinal
Physiology (AGIP) Committee of the British Society of
Gastroenterology (2019). Association of Gastrointestinal
Physiologists (AGIP) Proposed Standardised Testing Protocol for
Hydrogen/Methane Breath Testing (HMBT) to Assess Small Intestinal
Bacterial Overgrowth (SIBO) and Carbohydrate Malabsorption. 2019
British Society of Gastroenterology, (24 June 2019).
|
|
17
|
The European Parliament and the Council of
the European Union Regulation: Regulation (EU) 20-16/679 of the
European Parliament and of the Council of 27 April 2016 on the
protection of natural persons with regard to the processing of
personal data and on the free movement of such data, and repealing
Directive 95/46/EC (General Data Protection Regulation). Official
Journal of the European Union 2018. Available from: https://eur-lex.europa.eu/eli/reg/2016/679/oj.
Accessed at November, 2021.
|
|
18
|
Chassany O, Marquis P, Scherrer B, Read
NW, Finger T, Bergmann JF, Fraitag B, Geneve J and Caulin C:
Validation of a specific quality of life questionnaire for
functional digestive disorders. Gut. 44:527–533. 1999.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Monash University: The Low FODMAP Diet.
Online May 2, 2019. https://www.monashfodmap.com/. Accessed November 10,
2019.
|
|
20
|
Shayto RH, Abou Mrad R and Sharara AI: Use
of rifaximin in gastrointestinal and liver diseases. World J
Gastroenterol. 22:6638–6651. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pimentel M: Review article: Potential
mechanisms of action of rifaximin in the management of irritable
bowel syndrome with diarrhoea. Aliment Pharmacol Ther. 43 (Suppl
1):S37–S49. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Meyrat P, Safroneeva E and Schoepfer AM:
Rifaximin treatment for the irritable bowel syndrome with a
positive lactulose hydrogen breath test improves symptoms for at
least 3 months. Aliment Pharmacol Ther. 36:1084–1093.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sharara AI, Aoun E, Abdul-Baki H, Mounzer
R, Sidani S and Elhajj I: A randomized double-blind
placebo-controlled trial of rifaximin in patients with abdominal
bloating and flatulence. Am J Gastroenterol. 101:326–333.
2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pimentel M, Park S, Mirocha J, Kane SV and
Kong Y: The effect of a nonabsorbed oral antibiotic (rifaximin) on
the symptoms of the irritable bowel syndrome: A randomized trial.
Ann Intern Med. 145:557–563. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lai PK and Roy J: Antimicrobial and
chemopreventive properties of herbs and spices. Curr Med Chem.
11:1451–1460. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chedid V, Dhalla S, Clarke JO, Roland BC,
Dunbar KB, Koh J, Justino E, Tomakin E and Mullin GE: Herbal
therapy is equivalent to rifaximin for the treatment of small
intestinal bacterial overgrowth. Glob Adv Health Med. 3:16–24.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Staudacher HM, Ralph FSE, Irving PM,
Whelan K and Lomer MCE: Nutrient intake, diet quality, and diet
diversity in irritable bowel syndrome and the impact of the low
FODMAP Diet. J Acad Nutr Diet. 120:535–547. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
McIntosh K, Reed DE, Schneider T, Dang F,
Keshteli AH, De Palma G, Madsen K, Bercik P and Vanner S: FODMAPs
alter symptoms and the metabolome of patients with IBS: A
randomised controlled trial. Gut. 66:1241–1251. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ong DK, Mitchell SB, Barrett JS, Shepherd
SJ, Irving PM, Biesiekierski JR, Smith S, Gibson PR and Muir JG:
Manipulation of dietary short chain carbohydrates alters the
pattern of gas production and genesis of symptoms in irritable
bowel syndrome. J Gastroenterol Hepatol. 25:1366–1373.
2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hustoft TN, Hausken T, Ystad SO, Valeur J,
Brokstad K, Hatlebakk JG and Lied GA: Effects of varying dietary
content of fermentable short-chain carbohydrates on symptoms, fecal
microenvironment, and cytokine profiles in patients with irritable
bowel syndrome. Neurogastroenterol Motil: Oct 16, 2016 (Epub ahead
of print). doi: 10.1111/nmo.12969.
|