1. Introduction
Parkinson's disease (PD) is the second most common
neurodegenerative disease with a high morbidity that affects nearly
2% of the elderly (1). PD is
neuropathologically characterized by progressive loss of
dopaminergic neurons in the substantia nigra (SN), accompanied by
accumulation of Lewy bodies (LBs) and neurites, whose main
component are aggregated α-synuclein (encoded by the SNCA gene)
leading to nigrostriatal neurodegeneration and clinical
manifestations, including motor dysfunctions and non-motor
symptoms, such as cognitive deficits and sleep disorders (1). Several cytotoxic stimuli, like
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) or
1-methyl-4-phenylpyridinium iodide (MPP+),
6-hydroxydopamine (6-OHDA) and rotenone are employed to simulate
the pathogenesis of PD in vivo and in vitro (2). Current treatments alleviate symptoms
in patients with early-stage PD but fail to halt disease
progression or reverse existing disabilities. Thus, elucidation of
the pathogenesis is crucial for developing novel and promising
therapeutic modalities in PD. Autophagy is an evolutionarily
conserved self-digesting process that functions as a cytoprotective
cellular machinery to recycle misfolded proteins and damaged
organelles to produce energy for cell survival under stressful
conditions, such as nutrient deficiency and hypoxia (3). Aggregation of α-synuclein in PD
brains has been suggested to be attributed to impaired
autophagic-lysosomal degradation (4). In addition, dopaminergic neurons are
susceptible to deficient clearance of damaged mitochondria due to
their hypermetabolism with high mitochondrial energy demand
(5). Accumulation of damaged
mitochondria owing to autophagy impairment leads to reactive oxygen
species (ROS) overproduction, which further augments mitochondrial
dysfunction and causes oxidative damage to neurons, facilitating PD
progression in a vicious cycle (6,7).
Autophagy dysfunction has been implicated in the pathological
processes of PD, including aberrant protein aggregation,
mitochondrial dysfunction, oxidative stress, neuroinflammation and
neuronal apoptosis (6,8). Thus, autophagy mainly acts as a
protective factor in PD. Understanding the role of autophagy in PD
progression is of great significance, and the regulation of
autophagy provides a novel potential therapeutic target for this
disease.
Non-coding RNAs (ncRNAs) mainly comprise microRNA
(miRNA), long ncRNA (lncRNA) and circular RNA (circRNA). miRNAs are
a group of small endogenous, single-stranded molecules with lengths
of 21-25 nucleotides that regulate gene expression by binding to
the 3'-untranslated region of a target gene mRNA, thus suppressing
its translation or promoting its degradation (9). The miRNA-mediated gene regulation
can be also modulated by lncRNA and circRNA, which act as competing
endogenous RNAs to sponge miRNAs (10). Abnormal expression of miRNAs in PD
has been found to regulate autophagy via affecting the expression
of autophagy-related genes (ATGs) and autophagy-related signaling
molecules, and miRNA-mediated autophagy is involved in pathological
processes, such as α-synuclein and mitochondrial dysfunction,
implicated in the pathogenesis of PD (11,12). Hence, targeting miRNAs to modulate
the functional status of autophagy may provide promising
therapeutic strategies for this disease. The present review
summarizes the autophagy process and autophagy-related signaling
pathways in PD and discusses the role of autophagy in the
pathogenesis of this disease. It emphasizes the paradoxical effects
of miRNAs through regulation of autophagy, and concludes the
potential of targeting autophagy-regulating miRNAs as promising
interventions for patients with PD.
2. Autophagy in PD
Autophagic process
Autophagy generally contains macro-autophagy,
micro-autophagy and chaperone-mediated autophagy (13). Macro-autophagy is simply referred
to as autophagy due to the extensive research in this area.
Specific conditions such as starvation, hypoxia and inflammation,
are responsible for autophagy induction (14). The autophagic process consists of
initiation, elongation, maturation, fusion and degradation, all of
which are finely regulated by various ATGs and autophagy-related
signaling pathways (Fig. 1). The
inactivation of mammalian target of rapamycin (mTOR) induces the
formation of the unc-51-like kinase 1 (ULK1) complex, containing
ULK1-ATG13-ATG101-FIP200, which initiates autophagy (15). The complex containing
Beclin-1-AMBRA 1-ATG14L-VPS15-VPS34 recruits the PI3K-ATG2-ATG18
complex to facilitate lipid transport through the transmembrane
protein, ATG9, as well as recruit the ATG5-ATG12-ATG16L1 complex to
trigger autophagic membrane elongation (15). The ATG5-ATG12-ATG16L1 complex also
interacts with the ATG3-ATG7 complex to promote the binding of
microtubule associated protein light chain 3 (LC3)-II. LC3-II is
derived from the cleavage of LC3 by cysteine protease ATG4, and
combines with phosphatidylethanolamine (PE) to produce
PE-conjugated LC3-II, which forms a mature autophagosome and
further combines with cargo receptors, such as p62, to anchor
autophagic substrates (16).
Finally, the autophagosome fuses with the lysosome to form the
autolysosome, where substances are degraded and recycled for
cellular metabolism and growth. In addition, the autophagy of
mitochondria, also known as mitophagy, is mainly responsible for
the clearance of aging and damaged mitochondria ensuring the
metabolic equilibrium of mitochondrial energy.
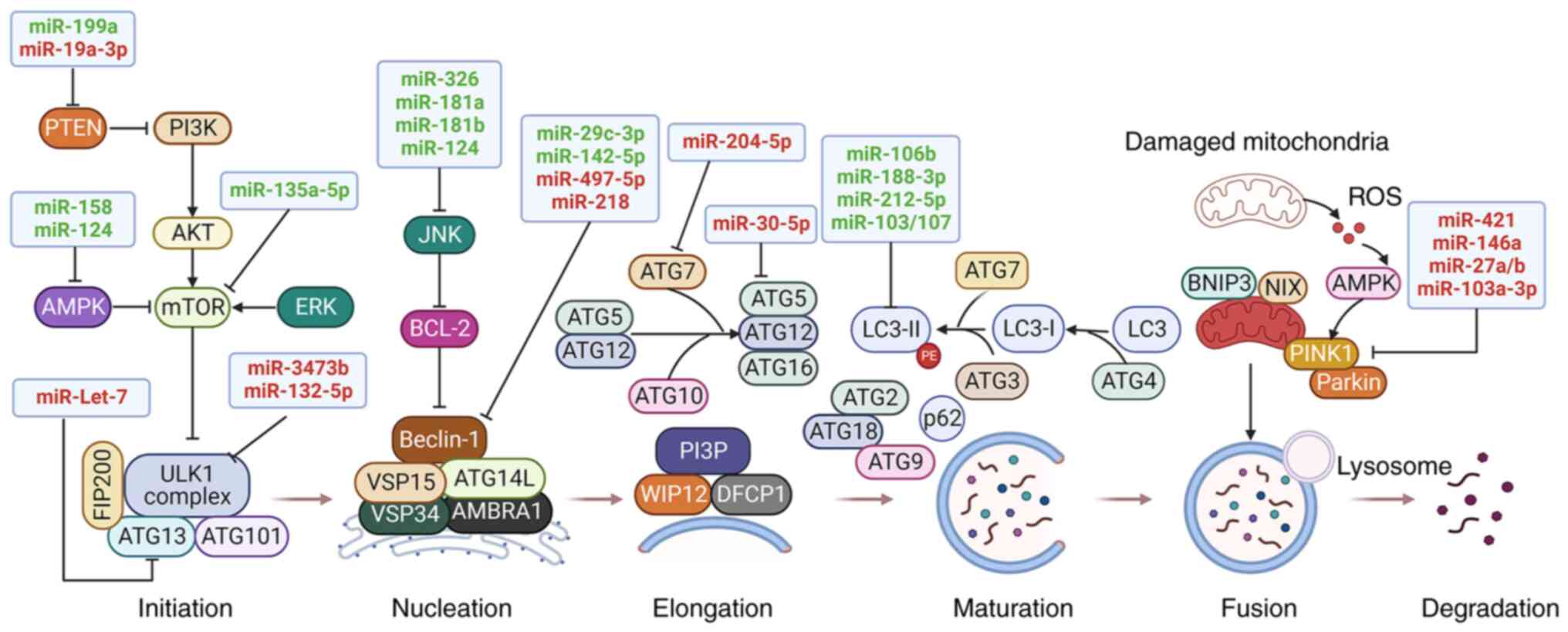 | Figure 1The regulatory role of miRNAs on the
autophagy process in PD. Autophagy-related proteins and signaling
pathways are regulated by miRNAs during each stage of autophagy,
including initiation and nucleation, elongation, maturation, fusion
and degradation. Both neuroprotective miRNAs (green) and neurotoxic
miRNAs (red) are involved in the regulation of autophagy by
targeting autophagy-related proteins and signaling pathways, thus
affecting the pathogenesis of PD. → indicates a promoting effect
and ┴ indicates an inhibitory effect. AMPK, adenosine
monophosphate-activated protein kinase; ATG, autophagy-related
gene; ERK, extracellular signal-regulated kinase; JNK, c-Jun
N-terminal kinase; miR, microRNA; miRNA, microRNA; mTOR, mammalian
target of rapamycin; PD, Parkinson's disease; PINK1, PTEN-induced
putative kinase protein 1; PTEN, phosphatase and tensin homolog;
ROS, reactive oxygen species; ULK1, unc-51-like kinase 1. |
A variety of signaling pathways are involved in the
regulation of autophagy. The inactivation of the mTOR signaling
pathway is the key step to stimulate autophagy, which can be
further regulated by various signaling pathways, such as PI3K/AKT,
adenosine monophosphate-activated protein kinase (AMPK), mitogen
activated kinase-like protein (MAPK), and phosphatase and tensin
homolog (PTEN) pathways (17).
PI3K phosphorylates phosphatidylinositol-4,5-bisphosphate to
generate phosphatidylinositol 3-phosphate, which induces AKT
phosphorylation, and subsequently mTOR is activated to suppress
autophagy (18). Under stress
conditions, such as cellular energy deficiency, the expression
level of AMPK is elevated to phosphorylate ULK1 for the activation
of autophagy, and to inactivate mTOR for the indirect induction of
autophagy (19). As a crucial
regulator of the autophagic process, BCL2 interacts with Beclin-1
and further abrogates the formation of the autophagosome (20). c-Jun N-terminal kinase (JNK), a
subtype of MAPK, phosphorylates BCL2, leading to the dissociation
of Beclin-1 from BCL2, thus eliciting autophagy (20). However, extracellular
signal-regulated kinase (ERK), another subtype of MAPK, is
activated to inhibit the formation of Tuberous sclerosis complex 1
and 2 (TSC1-TSC2) complex and subsequently contributes to the
activation of mTOR, which causes the suppression of autophagy
(19). In addition, PTEN has been
demonstrated to inactivate the PI3K/AKT signaling pathway (21). Consequently, the PTEN-mediated
suppression of the AKT signaling pathway enhances autophagy
activity. Furthermore, mitophagy is regulated by several signaling
pathways, including the PTEN-induced putative kinase protein 1
(PINK1)/Parkin, BCL2/adenovirus E1B 19kDa interacting protein
3/NIP3-like protein X (BNIP3/NIX) and FUN14 domain containing 1
(FUNDC1) pathways (6). PINK1 is
located in the mitochondrial outer membrane and mediates E3
ubiquitin ligase Parkin to initiate mitophagy. The BNIP3/NIX
signaling pathway accelerates mitophagy by dissociating
BCL2-Beclin-1, which recruits Parkin to the mitochondrial outer
membrane and participates in the transportation of LC3 to the
mitochondria (6). FUNDC1, a
mitophagy-related protein, can be activated under hypoxic
conditions and thus triggers mitophagy by binding to LC3-II and
regulating the mitochondrial dynamics (22).
Autophagy is an intracellular self-digesting process
by which misfolded proteins and damaged organelles are recycled to
sustain cell metabolism. Dysfunction of autophagy is accompanied by
abnormal activation of autophagy-related signaling pathways, which
further affects the expression of ATGs. Thus, clarifying
autophagy-related signaling pathways and the roles of autophagy in
the pathogenesis of PD may provide potential therapeutic targets to
tackle this disease.
Autophagy-related signaling pathways in
PD
As aforementioned, signaling pathways, including
PI3K/AKT/mTOR, MAPK, AMPK and PINK1/Parkin, can regulate autophagy.
Indeed, these pathways are related to various cellular processes,
such as proliferation and apoptosis, affecting the occurrence and
progression of PD. In the early stage of rotenone-induced PD in
rats, hydrogen-saturated saline postpones motor impairments as it
alleviates the damage of catecholaminergic and nigral dopamine
neurons, and decreases the expression of ROS and α-synuclein by
activating the autophagy machinery through the inactivation of the
PI3K/AKT/mTOR pathway (18). This
signaling pathway is also suppressed in neuroendocrine cells
treated with sodium butyrate, and autophagy is subsequently
promoted, mediating α-synuclein degradation in PD (23). Further investigation demonstrated
that inhibiting the PI3K/AKT/mTOR pathway with a natural compound,
astragalus polysaccharide, enhances the expression of
autophagy-related proteins and formation of the autophagosome, thus
increasing cell viability and exerting anti-Parkinson effects
(24). However, when autophagy is
overactivated in MPP+-treated SH-SY5Y cells, the
addition of IGF-1 decreases autophagic neuron death through the
PI3K/AKT/mTOR pathway (25).
Thus, the PI3K/AKT/mTOR pathway improves neuronal survival by
modulating autophagy in PD. In addition, several common subtypes of
MAPK, including p38 and JNK, can regulate autophagy via interaction
with the mTOR and BCL2 pathways in PD. In zebrafish and PC12 cells
exposed to pyrethroid, the p38 MAPK pathway is activated to inhibit
mTOR, which induces excessive autophagy and thus overproduces the
LBs in neurons that lead to the PD-like symptoms, suggesting the
neurotoxicity of p38 MAPK-mediated autophagy, which is contrary to
the pattern of the effect of autophagy on PD generally reported
(26). The stimulation of
autophagy induced by the JNK/BCL2 pathway after caffeic acid
administration mitigates α-synuclein generation and loss of
dopaminergic neurons in the SN, and improves behavioral
abnormalities in a A53T α-synuclein-induced PD mouse model
(27). By contrast, by
administering β-asarone to 6-OHDA-treated rats, JNK signaling is
blocked, which suppresses autophagy but upregulates the expression
of BCL2 (an anti-apoptotic protein), which alleviates the
α-synuclein accumulation and behavioral impairments (20). These findings indicate that the
bi-directional regulation of autophagy by the JNK/BCL2 axis exerts
neuroprotective effects in PD. Besides, AMPK-mediated autophagy is
also associated with the progression of PD. In both MPTP and
rotenone-induced mouse models of PD, it has been discovered that
the activation of AMPK-mediated autophagy by several compounds,
including mitochonic acid 5 and α-mangostin, ameliorate α-synuclein
aggregation, oxidative stress, neuroinflammation, neuronal
degeneration and motor deficits (28,29). Moreover, inhibitors of AMPK and
autophagy counteract these effects, indicating the neuroprotection
of AMPK-activated autophagy in PD (28,29). Targeting the AMPK/mTOR signaling
pathway to inhibit excessive autophagy induced by 6-OHDA alleviates
neuronal death, exerting a neuroprotective effect on PD (30). Furthermore, restoring
rotenone-induced autophagy impairment via activation of the
AMPK/mTOR/ULK1 signaling cascade attenuates neuronal apoptosis,
α-synuclein accumulation and ROS production, and improves the cell
viability and antioxidant capacity of SH-SY5Y cells (31,32). Therefore, modulating AMPK-induced
autophagy may be a potential therapeutic strategy for PD.
Furthermore, in response to mitochondrial damage, mitophagy
activated by the PINK1/Parkin pathway recycles dysfunctional
mitochondria and improves energy sources (33). Mitophagy induced by salidroside
diminishes DA neuron degeneration and motor deficits by enhancing
the expression of PINK1 and Parkin, conferring neuroprotective
effects in MPP+/MPTP-induced PD models (34); albeit, amplifying the
PINK1/Parkin-mediated mitophagy pathway by mono-2-ethylhexyl
phthalate exacerbates mitochondrial damage accompanied by enhanced
mitochondrial fragmentation and ROS production (35). Hence, the selective autophagic
elimination of damaged mitochondria by PINK1/Parkin-induced
mitophagy is essential for the maintenance of mitochondrial
integrity.
Collectively, autophagy activation can be modulated
by a variety of signaling pathways in PD. As the
autophagy-regulating signaling hub, mTOR interacts with other
pathways such as PI3K/AKT, MAPK and AMPK to indirectly regulate
autophagy, while the PINK1/Parkin pathway plays an essential role
in mitophagy. Further exploring the regulatory mechanisms of
autophagy-related pathways is key to clarifying the pathogenesis of
PD. It should be noted that α-synuclein-activated p38 MAPK is
required for the phosphorylation of Parkin and thus contributes to
mitochondrial dysfunction and neuronal apoptosis in PD (36). Activating AMPK to stimulate
PINK1/Parkin-mediated mitophagy in MPTP-induced PD mice maintains
mitochondrial homeostasis and neuronal survival (37). Thereby, the interplay among
autophagy-related signaling pathways in PD progression is complex
and more detailed studies are needed. In addition,
autophagy-regulating pathways also participate in the induction of
other cell death processes, such as apoptosis, necroptosis and
ferroptosis (38,39). However, the intrinsic crosstalk
between autophagy and these processes, which is simultaneously
regulated by shared upstream pathways, has not been fully
elucidated. Moreover, it is reported that AMPK inhibition in
response to mitochondrial oxidative stress promotes autophagy,
which maintains mitochondrial integrity and survival of SH-SY5Y
cells exposed to MPP+ (40). Thus, the interaction between
autophagy-related signaling pathways and autophagic processes
deserves further investigation for the development of potential
strategies of PD treatment.
Effects of autophagy in PD
Aberrant expression of autophagy-related proteins in
the brain is observed in patients with PD and various cellular and
animal models of PD. Previous studies reported that dysregulated
autophagy occurs in the dopaminergic neurons of the SN in patients
with PD, in which LBs colocalize with upregulated LC3, an
autophagy-related protein that is responsible for autophagosome
formation (41,42). Autophagic impairment, attributed
to the defective removal and excessive accumulation of undegraded
autophagosomes in LBs, causes lysosomal breakdown and the release
of proteases into the cytosol, leading to dopaminergic cell death
in the brain of patients with PD and MPTP-induced mice (43). The ectopic protein signals of
chaperone-mediated autophagy and mitophagy, such as LAMP2A, HSC70
and p-S65-Ub, are also present in PD brain tissues, where they
colocalize with α-synuclein, LBs and tangle aggregations (44,45). Recent studies have discovered a
downregulation of autophagic components, including LC3, Beclin-1,
p62 and ATG5, in peripheral blood cells of patients with PD
(46,47). Abnormal expression of these
autophagy-related proteins in the SN of PD-related mice is
accompanied by both upregulated α-synuclein and clinical symptoms,
such as constipation, olfactory impairment and depression-like
behaviors (48). Therefore, these
findings indicate that autophagy is involved in the pathological
process of PD.
Autophagy is considered to exert a neuroprotective
role in PD, since it can clear neuronal protein aggregates and
impaired mitochondria (3,8). As the major component of LBs,
fibrillar α-synuclein aggregation initiates the propagation of
α-synuclein pathology, which causes intracellular organelle
dysfunction and dopaminergic neuronal death (1). Moreover, these effects are
aggravated via the chloroquine-mediated blockade of autophagy but
are alleviated by rapamycin-induced activation of autophagy
(49). Dopaminergic neurons are
metabolically very active with high mitochondrial energy demand,
and are therefore particularly vulnerable to mitochondrial
dysfunction (5). Mitophagy is
required for maintaining the timely turnover of defective
mitochondria, and its impairment contributes to ROS overproduction
and oxidative stress, accelerating disease progression in PD
(6,7). The accumulation of α-synuclein and
damaged mitochondria is a consequence of the impaired
autophagic-lysosomal degradation system. Upregulation of
α-synuclein compromises autophagy degradation by restraining the
fusion of autophagosomes with lysosomes, which in turn further
facilitates the aggregation and toxicity of α-synuclein (50,51). Excessive α-synuclein expression
has also been demonstrated to interfere with mitophagy and
mitochondrial functions. For instance, α-synuclein upregulates the
expression of Miro protein, an adaptor on the outer mitochondrial
membrane that mediates mitochondrial motility, which contributes to
abnormal accumulation of Miro in damaged mitochondria, deferring
mitochondrial clearance via mitophagy (52). Mutant α-synuclein disturbs
mitochondrial dynamics and fusion, and causes pathological changes
in mitochondrial morphology (53). Additionally, multiple protein
products encoded from PD-related genes are related to autophagy
regulation. The leucine-rich repeat kinase 2 (LRRK2) gene
mutations, which are linked to familial and sporadic PD, enhance
the accumulation of large autophagic vacuoles and perturb
autophagic clearance of protein aggregates along with the induction
of dysfunctional expression of autophagic receptors p62 and
optineurin, leading to impairment of mitophagy, chaperone-mediated
autophagy and lysosomal function (54,55). Mutations in the PRKN and PINK1
genes (encoding parkin and PINK1, respectively) also impair the
autophagic process and are related to autosomal recessive PD
(56,57). The downregulation of PINK1
decreases both starvation-induced autophagy and mitophagy (56,57). Mutation of the GBA gene that
encodes β-glucocerebrosidase, which shuttles between the
endoplasmic reticulum and the lysosomal lumen, promotes lysosomal
dysfunction and α-synuclein pathology in PD through blockade of
chaperone-mediated autophagy (4,58).
Furthermore, microglia-mediated neuroinflammation has been
implicated in autophagy abnormality. Deficiency of DJ-1 in
microglia impairs the autophagy-dependent degradation of p62 and
LC3 proteins, which decreases the phagocytosis of α-synuclein by
microglia (59). Excessive
α-synuclein expression, in turn, represses microglial autophagy
activity and further exacerbates inflammatory responses,
dopaminergic neuron losses and locomotor deficits (60), resulting from NLRP3 inflammasome
activation induced by microglial autophagy sabotage (61).
In summary, as an essential degradation process,
autophagy is responsible for the elimination of aggregated
α-synuclein and damaged mitochondria, thus conferring a
neuroprotective role in PD. The excessive accumulation of
α-synuclein and mutations in PD-related genes (LRRK2, PINK1, PRKN
and GBA) impair the process of autophagy, which in turn enhances
aggregation of α-synuclein and other pathogenic proteins in the PD
brain, exacerbating neuronal inflammation and degeneration and
forming a vicious circle in the pathogenesis of PD. Thus, induction
of protective autophagy has been regarded as a promising strategy
for PD treatment. However, studies have suggested that
overactivation of autophagy causes autophagic neuronal death owing
to the overwhelmed lysosomal and mitochondrial clearance that may
stress the cells (62,63). Future investigations should be
performed to estimate the level of autophagy that is within a safe
and efficacious range. Rather, deficient elimination of toxic
α-synuclein suppresses autophagy, and further aggravates
α-synuclein accumulation, leading to a pathological feedforward
loop in PD (51,52). Therefore, identifying the
molecular mechanism by which α-synuclein affects autophagy may
provide strategies for autophagy restoration. It has been suggested
that increased autophagy may also act as a cellular compensatory
mechanism to other blocked degradation pathways (64). In fact, impairment of the
autophagy-lysosomal pathway can be alleviated by the inhibition of
the ubiquitin-proteasome system in neurotoxin-induced dopaminergic
cell death (64). Further
dissecting the dynamic interplay between autophagy and other
protein degradation pathways is crucial for the progression of
PD.
3. Roles of autophagy-regulating miRNAs in
PD
According to previous studies (65-68), miRNAs play a vital role in the
regulation of autophagy-related genes and signaling pathways, thus
the abnormal expression of miRNAs can affect the pathological
process of PD by modulating autophagy (Fig. 1).
Neuroprotection of miRNAs by regulating
autophagy
miRNAs have been demonstrated to participate in the
pathological process of PD by activating autophagy (65-71). For instance, the expression of
miR-326 decreases in the absence of PINK1, a PD-related gene
(65). Administration of a
miR-326 mimic to MPTP-treated mice diminishes the levels of
α-synuclein and inducible nitric oxide synthase, and mitigates the
locomotory impairment of the mice by promoting autophagy of
dopaminergic neurons through the activation of JNK signaling by
inhibiting XBP1 (66). Clearance
of aggregated α-synuclein is also mediated by miR-4813-3p.
miR-4813-3p is downregulated in a transgenic Caenorhabditis elegans
model of PD, where excessive α-synuclein contributes to oxidative
neuronal damage (67).
miR-4813-3p generally mobilizes protein quality control machinery,
including the autophagosome-lysosomal-pathway and the
ubiquitin-proteasomal-system, for the clearance of misfolded and
aggregated proteins, suggesting the potential of targeting
miR-4813-3p in PD treatment (67). Besides, delivery of miR-106b by
mesenchymal stem cells-derived extracellular vesicles rescues the
neuronal apoptosis induced by MPTP, and enhances autophagy by
downregulating CDKN2B, a gene encoding a protein that promotes
G1-phase cell cycle arrest, which is accompanied by the increased
BCL2 but decreased BAX expression (68). Sun et al (69) demonstrated that miR-212-5p is
expressed at a low level in SH-SY5Y cells and the midbrain of PD
animal models, and stereotactic injection of miR-212-5p mimics into
the midbrain alleviates the loss of dopaminergic neurons by
inhibiting sirtuin2 and further activating autophagy via decreasing
cytoplasmic p53 expression. In addition, in the
MPP+-treated SH-SY5Y cells and MPTP-treated mice, there
existed a decreased expression of miR-124, autophagosome
accumulation and lysosomal depletion. Upregulating miR-124 by using
its agonists and mimics decreases the loss of dopaminergic neurons
and elevates the level of striatal dopamine via restoring the
impaired autophagy process, simultaneous suppressing BIM expression
and thus BAX translocation to the mitochondria (70). Further investigation on the
protective mechanism of miR-124 revealed that exogenous
transferring of miR-124 into the SN of MPTP-treated mice inhibits
the release of proinflammatory cytokines from activated microglia
by suppressing the expression of p62 and p38 (71). These findings indicate that
downregulated expression of miRNAs in the brain of animal PD models
is associated with α-synuclein aggregation, oxidative stress,
neuronal apoptosis and neuroinflammation, and upregulation of these
miRNAs alleviates these effects by inducing autophagy. Therefore,
regulating autophagy by miRNAs could become a promising therapeutic
strategy for PD.
It is reported that miR-142-5p is downregulated in
6-OHDA-treated SH-SY5Y cells, and its upregulation enhances
neuronal vitality by suppressing Beclin-1-dependent autophagy,
implying a neuroprotective role of miR-142-5p in the progression of
PD (72). In addition to
targeting ATGs, miRNAs have been implicated in the regulation of
autophagy-related signaling pathways in PD. For example, miR-199a
level is decreased in MPP+-treated PC12 cells, and
transfection of miR-199a mimics improves neuronal viability and
survival via activating the PTEN/AKT/mTOR signaling pathway
(21). This signaling pathway is
also activated after overexpressing miR-181b in the same
experimental settings, and autophagy is subsequently suppressed,
which alleviates the cytotoxicity of MPP+ (73). The mTOR/ULK1/S6K1 signal
transduction cascade is also blocked by miR-135a-5p in
MPP+-treated SH-SY5Y and CHP-212 cells, thus attenuating
MPP+-induced neuronal autophagy and apoptosis (74). Analogously, miR-185 is
downregulated in SH-SY5Y cells cultured with MPP+, and
its upregulation impedes cell apoptosis and autophagy by
inactivating the AMPK/mTOR signaling pathway (75). Likewise, the expression of
miR-181a is decreased by MPP+ in SK-N-SH cells, and
overexpression of miR-181a decreases the rate of cell apoptosis via
abrogating activation of the p38MAPK/JNK pathway (76). These results suggest that miRNAs
play a protective role in PD progression through inactivation of
autophagy by targeting various autophagy-related signaling
pathways. It is also worth noting that miRNAs can affect end-stage
autophagy by regulating the cyclin-dependent kinase 5 (CDK5)
signaling pathway, which has been demonstrated to induce
MPTP-induced neuronal death (77). Li et al (78) demonstrated that in both
MPP+-treated MN9D cells and MPTP-treated mice, autophagy
is blocked by downregulating miR-103/107 and further activating the
CDKR5 signaling pathway, while HMGA1 is elevated to sustain the
expression of miR-103/107, forming a negative feedback loop between
HMGA1 and miR-103/107, which drives neuroprotection through
autophagy modulation. Moreover, CDK5-mediated autophagy is
suppressed by the injection of miR-188-3p-enriched exosomes,
derived from adipose-derived stem cells, into MPTP-induced mice,
which abrogates α-synuclein aggregation, NLRP3-induced
inflammasomes and neuronal damage in SN (79). These findings indicate that
CDK5-mediated autophagy might be an enlightening target in PD
treatment. Moreover, miR-29c-3p has been demonstrated to repress
microglial NLRP3 inflammasome activation and neuronal apoptosis in
PD models (80). Further
mechanistic evaluation has indicated that upregulation of
miR-29c-3p inhibits autophagy by decreasing the expression of
ten-eleven translocation 2, thus alleviating MPTP-mediated loss of
dopaminergic neurons in SN (81).
In conclusion, neuroprotective miRNAs are
downregulated in various cellular and animal PD models, and
upregulation of these miRNAs can alleviate α-synuclein pathology,
neuroinflammation, neuronal oxidative damage and apoptosis,
accompanied by increased or decreased levels of autophagy, by
targeting ATGs and autophagy-related signaling pathways (Table I). As aforementioned, it is
generally acknowledged that enhanced autophagy delays PD
progression in most cases owing to its neuroprotective feature.
However, some neuroprotective miRNAs are associated with low levels
of autophagy. Notably, the same miRNA appear to exert
neuroprotective effects on the PD progression with different
autophagy levels. By taking miR-124 as an example, several studies
have demonstrated that miR-124 elicits autophagy to inhibit
microglial activation and neuronal death in vivo and in
vitro (70,71), whereas in another study, it is
demonstrated that miR-124 suppress both autophagy and neuronal
apoptosis (82). The exact
mechanisms of these phenomenon remain elusive. It should be noted
that PD stimuli, such as MPP+, are known to induce
autophagy in a concentration-dependent manner, and excessive
autophagy attributed to high concentration or prolonged activation
of PD toxins may trigger autophagic neuronal death (21,73). Thus, one explanation may be that
upregulation of these miRNAs acts as an adaptive response to block
autophagic neuronal death. In this regard, future experimental
settings should take the concentration of PD-related stimulus and
the different models of PD into consideration as the dose of PD
stimulus treatment could affect the regulation of miRNAs on
autophagy during disease progression. Hence, future experimental
designs should estimate the concentration gradient of PD stimulus,
for selecting the optimal concentration to simulate PD pathology.
Moreover, administering these neuroprotective miRNAs in patients
with PD may provide a potential therapeutic strategy by modulating
autophagy. Currently, the locked-nucleic-acids-modified
oligonucleotides targeting miR-122 method is being employed in
Phase I clinical trials for hepatitis C virus infection (83). Further exploring miRNA-based
therapeutic approaches, such as miRNA-coated nanoparticles and
miRNA microinjection, are expected to treat PD.
 | Table INeuroprotective effects of
autophagy-regulating miRNAs in Parkinson's disease. |
Table I
Neuroprotective effects of
autophagy-regulating miRNAs in Parkinson's disease.
| First author,
year | miRNA | Expression | Autophagy-related
target | Autophagy | Outcome | Experimental
model | (Refs.) |
|---|
| Qin, 2022 | miR-135a-5p | Down | mTOR/ULK1/S6K1 | Inhibition | Protecting
MPP+-induced neuronal cell death |
MPP+-treated SH-SY5Y and CHP
212 cells | (74) |
| Bai, 2021 | miR-106b | Down | CDKN2B, LC3-II | Activation | Alleviating
neuronal apoptosis | MPTP-treated mice
and mouse primary hippocampal neurons | (68) |
| Wang, 2021 | miR-29c-3p | Down | TET2, Beclin-1,
LC3-II, p62 | Inhibition | Protecting
inflammation-mediated neuronal damage | MPTP-treated mice
and MPP+-treated SH-SY5Y cells | (81) |
| Li, 2021 | miR-188-3p | Down | CDK5, LC3-II,
p62 | Inhibition | Increasing cell
proliferation | MPTP-treated mice
and MPP+-treated MN9D cells | (79) |
| Li, 2021 | miR-103/107 | Down | CDKR51/CDK5,
LC3-II | Inhibition | Reducing neuronal
cell death | MPTP-treated mice
and MPP+-treated MN9D cells | (78) |
| Ba, 2020 | miR-199a | Down | PTEN/AKT/mTOR,
LC3-II, Beclin-1 | Inhibition | Elevating cell
viability and survival |
MPP+-treated PC12 cells | (21) |
| Chen, 2020 | miR-142-5p | Down | Beclin-1, LC3-II,
p62 | Inhibition | Enhancing cell
vitality | 6-OHDA-treated
SH-SY5Y cells | (72) |
| Zhao, 2019 | miR-326 | Down | XBP1/JNK,
LC3-II | Activation | Improving the
behavioral symptoms in mice and the clearance of α-synuclein | MPTP-treated
mice | (66) |
| Sun, 2018 | miR-212-5p | Down | SIRT2, p53, LC3-II,
p62 | Activation | Ameliorating
neuronal apoptosis and degeneration | MPTP-treated mice
and MPP+-treated SH-SY5Y cells | (69) |
| Li, 2018 | miR-181b | Down | PTEN/AKT/mTOR | Inhibition | Increased cell
viability |
MPP+-treated PC12 cells | (73) |
| Wen, 2018 | miR-185 | Down | AMPK/mTOR,
Beclin-1, LC3-II | Inhibition | Inhibit
dopaminergic cell apoptosis |
MPP+-treated SH-SY5Y cells | (75) |
| Liu, 2017 | miR-181a | Down | p38 MAPK/JNK,
Beclin-1, LC3-II | Inhibition | Reducing neuronal
cell apoptosis |
MPP+-treated SK-N-SH cells | (76) |
| Yao, 2019 | miR-124 | Down | p62, p38 | Activation | Attenuating the
activation of microglia and neuronal apoptosis | MPTP-treated mice
and LPS-treated BV2 cells | (71) |
| Wang, 2016 | miR-124 | Down | LC3-II | Activation | Attenuating
mitochondria-dependent apoptotic cell death and
neurodegeneration |
MPP+-treated SH-SY5Y cells and
MPTP-treated mice | (70) |
| Gong, 2016 | miR-124 | Down | AMPK/mTOR,
Beclin-1, LC3-II | Inhibition | Decreasing cell
apoptosis |
MPP+-treated SH-SY5Y and
SK-N-SH cells | (82) |
Neurotoxicity of miRNAs by regulating
autophagy
Although miRNAs have been shown to be
neuroprotective in PD, increasing the level of miRNAs have been
implicated in disease exacerbation through suppression of
autophagy. It has been demonstrated that miR-204-5p is highly
expressed in both the serum and brain tissues of an MPTP-induced
animal model of PD, and it augments the levels of α-synuclein and
tau in SN, and further causes autophagy impairment and cell death
by disturbing ATG7-regulated autophagy process and JNK-mediated
apoptotic cascade in dopaminergic cells (84). The miR-30c-5p/ATG5 axis, playing a
detrimental role in PD progression, contributes to a decrease in
antioxidants (malondialdehyde, catalase and superoxide dismutase),
dopamine and its metabolites, and neuronal apoptosis, which
aggravates motor deficits in MPTP-treated mice (85). Likewise, knockdown of miR-let-7,
which is upregulated in a C. elegans model of PD, decreases
α-synuclein expression, ROS-mediated oxidative stress and motor
function via suppressing the ATG13-related autophagy (11). Evidence also demonstrates that
silencing of miR-497-5p, which is expressed at high level in PD
models, plays a protective role in MPP+-treated SH-SY5Y
cells by inhibiting cell apoptosis and promoting autophagy via
upregulation of fibroblast growth factor-2, a neurotrophic factor
that serves as a regulator of p62 in an AKT pathway-dependent
manner (86,87). These findings demonstrate that
upregulated miRNAs in PD exacerbate the disease progression through
inhibition of neuronal autophagy via targeting various ATGs.
Nevertheless, miRNAs can affect microglial autophagy and thus
participate in inflammation in PD. Lv et al (88) demonstrated that the expression of
miR-3473b is elevated in brain tissues of MPTP-treated mice and
increases the inflammatory reaction. Transfection with a miR-3473b
mimic promotes the microglial secretion of inflammatory factors
TNF-α and IL-1β by downregulating the TREM2/ULK1 expression and
thus inhibiting autophagy (88).
Similarly, the increased level of miR-19a-3p in the exosomes of
SH-SY5Y cells transfected with the α-synuclein gene, has been
demonstrated to represses microglial autophagy by targeting the
PTEN/AKT/mTOR signaling pathway, possibly affecting α-synuclein
phagocytosis and inflammation activation in microglia, suggesting
an autophagy-related neurotoxicity of miR-19a-3p in PD (89).
Furthermore, aberrant expression of miRNAs is linked
to the pathogenesis of PD by targeting mitophagy-related signaling
pathways. Under chronic mitochondrial stress, miR-27a/b are
upregulated as an adaptive response to prevent the induction of
mitophagy by suppressing the expression of PINK1, which hinders
lysosomal degradation of damaged mitochondria (90). PINK1-mediated mitophagy is also
inhibited by miR-421 in both PD cells and animal models, and
mitochondrial autophagy is disrupted, leading to ROS-mediated
oxidative damage to neurons (12). In addition, in rotenone-treated
SH-SY5Y cells, miR-146a is increased by NF-kB-mediated
transcriptional activation, and further decreases the level of
mitophagy by inhibiting Parkin, resulting in accumulation of
dysfunctional mitochondria and ROS overproduction in degenerating
neurons (91). Consistently,
depletion of miR-103a-3p, which is a highly expressed in
MPTP-induced PD models, alleviates the loss of dopaminergic neurons
in SN and improves gait disorders via activating the
Parkin/Ambra1-mediated mitophagy (92). Thus, these results reveal that
miRNA-induced dysfunction of mitophagy is a critical pathogenic
mechanism in PD. Besides, increased miR-320a level in PD brains has
been demonstrated to suppress chaperone-mediated autophagy by
targeting heat shock protein 70, facilitating α-synuclein
intracellular accumulation (93,94).
It can be concluded that almost all reported miRNAs
in this review are upregulated in PD models and exert neurotoxic
effects through participation in various pathological process,
including α-synuclein accumulation, mitochondrial dysfunction and
microglial activation by inhibiting autophagy (Table II). However, a contradictory
study demonstrates that inhibition of miR-132-5p, which is
upregulated in MPTP-induced PD models, decreases neuronal apoptosis
and autophagy via direct targeting of ULK1, suggesting the
enhancement of autophagy in miR-132-5p-mediated neurotoxicity in PD
(95). One explanation for this
may be that miR-132-5p is involved in MPTP-stimulated
overactivation of autophagy in neurons, ultimately leading to
autophagic cell death. In addition, miRNAs regulate autophagy by
crosstalk with apoptotic cell death pathways. For example, Beclin-1
upregulation induced by miRNAs has been demonstrated to enhance the
activity of autophagy and simultaneously prevent apoptosis
(3). Thus, further clarifying the
role of miRNA-regulating autophagy is vital for understanding the
pathogenesis of PD. Besides, these neurotoxic miRNAs may affect PD
progression through many targets. By taking miR-30c-5p as an
example, it is reported that miR-30c-5p antagomir decreases
neuronal apoptosis and induces autophagy in brain tissues of
MPTP-treated mice (85), while
certain studies demonstrate that both immune reactions and
pyroptosis, which are associated with the development of PD, can
also be modulated by miR-30c-5p (96,97). Therefore, further investigation is
needed to fully determine the main target of miRNAs in PD.
Otherwise, miRNAs confer paradoxical effects in PD. For instance,
the expression of miR-128 is downregulated in PD mice and its
upregulation rescues neurons from apoptosis, thereby playing a
protective role in PD (98).
However, miR-128 also acts as a negative regulator of transcription
factor EB, which is a major transcriptional regulator of the
autophagy-lysosome pathway, and thus fails to clear α-synuclein
oligomers, aggravating the α-synuclein toxicity in PD (99). It can be assumed that the
neurotoxic miRNA regulation of autophagy in PD progression is
intricate due to its interaction with various pathological
processes. The interplay between miRNA-regulated autophagy and PD
pathogenesis has not yet been fully elucidated.
 | Table IINeurotoxic effects of
autophagy-regulating miRNAs in Parkinson's disease. |
Table II
Neurotoxic effects of
autophagy-regulating miRNAs in Parkinson's disease.
| First author,
year | miRNA | Expression | Autophagy-related
target | Autophagy | Outcome | Experimental
model | (Refs.) |
|---|
| Dong, 2022 | miR-421 | Up | PINK1/Parkin,
LC3-II | Inhibition | Promoting ROS
overproduction and neuronal death | MPTP-treated mice
and MPP+-treated SH-SY5Y cells | (12) |
| Zhu, 2021 | miR-497-5p | Up | FGF2, Beclin-1,
LC3-II, p62 | Inhibition | Inducing cell
apoptosis and mice bradykinesia | MPTP-treated mice
and MPP+-treated SH-SY5Y cells | (86) |
| Zhang, 2019 | miR-30c-5p | Up | ATG5, LC3-II | Inhibition | Aggregating cell
apoptosis and behavioral symptoms in mice | MPTP-treated mice
and MPP+-treated SH-SY5Y cells | (97) |
| Lv, 2021 | miR-3473b | Up | TREM2, ULK1,
LC3-II | Inhibition | Promoting the
microglia-mediated inflammatory responses | MPTP-treated mice
and LPS-treated BV2 cells | (88) |
| Zhou, 2020 | miR-103a-3p | Up | Parkin/Ambra1,
LC3-II | Inhibition | Reducing cell
viability and exacerbating neurodegeneration in mice | MPTP-treated mice
and MPP+-treated SH-SY5Y cells | (92) |
| Zhao, 2020 | miR-132-5p | Up | ULK1, Beclin-1 | Activation | Decreasing cell
survival ability and increasing apoptosis | MPTP-treated mice
and MPP+-treated SH-SY5Y cells | (95) |
| Jauhari, 2020 | miR-146a | Up | Parkin | Inhibition | Causing
accumulation of mitochondria and overproduction of ROS | Rotenone-treated
SH-SY5Y cells | (91) |
| Zhou, 2019 | miR-19a-3p | Up | PTEN/AKT/mTOR,
LC3-II, p62 | Inhibition | Impairing the
phagocytosis and degradation of α-synuclein in microglia | SH-SY5Y cells
overexpressing the α-synuclein gene | (89) |
| Chiu, 2019 | miR-204-5p | Up | DYRK1A, Beclin-1,
ATG7, ATG16L1, LC3-II | Inhibition | Inducing the death
of dopaminergic cells | MPTP-treated mice
and MPP+-treated SH-SY5Y cells | (84) |
| Shamsuzzama,
2017 | miR-Let-7 | Up | ATG13 | Inhibition | Increasing
α-synuclein expression | The transgenic
C. elegans model | (11) |
| Kim, 2016 | miR-27a/b | Up | PINK1 | Inhibition | Suppressing
lysosomal degradation of the damaged mitochondria | HeLa and M17
cells | (90) |
| Li, 2014 | miR-320a | Up | Hsc70 | Inhibition | Inhibiting
α-synuclein degradation | SH-SY5Y cell line
overexpressing α-synuclein | (93) |
| Decressac,
2013 | miR-128 | Down | TFEB, Beclin-1,
p62 | Inhibition | Aggravating
α-synuclein accumulation and toxicity |
α-synuclein-transfected HEK 293 cells and
α-synuclein-injected rats | (99) |
4. Regulating miRNA-mediated autophagy as
therapeutic strategies for PD
As aforementioned, it is widely believed that miRNAs
play a regulatory role in autophagy during PD progression.
Therefore, targeting miRNAs to modulate autophagy could offer a
promising therapeutic strategy to this disease. However,
miRNA-based therapies pose many unsolved challenges due to their
unstable structures, non-specific functions, potential toxicities
and inefficient deliveries to the brain. Emerging studies have
found that miRNA-mediated autophagy can be further modulated by
other ncRNAs, including lncRNAs and circRNAs, as well as natural
agents (100-115). These findings provide great
potential for the prevention and treatment of PD in the future.
Whether using ncRNA or natural products to target miRNAs regulating
autophagy, further investigations should be performed to clarify
how to modulate the level of miRNAs to maintain a desired autophagy
level since controlling the level of autophagy within a safe and
efficacious range is crucial for the prevention and treatment of
PD.
ncRNA
Both lncRNAs and circRNAs can act as competing
endogenous RNAs to sponge miRNAs, and thus participate in
miRNA-mediated gene regulation and cellular processes, including
proliferation, apoptosis and autophagy. With the development of PD,
aberrant expression of ncRNAs affect the pathological process of
this disease through modulation of miRNA-mediated autophagy
(100-109). For instance, lncRNA OIP5-AS1 is
decreased in MPP+-treated SH-SY5Y cells, which is
associated with miR-137-mediated suppression of mitophagy (100). Moreover, overexpression of
OIP5-AS1 alleviates mitochondrial damage and ROS accumulation via
inhibiting miR-137 activity and further restoring mitochondrial
autophagy (100). Consistent
with these findings, circDLGAP4 has been demonstrated to function
as a decoy of miR-134-5p to decrease mitochondrial damage and
apoptosis in neurons treated with MPP+, by targeting the
miR-134-5p/CREB axis and further enhancing autophagy (101). It can be concluded that
elevating the expression of downregulated ncRNAs in PD exerts
neuroprotective effects via restoring the miRNA-induced autophagy
impairment. However, several ncRNAs, such as lncRNA SNHG1, lncRNA
SNHG14 and lncRNA BDNF-AS, are overexpressed in PD and aggravate
neuronal damage through modulating miRNA-mediated autophagy
(102-104). Qian et al (102) demonstrated that downregulating
lncRNA SNHG1 facilitates autophagy and attenuates
MPP+-induced cytotoxicity in neurons through the
miR-221/222/p27/mTOR axis, implying an inhibitory effect of SNHG1
on protective autophagy. Besides, other upregulated ncRNAs are
reported to exacerbate PD progression by inducing autophagic cell
death. It has been demonstrated that lncRNA SNHG14 targets
miR-519a-3p to upregulate ATG10 expression in
MPP+-treated SK-N-SH cells, thus accelerating neuronal
cell damage by activating autophagy (103). Likewise, overexpressed lncRNA
BDNF-AS mediates MPP+-triggered death and loss of
dopaminergic neurons in MPTP-treated mice and promotes autophagy
through sponging miR-125b-5p (104). It should be noted that ncRNAs
can modulate autophagy in PD through targeting different miRNAs.
New evidence demonstrates that lncRNA NEAT1 strengthens neuronal
death in PD mice by enhancing autophagy through competitively
biding to both miR-107-5p and miR-374c-5p, indicating a potential
therapeutic role of NEAT1 in treating PD (105,106). Analogously, lncRNA HOTAIR
depletion decreases neuronal apoptosis in MPP+-treated
SK-N-SH and MN9D cells, along with an inhibition of autophagy
(107,108). Further molecular investigations
demonstrated that HOTAIR functions as a sponge for both miR-874-5p
and miR-221-3p, further promoting the expression of ATG10 and
NPTX2, which contributes to the neurotoxic role of HOTAIR by
upregulating autophagy (107,108). These results indicate that
upregulation of lncRNAs during PD progression deteriorate neuronal
apoptosis by promoting autophagy by acting as a sponge for miRNAs.
Besides, downregulation of circSAMD4A attenuates neuronal damage
and motor deficits in PD models, since circSAMD4A modulates disease
progression by sponging miR-29c-3p to activate the AMPK/mTOR
signaling pathway and further induces autophagy (109). Thus, this previous study
demonstrates that circRNAs play a pernicious role in PD by inducing
autophagy through targeting miRNAs.
Collectively, aberrant expression of ncRNAs affects
the pathological processes of PD, including neuronal apoptosis,
oxidative stress and mitochondrial dysfunction by modulating
miRNA-mediated autophagy (Table
III). lncRNA OIP5-AS1 and circDLGAP4 are downregulated in PD,
and their upregulation alleviates neuronal damage by inhibiting
neurotoxic miRNAs to activate autophagy (100,101). Thus, administration of these
ncRNAs may be beneficial for PD treatment by restoring
miRNA-induced autophagy impairment. Furthermore, other upregulated
ncRNAs (lncRNA SNHG1, lncRNA SNHG14, lncRNA BDNF-AS, lncRNA NEAT1,
lncRNA HOTAIR and circSAMD4A) in PD are linked to excessive
autophagy by negatively targeting neuroprotective miRNAs (102-109). Hence, maintaining sufficient
levels of neuroprotective miRNAs to alleviate excessive autophagy
by silencing these ncRNAs may become a potential therapeutic
strategy for PD.
 | Table IIIRegulatory roles of ncRNAs on
miRNA-mediated autophagy in PD. |
Table III
Regulatory roles of ncRNAs on
miRNA-mediated autophagy in PD.
| First author,
year | NcRNA | Expression | Effect on
miRNA | Autophagy | Mechanism of
alleviating PD | Experimental
model | (Refs.) |
|---|
| Dong, 2022 | lncRNA NEAT1 | Up | miR-107-5p ↓ | Activation | Interfering with
NEAT1 increases neuron viability and suppresses apoptosis | MPTP-treated mice
and MPP+-treated SH-SY5Y cells | (105) |
| Dong, 2021 | lncRNA NEAT1 | Up | miR-374c-5p ↓ | Activation | Suppression of
NEAT1 facilitates cell proliferation and inhibits apoptosis | MPTP-treated mice
and MPP+-treated SH-SY5Y cells | (106) |
| Zhuang, 2022 | lncRNA SNHG14 | Up | miR-519a-3p ↓ | Activation | Silencing SNHG14
and restoring miR-519a-3p reduce neuronal cell death |
MPP+-treated SK-N-SH cells | (103) |
| Qian, 2019 | lncRNA SNHG1 | Up | miR-221/222 ↓ | Inhibition | Silencing SNHG1
reduces cell death | MPTP-treated mice
and MPP+-treated MN9D cells | (102) |
| Zhao, 2022 | lncRNA
OIP5-AS1 | Down | miR-137 ↑ | Inhibition | Restoring OIP5-AS1
promotes mitophagy and thus protects neurons from degeneration |
MPP+-treated SH-SY5Y cells | (100) |
| Wang, 2021 | circSAMD4A | Up | miR-29c 3p ↓ | Activation | Knockdown of
circSAMD4A inhibits neuronal cell apoptosis | MPTP-treated mice
and MPP+-treated SH-SY5Y cells | (109) |
| Zhao, 2020 | lncRNA HOTAIR | Up | miR-874-5p ↓ | Activation | Inhibition of
HOTAIR reduces neuronal injury |
MPP+-treated SK-N-SH cells | (107) |
| Lang, 2020 | lncRNA HOTAIR | Up | miR-221-3p ↓ | Activation | Downregulation of
HOTAIR enhances cell viability | MPTP-treated mice
and MPP+-treated MN9D cells | (108) |
| Fan, 2020 | lncRNA BDNF-AS | Up | miR-125b-5p ↓ | Activation | Knockdown of
BDNF-AS promotes cell proliferation and suppresses apoptosis | MPTP-treated mice
and MPP+-treated SH-SY5Y cells | (104) |
| Feng, 2020 | circDLGAP4 | Down | miR-134-5p ↑ | Inhibition | Upregulation of
circDLGAP4 reduces mitochondrial damage and apoptosis | MPTP-treated mice
and MPP+-treated SH-SY5Y cells | (101) |
Drugs and natural compounds
In addition to the regulatory role of ncRNAs on
miRNA-mediated autophagy, drugs and natural products have also been
demonstrated to regulate autophagy by targeting miRNAs for the
prevention and treatment of PD. For instance, empagliflozin, a
selective sodium-glucose co-transporter-2 inhibitor, can suppress
rotenone-induced endoplasmic reticulum stress, α-synuclein
accumulation and neuroinflammation in the striatum of PD rats by
inhibiting miR-211-5p expression to upregulate Beclin-1-mediated
autophagy, executing neuroprotection (110). Pramipexole is a dopamine D2/D3
receptor agonist with proven efficacy in the treatment of motor
symptoms in early and advanced PD (111). It has been demonstrated that
pramipexole can rescue MPTP-induced neuronal death in mice by
activating BNIP3-mediated mitophagy via directly decreasing miR-96
levels (111). Moreover,
pramipexole mitigates cell apoptosis and indirectly promotes cell
autophagy in PD by downregulating the expression of circSNCA, which
serves as a miR-7 sponge, thereby triggering miR-7-induced
autophagy (112). In addition,
baicalein, a natural compound that is regarded as a novel regulator
for cell metabolism, proliferation and apoptosis, can increase
dopamine concentration and neuronal viability in 6-OHDA-treated
rats by inhibiting miR-30b, and thus inducing mitochondrial
autophagy via the activation of the NIX/BNIP3 signaling pathway
(113). Similarly, it has been
demonstrated that baicalein alleviates neuronal apoptosis and
recovers mitochondrial dysfunction by restoring mitophagy via
targeting miR-30b-5p and thus inactivating the SIRT1/AMPK/mTOR
pathway (114). However, in
6-OHDA-treated PC12 cells, baicalein negatively regulates the
expression of miR-192-5p and further decreases cell injury and
autophagy through suppression of the PI3K/AKT signaling cascade,
indicating that baicalein inhibits excessive autophagy to protect
against PD (115).
In summary, some drugs and natural compounds have
been implicated in the regulation of miRNA-mediated autophagy and
play a positive role in the prevention and treatment of PD
(Table IV). Further
identification of the mechanisms by which various drugs or natural
products regulate miRNA-mediated autophagy is crucial to develop
targeted interventions in PD. However, whether various targets
acting on miRNA-mediated autophagy exist in these drugs or natural
products needs to be confirmed.
 | Table IVRegulatory roles of drugs and natural
agents on miRNA-mediated autophagy in PD. |
Table IV
Regulatory roles of drugs and natural
agents on miRNA-mediated autophagy in PD.
| First author,
year | Drug and agent | Effect on
miRNA | Autophagy | Mechanism of
alleviating PD | Experimental
model | (Refs.) |
|---|
| Motawi, 2022 | Empagliflozin | miR-211-5p ↓ | Activation | Alleviating
oxidative stress, glial activation and neuroinflammation | Rotenone-treated
rats | (110) |
| Chen, 2022 | Baicalein | miR-30b-5p ↓ | Activation | Lessening neuronal
injury and recovering mitochondrial dysfunction | 6-OHDA-treated
rats | (114) |
| Chen, 2021 | Baicalein | miR-30b ↓ | Activation | Restoring neuronal
activity and alleviating neuron damage | 6-OHDA-treated
rats | (113) |
| Kang, 2019 | Baicalein | miR-192-5p ↓ | Inhibition | Reducing neuronal
injury and elevating cell viability | 6-OHDA-treated PC12
cells | (115) |
| Wang, 2021 | Pramipexole | miR-96 ↓ | Activation | Increasing cell
viability and decreasing apoptosis | MPTP-treated mice,
MPP+-treated SH-SY5Y and SK-N-SH cells | (111) |
| Sang, 2018 | Pramipexole | miR-7↑ | Activation | Reducing cell
apoptosis |
MPP+-treated SH-SY5Y cells | (112) |
5. Conclusions and future perspectives
Autophagy is a crucial biological process that can
be regulated through a variety of ATGs and autophagy-related
signaling pathways, and its dysfunction is associated with the
progression of PD. Although overactivated autophagy causes neuronal
damage, the autophagy-lysosomal pathway is responsible for the
removal of aggregated α-synuclein and impaired mitochondria, and
thus exerts neuroprotective roles in PD. Emerging studies (65-68) have also documented that various
miRNAs are involved in the pathological processes of PD, including
α-synuclein accumulation, mitochondrial damage, oxidative stress,
microglial activation and neuronal apoptosis via regulating
autophagy (Fig. 2).
Autophagy-regulating miRNAs perform a dual function in the
progression PD. By targeting both ATGs and autophagy-related
signaling pathways, downregulated miRNAs play neuroprotective roles
by activating protective autophagy or decreasing autophagic
neuronal cell death, whereas neurotoxic miRNAs are upregulated to
induce autophagy impairment. Thus, regulating miRNA-mediated
autophagy may be a novel strategy for the treatment of PD. Studies
(3,9) have demonstrated that the
implementation of miRNA mimics, agonists and antagonists by
nanoparticles and microinjection is effective to delay disease
progression in PD models. However, the biosafety and reliability of
these miRNA-based therapeutic strategies should be fully elucidated
before clinical application, owing to multiple gene target and
off-target effects of miRNAs. A comprehensive understanding of the
regulatory mechanism of miRNAs on autophagy and the crosstalk
between miRNA-regulated autophagy and other biological processes in
PD, including programmed cell death, protein degradation pathways
and pathophysiological mechanisms, can help in developing safe and
effective therapeutic targets for this disease. It is worth noting
that ncRNAs, including lncRNAs and circRNAs, drugs and natural
compounds can restore impaired autophagy via regulating miRNAs,
indicating their great potential value in the treatment of PD.
However, the identification of these interventions for specifically
targeting signaling pathways associated with miRNA-mediated
autophagy is needed to fully determine their main target in PD.
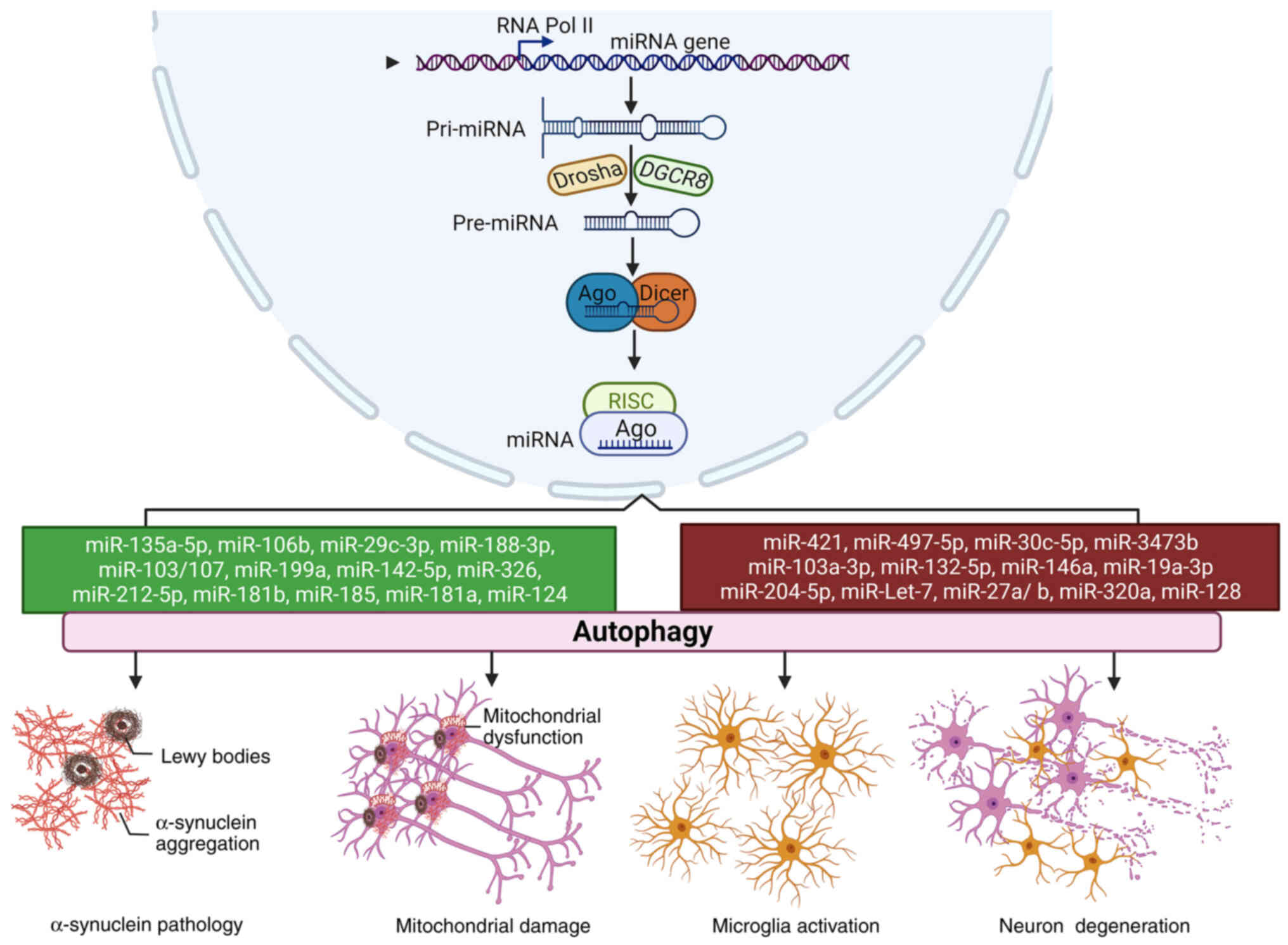 | Figure 2The roles of miRNA-mediated autophagy
in PD progression. In the nucleus, a pri-mRNA is transcribed and
further cleaved by Drosha and DGCR8 to generate pre-miRNA, which is
cleaved into a miRNA duplex by Dicer and then is loaded to the
groove of AGO to form the RISC complex. Both neuroprotective miRNAs
(green) and neurotoxic miRNAs (red) are involved in the
pathological processes of PD, including α-synuclein accumulation,
mitochondrial damage, microglial activation and neuronal
degeneration by modulating autophagy. AGO, Argonaute; PD,
Parkinson's disease; pri-mRNA, primary microRNA; miR, microRNA;
miRNA, microRNA; RISC, RNA-induced silencing complex. |
Availability of data and materials
Not applicable.
Authors' contributions
ZM and HL wrote the manuscript, BH and SC revised
the manuscript, DY retrieved references and raised the research
topic. Data authentication is not applicable. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Bloem BR, Okun MS and Klein C: Parkinson's
disease. Lancet. 397:2284–2303. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xia N, Cabin DE, Fang F and Reijo Pera RA:
Parkinson's disease: Overview of transcription factor regulation,
genetics, and cellular and animal models. Front Neurosci.
16:8946202022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cerri S and Blandini F: Role of autophagy
in Parkinson's disease. Curr Med Chem. 26:3702–3718. 2019.
View Article : Google Scholar
|
|
4
|
Kuo SH, Tasset I, Cheng MM, Diaz A, Pan
MK, Lieberman OJ, Hutten SJ, Alcalay RN, Kim S, Ximénez-Embún P, et
al: Mutant glucocerebrosidase impairs α-synuclein degradation by
blockade of chaperone-mediated autophagy. Sci Adv. 8:eabm63932022.
View Article : Google Scholar
|
|
5
|
Haddad D and Nakamura K: Understanding the
susceptibility of dopamine neurons to mitochondrial stressors in
Parkinson's disease. FEBS Lett. 589:3702–3713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lizama BN and Chu CT: Neuronal autophagy
and mitophagy in Parkinson's disease. Mol Aspects Med.
82:1009722021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao B, Kuruvilla J and Tan EK: Mitophagy
and reactive oxygen species interplay in Parkinson's disease. NPJ
Parkinsons Dis. 8:1352022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou X, Watzlawik JO, Fiesel FC and
Springer W: Autophagy in Parkinson's disease. J Mol Biol.
432:2651–2672. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goh SY, Chao YX, Dheen ST, Tan EK and Tay
SS: Role of MicroRNAs in Parkinson's disease. Int J Mol Sci.
20:56492019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Das T, Das TK, Khodarkovskaya A and Dash
S: Non-coding RNAs and their bioengineering applications for
neurological diseases. Bioengineered. 12:11675–11698. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shamsuzzama, Kumar L and Nazir A:
Modulation of alpha-synuclein expression and associated effects by
MicroRNA Let-7 in transgenic C. elegans. Front Mol Neurosci.
10:3282017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong X, He X, Yang L, Li Q and Xu Y:
Inhibition of miR-421 preserves mitochondrial function and protects
against parkinson's disease pathogenesis via Pink1/Parkin-dependent
mitophagy. Dis Markers. 2022:51862522022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galluzzi L, Baehrecke EH, Ballabio A, Boya
P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P,
Colombo MI, et al: Molecular definitions of autophagy and related
processes. EMBO J. 36:1811–1836. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malpartida AB, Williamson M, Narendra DP,
Wade-Martins R and Ryan BJ: Mitochondrial dysfunction and mitophagy
in Parkinson's disease: From mechanism to therapy. Trends Biochem
Sci. 46:329–343. 2021. View Article : Google Scholar
|
|
15
|
Lystad AH, Carlsson SR and Simonsen A:
Toward the function of mammalian ATG12-ATG5-ATG16L1 complex in
autophagy and related processes. Autophagy. 15:1485–1486. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Wang L, Ireland SC, Ahat E, Li J,
Bekier ME II, Zhang Z and Wang Y: GORASP2/GRASP55 collaborates with
the PtdIns3K UVRAG complex to facilitate autophagosome-lysosome
fusion. Autophagy. 15:1787–1800. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu Z, Yang C, Iyaswamy A, Krishnamoorthi
S, Sreenivasmurthy SG, Liu J, Wang Z, Tong BC, Song J, Lu J, et al:
Balancing mTOR signaling and autophagy in the treatment of
Parkinson's disease. Int J Mol Sci. 20:7282019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Z, Sun X, Wang K, Yu Y, Zhang L,
Zhang K, Gu J, Yuan X and Song G: Hydrogen-saturated saline
mediated neuroprotection through autophagy via PI3K/AKT/mTOR
pathway in early and medium stages of rotenone-induced Parkinson's
disease rats. Brain Res Bull. 172:1–13. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mao K and Klionsky DJ: AMPK activates
autophagy by phosphorylating ULK1. Circ Res. 108:787–788. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang S, Gui XH, Huang LP, Deng MZ, Fang
RM, Ke XH, He YP, Li L and Fang YQ: Neuroprotective effects of
β-asarone against 6-hydroxy dopamine-induced parkinsonism via
JNK/Bcl-2/beclin-1 pathway. Mol Neurobiol. 53:83–94. 2016.
View Article : Google Scholar
|
|
21
|
Ba RQ, Liu J, Fan XJ, Jin GL, Huang BG,
Liu MW and Yang JS: Effects of miR-199a on autophagy by targeting
glycogen synthase kinase 3β to activate PTEN/AKT/mTOR signaling in
an MPP+ in vitro model of Parkinson's disease. Neurol
Res. 42:308–318. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clark EH, Vázquez de la Torre A, Hoshikawa
T and Briston T: Targeting mitophagy in Parkinson's disease. J Biol
Chem. 296:1002092021. View Article : Google Scholar :
|
|
23
|
Qiao CM, Sun MF, Jia XB, Shi Y, Zhang BP,
Zhou ZL, Zhao LP, Cui C and Shen YQ: Sodium butyrate causes
α-synuclein degradation by an Atg5-dependent and
PI3K/Akt/mTOR-related autophagy pathway. Exp Cell Res.
387:1117722020. View Article : Google Scholar
|
|
24
|
Tan Y, Yin L, Sun Z, Shao S, Chen W, Man
X, Du Y and Chen Y: Astragalus polysaccharide exerts anti-Parkinson
via activating the PI3K/AKT/mTOR pathway to increase cellular
autophagy level in vitro. Int J Biol Macromol. 153:349–356. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang XW, Yuan LJ, Yang Y, Zhang M and Chen
WF: IGF-1 inhibits MPTP/MPP+-induced autophagy on
dopaminergic neurons through the IGF-1R/PI3K-Akt-mTOR pathway and
GPER. Am J Physiol Endocrinol Metab. 319:E734–E743. 2020.
View Article : Google Scholar
|
|
26
|
Zhu J, Xia R, Liu Z, Shen J, Gong X, Hu Y,
Chen H, Yu Y, Gao W, Wang C and Wang SL: Fenvalerate triggers
Parkinson-like symptom during zebrafish development through
initiation of autophagy and p38 MAPK/mTOR signaling pathway.
Chemosphere. 243:1253362020. View Article : Google Scholar
|
|
27
|
Zhang Y, Wu Q, Zhang L, Wang Q, Yang Z,
Liu J and Feng L: Caffeic acid reduces A53T α-synuclein by
activating JNK/Bcl-2-mediated autophagy in vitro and improves
behaviour and protects dopaminergic neurons in a mouse model of
Parkinson's disease. Pharmacol Res. 150:1045382019. View Article : Google Scholar
|
|
28
|
Wan J, Gao Y, Tan J, Yi S, Huang K, Liu Y,
Chang D, Xie J, Chen S and Wu H: Mitochonic acid 5 ameliorates the
motor deficits in the MPTP-induced mouse Parkinson's disease model
by AMPK-mediated autophagy. Folia Neuropathol. 60:329–337. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Parekh P, Sharma N, Sharma M, Gadepalli A,
Sayyed AA, Chatterjee S, Kate A and Khairnar A: AMPK-dependent
autophagy activation and alpha-synuclein clearance: A putative
mechanism behind alpha-mangostin's neuroprotection in a
rotenone-induced mouse model of Parkinson's disease. Metab Brain
Dis. 37:2853–2870. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou L and Cheng Y: Alpha-lipoic acid
alleviated 6-OHDA-induced cell damage by inhibiting AMPK/mTOR
mediated autophagy. Neuropharmacology. 155:98–103. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen P, Wang Y, Chen L, Song N and Xie J:
Apelin-13 protects dopaminergic neurons against rotenone-induced
neurotoxicity through the AMPK/mTOR/ULK-1 mediated autophagy
activation. Int J Mol Sci. 21:83762020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang M, Deng YN, Zhang JY, Liu J, Li YB,
Su H and Qu QM: SIRT3 protects rotenone-induced injury in SH-SY5Y
cells by promoting autophagy through the LKB1-AMPK-mTOR pathway.
Aging Dis. 9:273–286. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Agarwal S and Muqit MMK: PTEN-induced
kinase 1 (PINK1) and Parkin: Unlocking a mitochondrial quality
control pathway linked to Parkinson's disease. Curr Opin Neurobiol.
72:111–119. 2022. View Article : Google Scholar
|
|
34
|
Li R and Chen J: Salidroside protects
dopaminergic neurons by enhancing PINK1/parkin-mediated mitophagy.
Oxid Med Cell Longev. 2019:93410182019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu J, Wang L, Zhang L, Zheng F, Wang F,
Leng J, Wang K, Héroux P, Shen HM, Wu Y and Xia D:
Mono-2-ethylhexyl phthalate drives progression of
PINK1-parkin-mediated mitophagy via increasing mitochondrial ROS to
exacerbate cytotoxicity. Redox Biol. 38:1017762021. View Article : Google Scholar
|
|
36
|
Chen J, Ren Y, Gui C, Zhao M, Wu X, Mao K,
Li W and Zou F: Phosphorylation of Parkin at serine 131 by p38 MAPK
promotes mitochondrial dysfunction and neuronal death in mutant
A53T α-synuclein model of Parkinson's disease. Cell Death Dis.
9:7002018. View Article : Google Scholar
|
|
37
|
Chen C, Chen Y, Liu T, Song D, Ma D and
Cheng O: Dexmedetomidine can enhance PINK1/parkin-mediated
mitophagy in MPTP-induced PD mice model by activating AMPK. Oxid
Med Cell Longev. 2022:75113932022.PubMed/NCBI
|
|
38
|
Bekker M, Abrahams S, Loos B and Bardien
S: Can the interplay between autophagy and apoptosis be targeted as
a novel therapy for Parkinson's disease? Neurobiol Aging.
100:91–105. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miller DR, Cramer SD and Thorburn A: The
interplay of autophagy and non-apoptotic cell death pathways. Int
Rev Cell Mol Biol. 352:159–187. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liao Z, Gong Z, Wang Z, Yang W, Liu W, Hou
L, Liu X, Hua J, Wang B and Li N: The degradation of TMEM166 by
autophagy promotes AMPK activation to protect SH-SY5Y cells exposed
to MPP. Cells. 11:27062022. View Article : Google Scholar
|
|
41
|
Anglade P, Vyas S, Javoy-Agid F, Herrero
MT, Michel PP, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch EC and
Agid Y: Apoptosis and autophagy in nigral neurons of patients with
Parkinson's disease. Histol Histopathol. 12:25–31. 1997.PubMed/NCBI
|
|
42
|
Tanji K, Mori F, Kakita A, Takahashi H and
Wakabayashi K: Alteration of autophagosomal proteins (LC3, GABARAP
and GATE-16) in Lewy body disease. Neurobiol Dis. 43:690–697. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dehay B, Bové J, Rodriguez-Muela N, Perier
C, Recasens A, Boya P and Vila M: Pathogenic lysosomal depletion in
Parkinson's disease. J Neurosci. 30:12535–12544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Alvarez-Erviti L, Rodriguez-Oroz MC,
Cooper JM, Caballero C, Ferrer I, Obeso JA and Schapira AH:
Chaperone-mediated autophagy markers in Parkinson disease brains.
Arch Neurol. 67:1464–1472. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hou X, Fiesel FC, Truban D, Castanedes
Casey M, Lin WL, Soto AI, Tacik P, Rousseau LG, Diehl NN, Heckman
MG, et al: Age- and disease-dependent increase of the mitophagy
marker phospho-ubiquitin in normal aging and Lewy body disease.
Autophagy. 14:1404–1418. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tu HY, Gu YQ, Li X, Pei SF, Hu LF and Wang
YL: Expression of autophagy related genes in peripheral blood cells
in Parkinson's disease. Neurosci Lett. 762:1361662021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sepúlveda D, Grunenwald F, Vidal A,
Troncoso-Escudero P, Cisternas-Olmedo M, Villagra R, Vergara P,
Aguilera C, Nassif M and Vidal RL: Insulin-like growth factor 2 and
autophagy gene expression alteration arise as potential biomarkers
in Parkinson's disease. Sci Rep. 12:20382022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li Y, Yin Q, Wang B, Shen T, Luo W and Liu
T: Preclinical reserpine models recapitulating motor and non-motor
features of Parkinson's disease: Roles of epigenetic upregulation
of alpha-synuclein and autophagy impairment. Front Pharmacol.
13:9443762022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gao J, Perera G, Bhadbhade M, Halliday GM
and Dzamko N: Autophagy activation promotes clearance of
α-synuclein inclusions in fibril-seeded human neural cells. J Biol
Chem. 294:14241–14256. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tang Q, Gao P, Arzberger T, Höllerhage M,
Herms J, Höglinger G and Koeglsperger T: Alpha-synuclein defects
autophagy by impairing SNAP29-mediated autophagosome-lysosome
fusion. Cell Death Dis. 12:8542021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nascimento AC, Erustes AG, Reckziegel P,
Bincoletto C, Ureshino RP, Pereira GJS and Smaili SS: α-Synuclein
overexpression induces lysosomal dysfunction and autophagy
impairment in human neuroblastoma SH-SY5Y. Neurochem Res.
45:2749–2761. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shaltouki A, Hsieh CH, Kim MJ and Wang X:
Alpha-synuclein delays mitophagy and targeting Miro rescues neuron
loss in Parkinson's models. Acta Neuropathol. 136:607–620. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Portz P and Lee MK: Changes in Drp1
function and mitochondrial morphology are associated with the
α-synuclein pathology in a transgenic mouse model of Parkinson's
disease. Cells. 10:8852021. View Article : Google Scholar
|
|
54
|
Wauters F, Cornelissen T, Imberechts D,
Martin S, Koentjoro B, Sue C, Vangheluwe P and Vandenberghe W:
LRRK2 mutations impair depolarization-induced mitophagy through
inhibition of mitochondrial accumulation of RAB10. Autophagy.
16:203–222. 2020. View Article : Google Scholar :
|
|
55
|
Albanese F, Domenicale C, Volta M and
Morari M: Modeling Parkinson's disease in LRRK2 mice: Focus on
synaptic dysfunction and the autophagy-lysosomal pathway. Biochem
Soc Trans. 50:621–632. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Michiorri S, Gelmetti V, Giarda E,
Lombardi F, Romano F, Marongiu R, Nerini-Molteni S, Sale P, Vago R,
Arena G, et al: The Parkinson-associated protein PINK1 interacts
with Beclin1 and promotes autophagy. Cell Death Differ. 17:962–974.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Narendra D, Tanaka A, Suen DF and Youle
RJ: Parkin is recruited selectively to impaired mitochondria and
promotes their autophagy. J Cell Biol. 183:795–803. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Navarro-Romero A, Fernandez-Gonzalez I,
Riera J, Montpeyo M, Albert-Bayo M, Lopez-Royo T, Castillo-Sanchez
P, Carnicer-Caceres C, Arranz-Amo JA, Castillo-Ribelles L, et al:
Lysosomal lipid alterations caused by glucocerebrosidase deficiency
promote lysosomal dysfunction, chaperone-mediated-autophagy
deficiency, and alpha-synuclein pathology. NPJ Parkinsons Dis.
8:1262022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nash Y, Schmukler E, Trudler D,
Pinkas-Kramarski R and Frenkel D: DJ-1 deficiency impairs autophagy
and reduces alpha-synuclein phagocytosis by microglia. J Neurochem.
143:584–594. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tu HY, Yuan BS, Hou XO, Zhang XJ, Pei CS,
Ma YT, Yang YP, Fan Y, Qin ZH, Liu CF and Hu LF: α-Synuclein
suppresses microglial autophagy and promotes neurodegeneration in a
mouse model of Parkinson's disease. Aging Cell. 20:e135222021.
View Article : Google Scholar
|
|
61
|
Qin Y, Qiu J, Wang P, Liu J, Zhao Y, Jiang
F and Lou H: Impaired autophagy in microglia aggravates
dopaminergic neurodegeneration by regulating NLRP3 inflammasome
activation in experimental models of Parkinson's disease. Brain
Behav Immun. 91:324–338. 2021. View Article : Google Scholar
|
|
62
|
Zhao M, Chen J, Mao K, She H, Ren Y, Gui
C, Wu X, Zou F and Li W: Mitochondrial calcium dysfunction
contributes to autophagic cell death induced by MPP+ via
AMPK pathway. Biochem Biophys Res Commun. 509:390–394. 2019.
View Article : Google Scholar
|
|
63
|
Niranjan R, Mishra KP and Thakur AK:
Inhibition of cyclooxygenase-2 (COX-2) initiates autophagy and
potentiates MPTP-induced autophagic cell death of human
neuroblastoma cells, SH-SY5Y: An INSIDE IN THE PATHOlogy of
Parkinson's disease. Mol Neurobiol. 55:8038–8050. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jang HJ and Chung KC: The
ubiquitin-proteasome system and autophagy mutually interact in
neurotoxin-induced dopaminergic cell death models of Parkinson's
disease. FEBS Lett. 596:2898–2913. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Choi I, Woo JH, Jou I and Joe EH: PINK1
Deficiency decreases expression levels of mir-326, mir-330, and
mir-3099 during brain development and neural stem cell
differentiation. Exp Neurobiol. 25:14–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhao XH, Wang YB, Yang J, Liu HQ and Wang
LL: MicroRNA-326 suppresses iNOS expression and promotes autophagy
of dopaminergic neurons through the JNK signaling by targeting XBP1
in a mouse model of Parkinson's disease. J Cell Biochem.
120:14995–15006. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sarkar A, Shamsuzzama, Kumar L, Hameed R
and Nazir A: Multiple checkpoints of protein clearance machinery
are modulated by a common microRNA, miR-4813-3p through its
putative target genes: Studies employing transgenic C. elegans
model. Biochim Biophys Acta Mol Cell Res. 1869:1193422022.
View Article : Google Scholar
|
|
68
|
Bai X, Dong Q, Zhao L, Yao Y and Wang B:
microRNA-106b-containing extracellular vesicles affect autophagy of
neurons by regulating CDKN2B in Parkinson's disease. Neurosci Lett.
760:1360942021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sun S, Han X, Li X, Song Q, Lu M, Jia M,
Ding J and Hu G: MicroRNA-212-5p prevents dopaminergic neuron death
by inhibiting SIRT2 in MPTP-induced mouse model of Parkinson's
disease. Front Mol Neurosci. 11:3812018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang H, Ye Y, Zhu Z, Mo L, Lin C, Wang Q,
Wang H, Gong X, He X, Lu G, et al: MiR-124 regulates apoptosis and
autophagy process in MPTP model of Parkinson's disease by targeting
to bim. Brain Pathol. 26:167–176. 2016. View Article : Google Scholar
|
|
71
|
Yao L, Zhu Z, Wu J, Zhang Y, Zhang H, Sun
X, Qian C, Wang B, Xie L, Zhang S and Lu G: MicroRNA-124 regulates
the expression of p62/p38 and promotes autophagy in the
inflammatory pathogenesis of Parkinson's disease. FASEB J.
33:8648–8665. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chen J, Jiang C, Du J and Xie CL:
MiR-142-5p protects against 6-OHDA-induced SH-SY5Y cell injury by
downregulating BECN1 and autophagy. Dose Response.
18:15593258209070162020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Li W, Jiang Y, Wang Y, Yang S, Bi X, Pan
X, Ma A and Li W: MiR-181b regulates autophagy in a model of
Parkinson's disease by targeting the PTEN/Akt/mTOR signaling
pathway. Neurosci Lett. 675:83–88. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Qin Y, Huang J, Zhao X and Chen C:
MiR-135a-5p and Mst1 regulate MPP + -1 induced apoptosis and
autophagy in Parkinson's disease model in vitro. Cell Signal.
94:1103282022. View Article : Google Scholar
|
|
75
|
Wen Z, Zhang J, Tang P, Tu N, Wang K and
Wu G: Overexpression of miR-185 inhibits autophagy and apoptosis of
dopaminergic neurons by regulating the AMPK/mTOR signaling pathway
in Parkinson's disease. Mol Med Rep. 17:131–137. 2018.
|
|
76
|
Liu Y, Song Y and Zhu X: MicroRNA-181a
regulates apoptosis and autophagy process in Parkinson's disease by
inhibiting p38 mitogen-activated protein kinase (MAPK)/c-Jun
N-terminal kinases (JNK) signaling pathways. Med Sci Monit.
23:1597–1606. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wong AS, Lee RH, Cheung AY, Yeung PK,
Chung SK, Cheung ZH and Ip NY: Cdk5-mediated phosphorylation of
endophilin B1 is required for induced autophagy in models of
Parkinson's disease. Nat Cell Biol. 13:568–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li G, Luo W, Wang B, Qian C, Ye Y, Li Y
and Zhang S: HMGA1 induction of miR-103/107 forms a negative
feedback loop to regulate autophagy in MPTP model of Parkinson's
disease. Front Cell Neurosci. 14:6200202021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li Q, Wang Z, Xing H, Wang Y and Guo Y:
Exosomes derived from miR-188-3p-modified adipose-derived
mesenchymal stem cells protect Parkinson's disease. Mol Ther
Nucleic Acids. 23:1334–1344. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang R, Li Q, He Y, Yang Y, Ma Q and Li C:
miR-29c-3p inhibits microglial NLRP3 inflammasome activation by
targeting NFAT5 in Parkinson's disease. Genes Cells. 25:364–374.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang R, Yao J, Gong F, Chen S, He Y, Hu C
and Li C: miR-29c-3p regulates TET2 expression and inhibits
autophagy process in Parkinson's disease models. Genes Cells.
26:684–697. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Gong X, Wang H, Ye Y, Shu Y, Deng Y, He X,
Lu G and Zhang S: miR-124 regulates cell apoptosis and autophagy in
dopaminergic neurons and protects them by regulating AMPK/mTOR
pathway in Parkinson's disease. Am J Transl Res. 8:2127–2137.
2016.PubMed/NCBI
|
|
83
|
Lanford RE, Hildebrandt-Eriksen ES, Petri
A, Persson R, Lindow M, Munk ME, Kauppinen S and Ørum H:
Therapeutic silencing of microRNA-122 in primates with chronic
hepatitis C virus infection. Science. 327:198–201. 2010. View Article : Google Scholar
|
|
84
|
Chiu CC, Yeh TH, Chen RS, Chen HC, Huang
YZ, Weng YH, Cheng YC, Liu YC, Cheng AJ, Lu YC, et al: Upregulated
expression of MicroRNA-204-5p leads to the death of dopaminergic
cells by targeting DYRK1A-mediated apoptotic signaling cascade.
Front Cell Neurosci. 13:3992019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhang L, Chen X, Chang M and Jiao B:
MiR-30c-5p/ATG5 axis regulates the progression of Parkinson's
disease. Front Cell Neurosci. 15:6445072021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhu W, Zhang H, Gao J and Xu Y: Silencing
of miR-497-5p inhibits cell apoptosis and promotes autophagy in
Parkinson's disease by upregulation of FGF2. Environ Toxicol.
36:2302–2312. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang HY, Wang ZG, Wu FZ, Kong XX, Yang J,
Lin BB, Zhu SP, Lin L, Gan CS, Fu XB, et al: Regulation of
autophagy and ubiquitinated protein accumulation by bFGF promotes
functional recovery and neural protection in a rat model of spinal
cord injury. Mol Neurobiol. 48:452–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lv Q, Zhong Z, Hu B, Yan S, Yan Y, Zhang
J, Shi T, Jiang L, Li W and Huang W: MicroRNA-3473b regulates the
expression of TREM2/ULK1 and inhibits autophagy in inflammatory
pathogenesis of Parkinson disease. J Neurochem. 157:599–610. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhou T, Lin D, Chen Y, Peng S, Jing X, Lei
M, Tao E and Liang Y: α-Synuclein accumulation in SH-SY5Y cell
impairs autophagy in microglia by exosomes overloading miR-19a-3p.
Epigenomics. 11:1661–1677. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kim J, Fiesel FC, Belmonte KC, Hudec R,
Wang WX, Kim C, Nelson PT, Springer W and Kim J: miR-27a and
miR-27b regulate autophagic clearance of damaged mitochondria by
targeting PTEN-induced putative kinase 1 (PINK1). Mol Neurodegener.
11:552016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Jauhari A, Singh T, Mishra S, Shankar J
and Yadav S: Coordinated action of miR-146a and parkin gene
regulate rotenone-induced neurodegeneration. Toxicol Sci.
176:433–445. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhou J, Zhao Y, Li Z, Zhu M, Wang Z, Li Y,
Xu T, Feng D, Zhang S, Tang F and Yao J: miR-103a-3p regulates
mitophagy in Parkinson's disease through Parkin/Ambra1 signaling.
Pharmacol Res. 160:1051972020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Li G, Yang H, Zhu D, Huang H, Liu G and
Lun P: Targeted suppression of chaperone-mediated autophagy by
miR-320a promotes α-synuclein aggregation. Int J Mol Sci.
15:15845–15857. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kim J, Inoue K, Ishii J, Vanti WB, Voronov
SV, Murchison E, Hannon G and Abeliovich A: A MicroRNA feedback
circuit in midbrain dopamine neurons. Science. 317:1220–1224. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhao J, Yang M, Li Q, Pei X and Zhu X:
miR-132-5p regulates apoptosis and autophagy in MPTP model of
Parkinson's disease by targeting ULK1. Neuroreport. 31:959–965.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Li P, Zhong X, Li J, Liu H, Ma X, He R and
Zhao Y: MicroRNA-30c-5p inhibits NLRP3 inflammasome-mediated
endothelial cell pyroptosis through FOXO3 down-regulation in
atherosclerosis. Biochem Biophys Res Commun. 503:2833–2840. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhang C, Yu S, Zheng B, Liu D, Wan F, Ma
Y, Wang J, Gao Z and Shan Z: miR-30c-5p reduces renal
ischemia-reperfusion involving macrophage. Med Sci Monit.
25:4362–4369. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhou L, Yang L, Li YJ, Mei R, Yu HL, Gong
Y, Du MY and Wang F: MicroRNA-128 protects dopamine neurons from
apoptosis and upregulates the expression of excitatory amino acid
transporter 4 in Parkinson's disease by binding to AXIN1. Cell
Physiol Biochem. 51:2275–2289. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Decressac M, Mattsson B, Weikop P,
Lundblad M, Jakobsson J and Björklund A: TFEB-mediated autophagy
rescues midbrain dopamine neurons from α-synuclein toxicity. Proc
Natl Acad Sci USA. 110:E1817–E1826. 2013. View Article : Google Scholar
|
|
100
|
Zhao Y, Xie Y, Yao WY, Wang YY and Song N:
Long non-coding RNA Opa interacting protein 5-antisense RNA 1
promotes mitochondrial autophagy and protects SH-SY5Y cells from
1-methyl-4-phenylpyridine-induced damage by binding to microRNA-137
and upregulating NIX. Kaohsiung J Med Sci. 38:207–217. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Feng Z, Zhang L, Wang S and Hong Q:
Circular RNA circDLGAP4 exerts neuroprotective effects via
modulating miR-134-5p/CREB pathway in Parkinson's disease. Biochem
Biophys Res Commun. 522:388–394. 2020. View Article : Google Scholar
|
|
102
|
Qian C, Ye Y, Mao H, Yao L, Sun X, Wang B,
Zhang H, Xie L, Zhang H, Zhang Y, et al: Downregulated lncRNA-SNHG1
enhances autophagy and prevents cell death through the
miR-221/222/p27/mTOR pathway in Parkinson's disease. Exp Cell Res.
384:1116142019. View Article : Google Scholar
|
|
103
|
Zhuang Z, Zhang L and Liu C: SNHG14
upregulation was a molecular mechanism underlying MPP+
neurotoxicity in dopaminergic SK-N-SH cells via
SNHG14-miR-519a-3p-ATG10 ceRNA pathway. Neurotox Res. 40:553–563.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Fan Y, Zhao X, Lu K and Cheng G: LncRNA
BDNF-AS promotes autophagy and apoptosis in MPTP-induced
Parkinson's disease via ablating microRNA-125b-5p. Brain Res Bull.
157:119–127. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Dong L, Zheng Y and Luo X: lncRNA NEAT1
promotes autophagy of neurons in mice by impairing miR-107-5p.
Bioengineered. 13:12261–12274. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Dong LI, Zheng Y, Gao L and Luo X: lncRNA
NEAT1 prompts autophagy and apoptosis in MPTP-induced Parkinson's
disease by impairing miR-374c-5p. Acta Biochim Biophys Sin
(Shanghai). 53:870–882. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhao J, Li H and Chang N: LncRNA HOTAIR
promotes MPP+-induced neuronal injury in Parkinson's disease by
regulating the miR-874-5p/ATG10 axis. EXCLI J. 19:1141–1153.
2020.PubMed/NCBI
|
|
108
|
Lang Y, Li Y, Yu H, Lin L, Chen X, Wang S
and Zhang H: HOTAIR drives autophagy in midbrain dopaminergic
neurons in the substantia nigra compacta in a mouse model of
Parkinson's disease by elevating NPTX2 via miR-221-3p binding.
Aging (Albany NY). 12:7660–7678. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wang W, Lv R, Zhang J and Liu Y:
circSAMD4A participates in the apoptosis and autophagy of
dopaminergic neurons via the miR-29c-3p-mediated AMPK/mTOR pathway
in Parkinson's disease. Mol Med Rep. 24:5402021. View Article : Google Scholar :
|
|
110
|
Motawi TK, Al-Kady RH, Abdelraouf SM and
Senousy MA: Empagliflozin alleviates endoplasmic reticulum stress
and augments autophagy in rotenone-induced Parkinson's disease in
rats: Targeting the GRP78/PERK/eIF2α/CHOP pathway and miR-211-5p.
Chem Biol Interact. 362:1100022022. View Article : Google Scholar
|
|
111
|
Wang DX, Yang Y, Huang XS, Tang JY, Zhang
X, Huang HX, Zhou B, Liu B, Xiao HQ, Li XH, et al: Pramipexole
attenuates neuronal injury in Parkinson's disease by targeting
miR-96 to activate BNIP3-mediated mitophagy. Neurochem Int.
146:1049722021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Sang Q, Liu X, Wang L, Qi L, Sun W, Wang
W, Sun Y and Zhang H: CircSNCA downregulation by pramipexole
treatment mediates cell apoptosis and autophagy in Parkinson's
disease by targeting miR-7. Aging (Albany NY). 10:1281–1293.
2018.
|
|
113
|
Chen M, Peng L, Gong P, Zheng X, Sun T,
Zhang X and Huo J: Baicalein mediates mitochondrial autophagy via
miR-30b and the NIX/BNIP3 signaling pathway in Parkinson's disease.
Biochem Res Int. 2021:23194122021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chen M, Peng L, Gong P, Zheng X, Sun T,
Zhang X and Huo J: Baicalein induces mitochondrial autophagy to
prevent Parkinson's disease in rats via miR-30b and the
SIRT1/AMPK/mTOR pathway. Front Neurol. 12:6468172022. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kang C, Wang L, Kang M, Liu X, Fu Y and
Gao J: Baicalin alleviates 6-hydroxydopamine-induced neurotoxicity
in PC12 cells by down-regulation of microRNA-192-5p. Brain Res.
1708:84–92. 2019. View Article : Google Scholar
|
















