Apical periodontitis (AP) is a type of chronic oral
inflammatory disease characterized by destruction and absorption of
alveolar bone in the periapical tissue (1,2).
It is usually caused by severe caries, pulp lesions or periodontal
diseases. As a type of highly prevalent oral disease, 52% of the
adult population worldwide was reported to have at least one tooth
with AP according to a systematic review and meta-analysis in 2021
(3). Untreated teeth with AP can
lead to tooth loss, jaw osteomyelitis and systemic disease
associated with mortality (4-6).
In recent years, the association between AP and
systemic disease has attracted attention, leading to the concept of
endodontic medicine (7). As a
local infection (8), previous
studies have shown that microbes and toxins in periapical lesions
have access to the bloodstream from the root canal system during or
following endodontic therapy of teeth (9-11).
Furthermore, AP modulates the systemic immune response by modifying
the levels of inflammatory cytokines, such as C-reactive protein
(CRP), tumor necrosis factor (TNF)-α, IL-6 and IL-1 (12-14). The aforementioned findings
suggested that periapical inflammation is important for maintaining
the health of the whole body. Furthermore, the long-term
persistence of chronic inflammatory disease is implicated in
systemic immune dysregulation and altered inflammatory factors in
the circulation (15). Hence,
chronic inflammation-associated disorder affects the status of AP,
which is dependent on the antagonistic balance between the
pathogenic microorganism and host immune response (16). To the best of our knowledge,
however, evidence of the direct relationship between AP and
systemic disease is lacking.
Globally, DM is a chronic metabolic disease
characterized by hyperglycemia owing to insulin resistance and/or a
deficiency in secretion of insulin or both (21). As one of the most common types of
metabolic disease, it is estimated that the prevalence of DM for
all age groups worldwide will rise from 2.8% in 2000 to 4.4% in
2030 and may affect ≥693 million people in 2045 (22,23). Diabetes is accompanied by various
complications (24). It is
considered that oral complications in patients with diabetes, such
as AP, could affect quality of life (25). As the prevalence of diabetes
increases, it may lead to increasing consequences of the
complications of DM, such as enhancing the inflammatory response in
apical tissue and accelerating alveolar bone loss (26). There have been numerous studies on
the possible association between DM and AP (27-30). Notably, there may be a
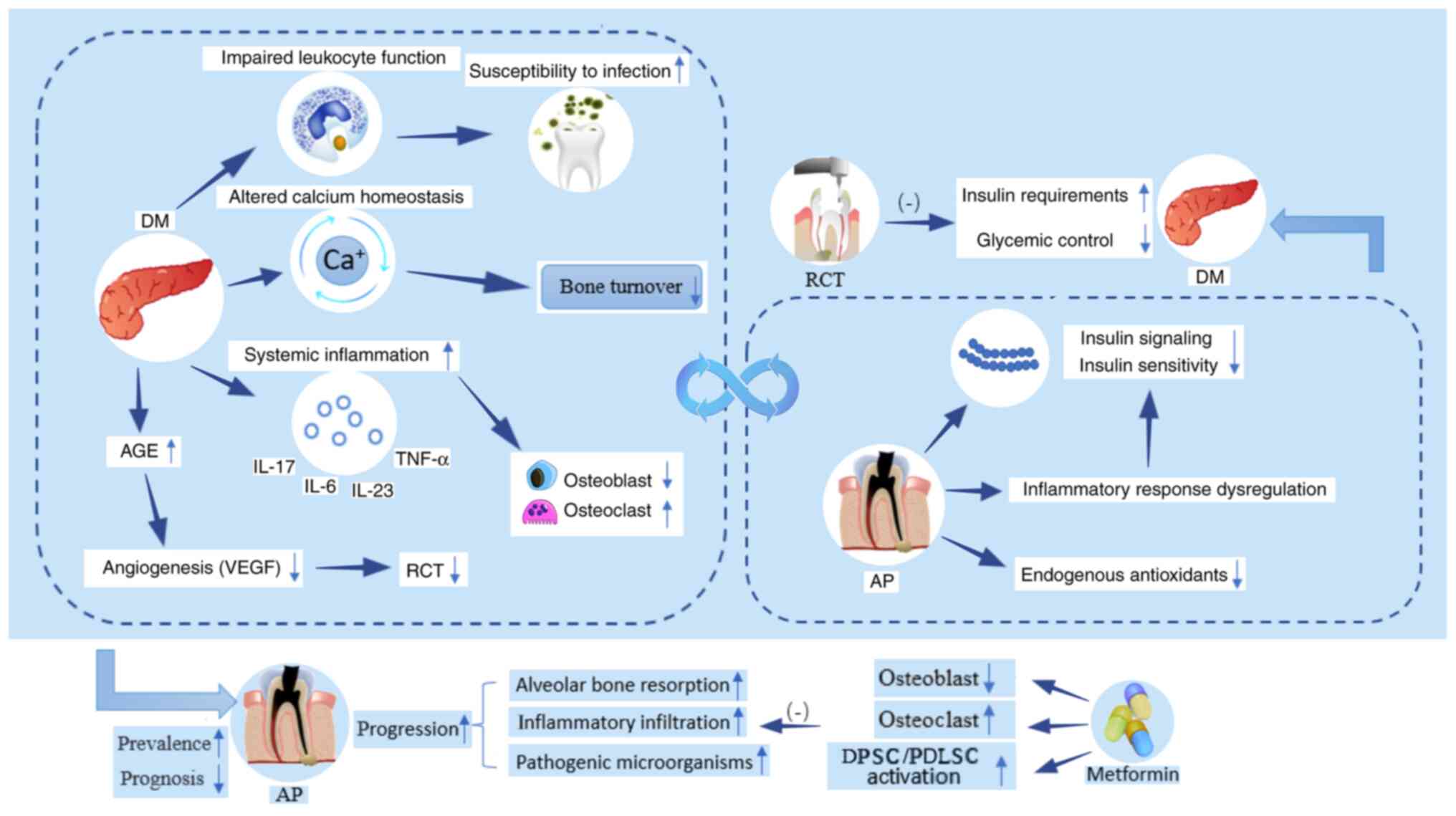
bidirectional association between DM and AP (Fig 2). DM may increase the prevalence of
AP, accelerate the progression of AP and undermine the efficacy of
root canal treatment (RCT). However, AP can affect insulin
signaling pathways or reduce insulin sensitivity, leading to
increased insulin requirements and blood glucose levels.
In addition to affecting the prevalence of AP, DM
accelerates the progression of AP. In animal studies, high sugar
intake and hyperglycemic states increase susceptibility to oral
infection as a result of the disruption of leukocyte function,
which is also supported by a greater and unbalanced presence of
pathogenic microorganisms in root canals in periapical lesions in
diabetes (41,42). In a rat model of combined AP and
DM, increased inflammatory infiltration, higher levels of
proinflammatory cytokines (IL-17, IL-23, IL-6 and TNF-α) and larger
lesion size are observed in periapical tissue (41,43). Cintra et al (44) hypothesized that this might be
partly due to enhanced systemic inflammation of DM, which
contributes to a greater destruction of the alveolar bone in the
periapical lesions. For example, IL-17, as a destructive
inflammatory cytokine, serves a critical role in the resorption of
periapical bone (45,46). Moreover, TNF-α could also promote
osteoclast differentiation and maturation by activating the NF-κB
signaling pathway to trigger a decrease in osteoblast function and
apoptosis, resulting in an imbalance in osteoblast-osteolysis
coupling (47). Moreover, the
imbalance of calcium homeostasis associated with insulin deficiency
leads to bone loss (48,49). Metformin, a classical diabetic
prescription drug, improves hyperglycemic conditions and enhances
osteogenesis by regulating glucose metabolism, promoting cell
migration and increasing angiogenesis (50,51). In addition, metformin could
activate the proliferation and osteogenic differentiation of dental
pulp and periodontal ligament stem cells, which serve an important
role in the repair and mineralization of oral-associated bone
defects (52,53). In animal studies, intramuscular
injection of metformin in rats with AP is effective in decreasing
the size of periapical lesions via suppression of the NF-κB
signaling pathway and diminishing the hypoxia-induced apoptosis of
osteoblasts (54,55). In addition, intracanal metformin
decreases expression of inducible nitric oxide synthase and nitric
oxide to inhibit monocyte migration in periapical tissue (56). The aforementioned results confirm
the positive effect of DM on the progression of AP. By contrast,
Sarmento et al (57) found
no significant differences in bone resorption mediators between
patients with DM and normoglycemic patients with AP; this may be
because the sample population was too small and the DM group
comprised well-controlled individuals with a mean glycated
hemoglobin (HbA1c) <7%.
Previous evidence has suggested that diabetes is a
key preoperative factor in evaluating the prognosis of RCT and is a
common cause for tooth extraction following non-surgical RCT
(58-60). The accumulation of late
glycosylated products in DM prolong the treatment time of RCT and
increased the rate of infection in the oral cavity (60). In a retrospective case-control
study, Uğur Aydın et al (61) assessed changes of fractal
dimension and concluded that DM has a negative effect on the
healing progress of periapical lesions after RCT. In the light of
clinical and radiographic healing outcomes, the success rate of RCT
is significantly lower in patients with DM or poor glycemic control
due to impairment of the angiogenic process in DM (62,63), which is in accordance with other
clinical studies (32,64-67). Similar results have been observed
in patients with type 1 diabetes (68). Conversely, other studies suggest
that DM does not affect the healing outcome of RCT (31,69-71). Different epidemiological
methodologies may contribute to the different outcomes, including
tooth type, radiographic method, assessment criteria and follow-up
time. The healing of RCT in DM is a continuous process and could be
affected by various factors, such as time to assess prognosis,
status of general health, and control of oral reinfections. The
outcomes of cross-sectional studies should be evaluated with care
and more prospective studies with longer follow-up time, larger
sample size and stricter control of confounding factors are
required.
Inflammation is involved in the pathogenesis of DM,
as inflammatory states could decrease insulin sensitivity (72). Chronic infection of AP could cause
aggravated and dysregulated inflammatory response, which may result
in poor glycemic control and increased insulin requirements
(49,73,74). Similarly, animal experiments have
showed that AP could alter insulin signaling and increase blood
glucose concentrations by elevating serum inflammatory cytokines
and activating the adaptive immune system (74-77). Maternal alterations in
inflammation and insulin signaling pathways caused by AP may
directly affect insulin resistance in adult offspring (78). In the rat model of DM, mean
platelet count could be elevated in the presence of both AP and
periodontitis (79). In addition,
alterations in antioxidant status may be one of the potential
mechanisms by which AP affects the pathogenesis of DM (80,81). AP could enhance systemic effects
of DM, as shown by decreased serum albumin levels and increased
uric acid concentrations in DM rats with AP (80).
The efficacy of RCT in diabetes is unknown. In a
case report, a patient with DM noticed a rapid increase in insulin
demand after an exacerbation of the endodontic-periodontic lesion
of a tooth, while the requirement for insulin decreased suddenly
within one day of the root canal preparation (82). By contrast, a prospective clinical
study with a 1-year follow-up period by Arya et al (67) suggested that endodontic treatment
does not improve the levels of HbA1c in patients with DM.
Furthermore, a pilot investigation indicated that AP does not
affect levels of inflammatory markers or glycemic control in
patients with T2DM (83). The
limited sample size and uncontrolled confounding variables may
partly explain the findings of the pilot study. Moreover, the
administration of metformin in some participants with combined DM
and AP may be a confounding factor influence blood glucose levels,
masking the contribution of AP to blood glucose. In addition, both
periapical healing and HbA1c levels in diabetic patients vary over
time. Hence, it is critical to determine a consistent and
appropriate time to assess levels of HbA1c following endodontic
therapy in DM patients with AP.
Osteoporosis is a common metabolic bone disorder in
post-menopausal patients with estrogen deficiency and manifests as
increased bone fragility due to bone loss and microarchitectural
deterioration of bone tissue (84,85). Osteoporosis decreases total
skeletal mass, including jawbone (86-88). Bone changes in osteoporosis are
correlated with decrease of alveolar bone density and height of the
alveolar ridge and loss of teeth (89-91).
Systemic factors of bone remodeling in osteoporosis,
such as estrogen, modify the balance of periapical bone metabolism.
In ovariectomized animal models, estrogen deficiency serves a key
role in the pathogenesis of periapical lesion by upregulating
expression of members of the NLRP3/caspase-1/IL-1β axis and RANKL
(95,97,98), leading to increased periapical
bone resorption. The disturbed systemic inflammatory state in
patients with osteoporosis is hypothesized to be the main cause of
the imbalance between periapical osteoclasts and osteoblasts, which
induces osteoclast apoptosis (99,100). Lucisano et al (101) found oral microorganisms in the
saliva as well as greater periapical bone loss in ovariectomized
mice compared with a control group, which indicated that decreased
estrogen aggravates development of periapical lesions by altering
the microbiota in saliva. Similarly, Gomes-Filho et al
(102) simulated the effects of
estrogen by administering raloxifene (RLX) in ovariectomized mice
and found that RLX was able to inhibit the production of local
regulators of osteoclastogenesis and angiogenesis induced by
estrogen deficiency during the development of AP.
Follicle-stimulating hormone (FSH), a hormone that
increases with the decrease of estrogen levels, is an independent
risk factor for periapical inflammation. FSH could exacerbate bone
loss in periapical lesions via directly coupling FSH receptor on
the surface of osteoclasts and elevated secretion of inflammatory
cytokines (103). FSH inhibitor
leuprorelin has a protective effect against periapical bone loss of
the experimental periapical lesions in ovariectomized rats
(104).
In general, osteoporosis is considered to show a
unidirectional association with AP. Osteoporosis increases
incidence of AP and estrogen linking these diseases. To the best of
our knowledge, however, evidence for this conclusion is limited.
More robust epidemiological evidence is required to corroborate
this conclusion.
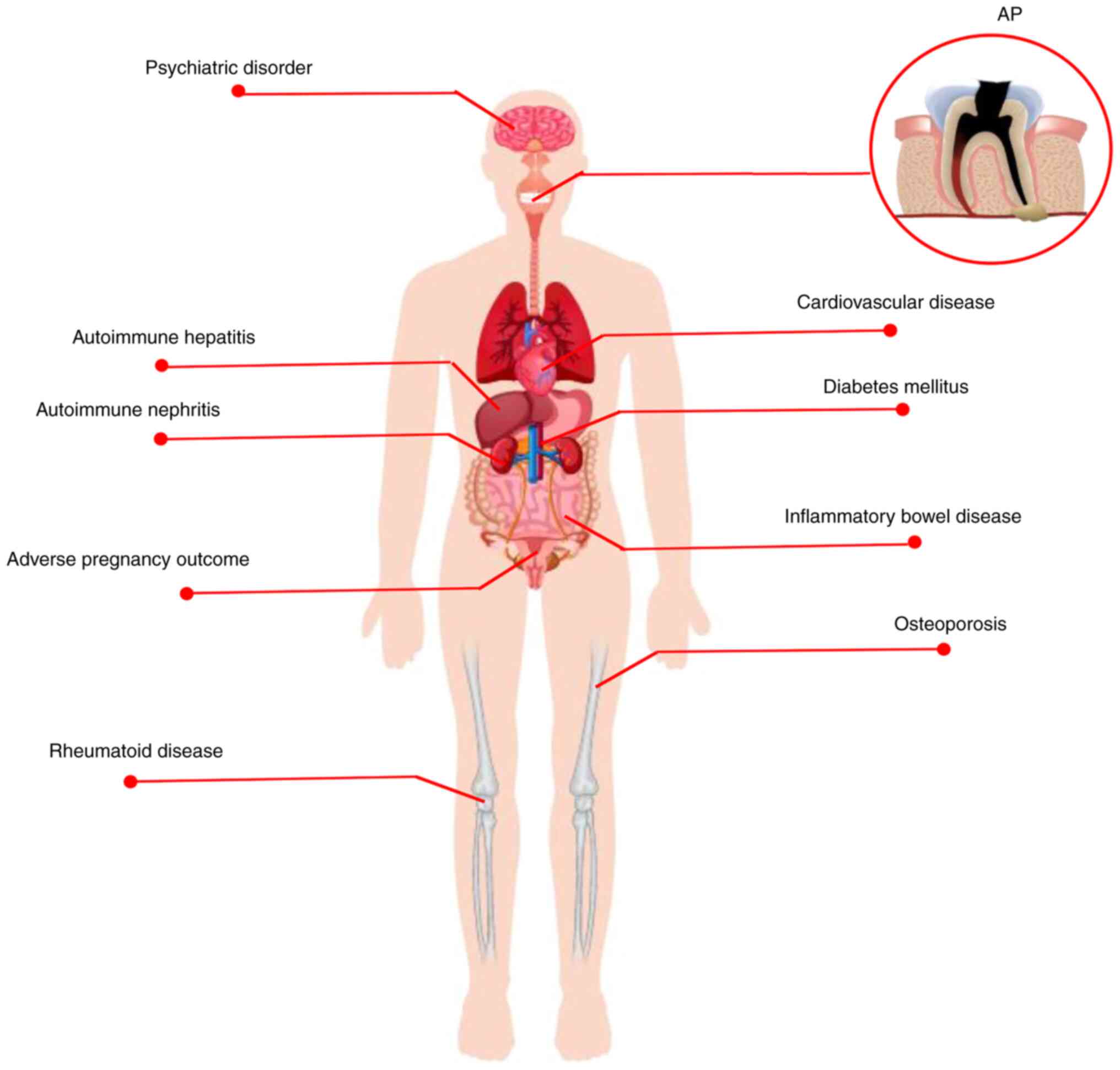
AP is a chronic inflammatory disease involving a
variety of immunocompetent cells, similar to the autoimmune system,
such as such as T and B cells, macrophages and immunoglobulins
synthesized by plasma cells (105,106). Given the similar immunocompetent
cells, it is hypothesized that there is an associate the AP with
AD. Previous studies have linked types of AD to AP, such as
rheumatoid and inflammatory bowel disease (IBD) and autoimmune
hepatitis/nephritis (107-109).
In addition, the use of immunosuppressive agents, a
conventional option for the treatment of AD, is associated with AP.
It is been hypothesized that the use of immunosuppressive agents
leads to weakened resistance as a consequence of decreased systemic
leukocyte count, thus increasing the risk of opportunistic oral
infection and susceptibility to AP (110,111). Conversely, Yamasaki et al
(112) conducted an animal study
and suggested that the long-term use of immunosuppressive agents
prior to pulpal exposure significantly inhibits inflammatory
expansion of AP. By contrast, Waterman et al (113) and Teixeira et al
(114) showed that use of
immunosuppressive agents does not exacerbate or reduce the
periapical inflammatory destruction.
The aforementioned contradictory views suggest a
complex and uncertain role of the systemic immune response in AP.
The pathological process of AP is determined by the balance between
host immunity and virulence of the pathogen (111-113) but this dynamic balance is
difficult to define. The differing findings may be related to the
dose and duration of immunosuppressive agents used, as well as
species or virulence of the invading bacteria.
Rheumatoid disease (RD) is a common autoimmune
disease with a global prevalence of 0.24% in 2010 (115). A cross-sectional study conducted
by Karataş et al (116)
suggested that individuals with RD had at least two times higher
risk of AP than controls. Rotstein and Katz (117) reported a similar tendency of
patients with RD to develop AP based on the integrated data of
hospital cases. Similarly, Oh et al (118) reported a clinical case of rapid
destruction of periapical bone during the endodontic therapy in a
patient with RD taking azathioprine. Conversely, Jalali et
al (119) found no
correlation between periapical rarefying osteitis and RD, which
might be due to the restrictive diagnostic criteria of AP reducing
the actual detection rate of periapical osteitis.
AP and RD are types of chronic inflammation
involving similar inflammation markers, such as TNF-α, IL-6 and
IL-17, which promote bone destruction by activating the NF-κB
signaling pathway (120). In
1975, Malmström et al (121,122) performed a series of biopsies on
periapical tissue from patients with RD and found no histological
differences in periapical lesions between patients with RD and
controls. However, they subsequently identified IgG, as well as
other free rheumatoid factors, in periapical tissue from patients
with RD (123,124). Furthermore, amyloid protein was
almost five times more frequent in periapical lesions of patients
with RD than in controls (125).
Foxo3a, a representative protein that serves an osteolytic role in
rheumatoid disease, is found in periapical tissue (126). Therefore, it is hypothesized
that the periapical alveolar bone might be a bony target of RD.
Autoimmune hepatitis/nephritis is a chronic
progressive inflammatory disease mediated by a dysfunctional immune
response. Their etiology is obscure but higher susceptibility may
be associated with genetics, medication and persistent infection
(127-129). In the later stages of
progression, severe autoimmune hepatitis/nephritis develops into
end-stage liver/renal disease (ESRD), requiring liver/renal
transplantation (130).
Assessment of the oral health of patients with liver
disease revealed that the patients with liver disease exhibit a
higher frequency of oral infection (131-133), particularly in liver transplant
candidates (134-136). Castellanos-Cosano et al
(137) performed a descriptive
cross-sectional study that found that the prevalence of AP in liver
transplant candidates is higher than in controls, while the
frequency of root-filled teeth (RFT) was lower. Furthermore, an
epidemiological study by Grønkjær et al (138) confirmed that nearly half of
patients with liver cirrhosis exhibited periapical radiolucency.
These findings were confirmed by an animal study, in which
increased periapical bone loss, inflammatory cell infiltration and
expression of inflammatory factors in the periapical tissue were be
observed in a rat model of liver fibrosis with AP (139).
Oral disease is also prominent in patients with
kidney disease. Children with purpura nephritis are more likely to
develop periapical infection (140,141). In patients with ESRD, oral
hygiene status is worse than that of healthy individuals (142,143). Additionally, the prevalence of
oral disease is associated with severity of the renal failure and
worsens with length of dialysis treatment (144,145). A clinal study conducted by
Buhlin et al (146) found
that more than half of patients with ESRD have at least one tooth
with periapical inflammation. In 2017, Khalighinejad et al
(147) provided epidemiological
evidence of the association between AP and ESRD and indicated that
the incidence of AP is notably higher in patients with ESRD and
number of AP teeth in patients with ESRD is significantly
correlated with urea serum levels.
The aforementioned findings suggest that patients
with autoimmune hepatitis/nephritis are at greater risk of AP
compared with healthy individuals. The susceptibility of patients
with autoimmune hepatitis/nephropathy to periapical inflammation
may be partly attributable to liver/renal failure, loss of
detoxification and accumulation of harmful metabolites, such as
Urea, creatinine, uric acid, resulting in a systemic
hyperinflammatory state and immunosuppression (130,148).
The aforementioned data indicate that AP might serve
a pathogenic role in autoimmune hepatitis/nephritis. The
microorganisms within periapical tissue have the potential to
colonize the liver/kidney either directly or by forming immune
complexes, triggering a misdirected autoimmune response (152,154).
In brief, there is an interaction between AP and
autoimmune hepatitis/nephritis. Extensive epidemiological evidence
(137,138,146,147) suggests that patients with
autoimmune hepatitis/nephritis are more likely to exhibit AP.
Conversely, as an infectious source, periapical inflammation may
trigger autoimmune hepatitis/nephritis. Therefore, early and
thorough treatment of AP is important. In certain cases, endodontic
treatment could be combined with antibiotics, especially in
children who have a high propensity to develop purpura nephritis.
In addition, it is essential to follow up children with Henoch
Schönlein Purpura after endodontic treatment.
IBDs are chronic recurrent autoimmune diseases
characterized by diffuse inflammation of the intestinal mucosa and
include ulcerative colitis (UC) and Crohn's disease (CD), which
affect any section of digestive tract (155,156). The connections between IBD and
oral health (157), particularly
with respect to periodontitis and dental caries, are recognized
(158-160). The relationship between AP and
IBDs has also gained attention (109).
In addition to increased incidence, it is
hypothesized that the impaired immune system of patients with IBD
has a detrimental impact on the progression and prognosis of AP
(107,167). CD is a T helper (Th) 1 type of
immune disease, whereas UC is defined as a Th2 type of immune
disease (168). Both types of
immune response are associated with the pathological process of AP.
For example, activation of osteoclasts in the Th1 lymphocyte
response is implicated in the progression of periapical bone
destruction, while the Th2 lymphocyte response is associated with
the healing of AP following RCT (169-171). Accordingly, IBD may be
associated with the onset and worsening of AP.
From a microscopic perspective, microorganisms
originating from the oral cavity may serve a role in the link
between AP and IBD (151,172).
The oral cavity is the start of the gastrointestinal tract
(173). Oral bacteria that enter
the gut during swallowing could stimulate intestinal pathogens
(necrotrophy) and create novel phenotypical bacterial virulence
genes by increasing bacterial virulence (151,174). On the other hand, oral bacteria
might be directly linked with digestive disease by altering
intestinal flora (175). An
animal study in rats by Tavares et al (176) demonstrated that AP can elevate
the intestinal leptin levels in metabolic disorders by modulating
gut microbiota. Dysbiosis of the intestinal microbiota is a common
feature of intestinal diseases, including IBD (177). Recently, Gan et al
(178) established an AP model
in rat infected by Porphyromonas gingivalis and found that
AP changed the diversity of intestinal microbiota and P.
gingivalis was not detected in the collected stools. The
aforementioned results suggest that periapical inflammation might
alter the intestinal flora through multiple pathways (178). Similarly, Kojima et al
(153) found a significantly
higher detection rate of Streptococcus mutans in patients
with IBD compared with that in controls in a clinical study. In
addition, it was observed that the injection of S. mutans
into mice is more likely to cause IBD than oral administration
(153).
Immunosuppressive agents are generally used to
treat IBD in the clinic. However, use of immunosuppressive agents
increases the risk of oral opportunistic infections and bone marrow
suppression, thus increasing the susceptibility to AP or promoting
further deterioration of periapical lesions (118,179). BMs are recombinant human
proteins that target inflammatory factors, such as anti-TNF agents
(180). Existing studies have
shown that patients with IBDs and AP exhibit faster and better
healing with few complications in endodontic treatment combined
with anti-TNF therapy (109,181). Theoretically, anti-TNF agents
block not only the direct stimulatory effect of TNF-α on
osteoclasts, but also the negative effect on osteoblast activity
and differentiation, which relieves destruction of periapical
tissue and stimulates regeneration of supporting tissue (182). Accordingly, it is hypothesized
that patients with IBD and severe treatment-resistant AP and with
elevated levels of circulating TNF-α may benefit from anti-TNF
treatment.
CVD, particularly cerebrovascular and ischemic
heart disease, are primary causes of mortality and disability
worldwide. An estimated 17.7 million deaths could be attributed to
CVD, accounting for 31% of all deaths worldwide in 2019 (183), which is a major threat to human
life (184,185). Therefore, it is key to determine
the risk factors associated with CVD. The relationship between AP
and CVD has been investigated for several decades and numerous
reviews or meta-analyses have been published (186-189). Most studies have found a weak
correlation between AP and CVD (186-191), with only a few disagree
(192,193).
A systematic review with meta-analysis in 2022
suggested a weak positive association between AP and CVD (194). Atherosclerosis is the main
pathological basis of CVD and is highly correlated with the
prevalence of AP, as shown by epidemiological studies (195,196). Additionally, Conti et al
(197) confirmed that
atherosclerosis increases the inflammatory reaction and size of
periapical lesions. As one of the most common types of CVD,
coronary artery disease (CAD) is associated with a higher
prevalence of periapical inflammation (198-200). In comparison with non-CAD
subjects, patients with CAD have a nearly threefold increase in the
risk of AP (201), which is
consistent with another study detected AP in 42.6% of patients with
CAD and in 40.1% of non-CAD controls (202). Hypertension, a recognized
primary risk factor for CVD, is also linked with a high prevalence
and increased radiographic area of AP (203), and the prevalence of AP in
hypertensive patients is notably decreased following treatment with
angiotensin II receptor blockers (204).
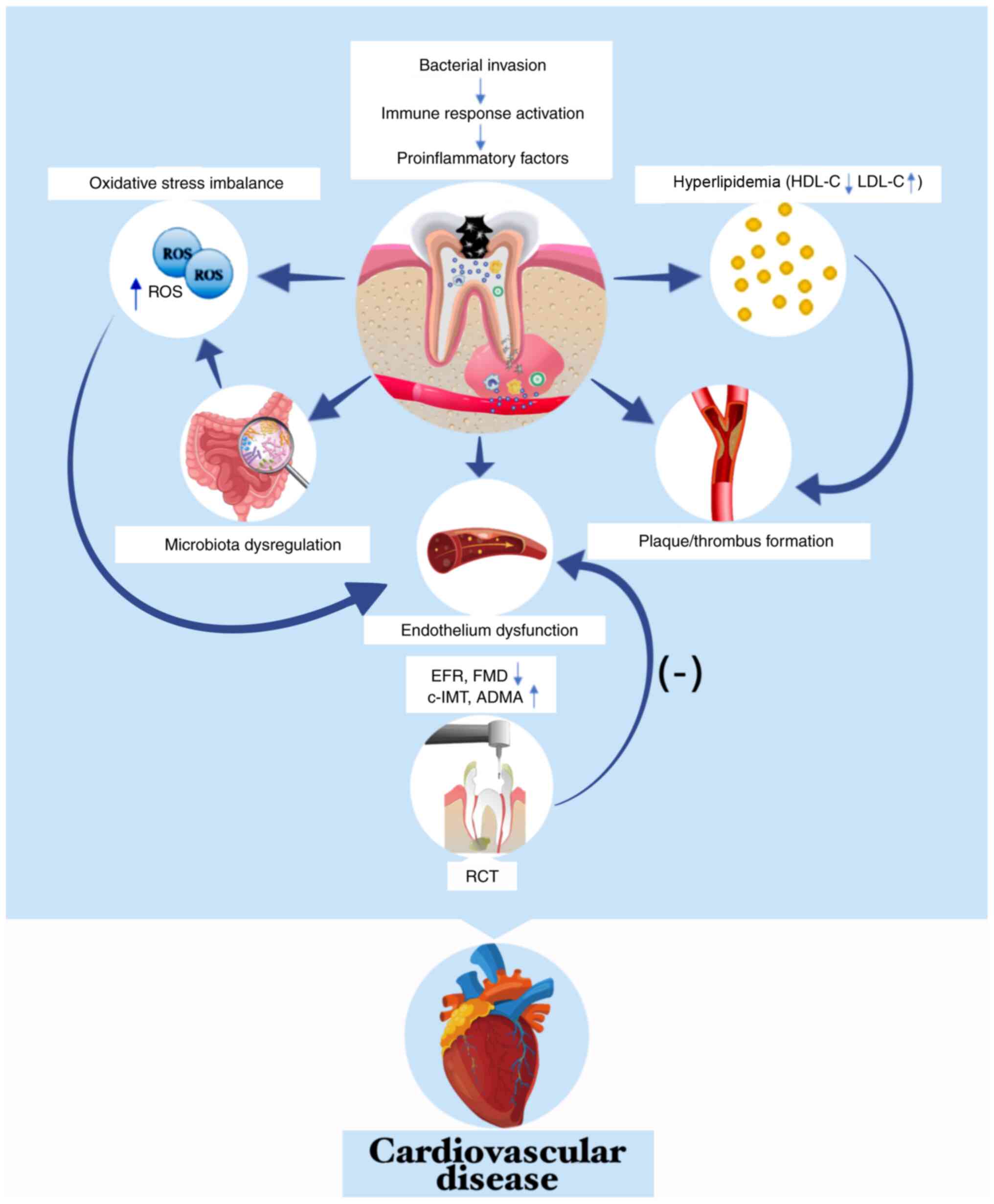
Numerous underlying mechanisms have been proposed
for the association between AP and CVD (Fig. 3). Bacteria and/or their products
may translocate from periapical tissue to various parts of the body
via systemic circulation, especially areas with pre-existing
cardiovascular lesions, and accelerate the progression of CVD. For
example, DNA of P. endodontalis in monocytes (210) and elevated levels of anti-P.
endodontalis IgG are found in peripheral circulation of
patients with AP (211). In
addition, Kazanci et al (212) reported an 8-year-old boy was
admitted to hospital for acute cerebral infarct; cerebrospinal
fluid cultures yielded Streptococcus oralis from alveolar
abscess of the left maxillary first premolar. These findings are in
accordance with a previous study that found bacteria and/or their
products in circulation trigger low-grade systemic inflammation and
contribute to the formation of plaques or thrombi (178).
In terms of oxidative stress, reactive oxygen
species (ROS) are by-products of cellular metabolism involved in
the pathological process of both AP and atherosclerosis (217). During pulp infection, bacterial
motifs bind toll-like receptors (TLRs) on the surface of phagocytes
and promote synthesis and release of ROS (218). Furthermore, the dysbiosis of
intestinal flora due to AP may be a source of accumulated ROS in
the body (96,176). Excess ROS production directly
damages the vascular endothelium and activates multiple components
of the immune system, such as increasing the inflammatory cytokine
expression and amplifying the neutrophil recruitment (219), which was confirmed by a study
demonstrating that AP could disrupt the oxidative status and impair
cardiodynamics in hypertensive rats (203). Moreover, antioxidant agent
tempol exerts a prevent effect on the establishment of AP in rats
with doxorubicin-induced cardiomyopathy (220).
APOs are harmful to both pregnant people and
newborn infants and related with early spontaneous abortion to an
extent (221,222). In particular, the alteration of
hormone levels during pregnancy increases vascular permeability and
accelerates the spread of inflammation (223). Maternal infection is more likely
to cross the placental barrier, leading to APOs (224).
The aforementioned results suggest that bacteria
from periapical lesions could enter the circulation and induce
temporary bacteremia, together with increased vascular permeability
due to changes in hormone levels during pregnancy, which allow
bacteria in the bloodstream to easily cross the placental barrier
and induce maternal and fetal inflammatory reactions and immune
responses. TNF-α, IL-1β and IL-6 induce premature labor, while
IL-10 and vascular endothelial growth factor prevent LBW (235-238). Theoretically, maternal infection
with P. gingivalis may inhibit expression of IL-10, thereby
promoting intrauterine growth restriction; therefore, AP may induce
LBWPB (239).
To the best of our knowledge, studies about the
association between AP and APOs are lacking. The majority of
studies show that AP is positively associated with APO. However,
more studies are required to investigate the association between AP
and APO and determine whether it is necessary to take preventive
measures against AP in pregnant people.
With the rapid pace and increasing pressure of
modern society, psychiatric disorders are becoming increasingly
prominent, it is estimated the lifetime prevalence rates were 22.5%
for any non-substance abuse mental disorder in the United States
(240). Excessive stress,
persistent negative emotion and severe sleep disorder affect
psychiatric health to varying degrees. On one hand, psychiatric
disorders affect the balance of endocrine hormone and immune
response, which decreases host resistance and increases
susceptibility to chronic disease (241). On the other hand, objective
causes such as difficulties in maintaining oral hygiene confer
susceptibility to AP. Negative mental state is involved in the
underlying mechanism of various types of oral disease, such as
periodontitis, oral mucosal ulcers and bruxism (241-243). For example, Maes (244) hypothesized that severe
depression is accompanied by hypersecretion of adrenaline,
disordered systemic immune response and activation of acute
inflammatory markers, which lead to decreased host resistance and
allow oral pathogens to trigger periapical lesions. Furthermore, in
the dental clinic, excessive mental stress and disturbed sleep
schedule are common in asymptomatic patients with AP who typically
seek emergency dental treatment for acute toothache (245).
Mental stress is a state of physical or
psychological tension caused by adverse mental or emotional stimuli
that interferes with normal physiological functions of the body
(249). Studies (250,251) have shown that the levels of
catecholamines, cortisol hormones and inflammatory factors in the
body are closely associated with mental stress Excessive stress
stimulates secretion of catecholamines by exciting the sympathetic
nervous system and activating the
hypothalamic-pituitary-adrenocortical axis, promoting the release
of cortisol (250,252). Cortisol regulates immunity by
decreasing the number and activity of immune cells and inhibiting
secretion of cytokines (253).
In a survey, Haug and Marthinussen (245) found that a sudden onset of acute
toothache in patients with chronic AP is typically associated with
greater stress at home or work; higher levels of cortisol and
inflammatory factors in saliva may be the direct cause of acute
episodes of AP (254).
Similarly, exogenous chronic stress exacerbates the pathological
process of AP by increasing periapical bone resorption and release
of inflammatory cytokines (255,256). In addition, the application of
adrenergic blockers is effective in reducing the number of
osteoclasts in the periapical region (257). Thus, mental stress induces
imbalance between the secretion of cortisol and inflammatory
factors in saliva, which is strongly correlated with the severity
of AP (254).
Not applicable.
LY, LC, WS and CY wrote and revised the manuscript
and constructed table and figures. QT and ZY conceived the study
and revised the manuscript. All authors have read and approved the
final manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
The present study was supported by National Natural Science
Foundation of China (grant no. 82000500), Hubei Province Key
Laboratory of Oral and Maxillofacial Development and Regeneration
(grant no. 2021kqhm004) and Scientific Research Program of Hubei
Provincial Health Commission (grant no. WJ2021Q059).
|
1
|
Yamasaki M, Kumazawa M, Kohsaka T,
Nakamura H and Kameyama Y: Pulpal and periapical tissue reactions
after experimental pulpal exposure in rats. J Endod. 20:13–17.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kakehashi S, Stanley HR and Fitzgerald RJ:
The effects of surgical exposures of dental pulps in germ-free and
conventional laboratory rats. Oral Surg Oral Med Oral Pathol.
20:340–349. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tibúrcio-Machado CS, Michelon C, Zanatta
FB, Gomes MS, Marin JA and Bier CA: The global prevalence of apical
periodontitis: A systematic review and meta-analysis. Int Endod J.
54:712–735. 2021. View Article : Google Scholar
|
|
4
|
Bahrami G, Vaeth M, Kirkevang LL, Wenzel A
and Isidor F: Risk factors for tooth loss in an adult population: A
radiographic study. J Clin Periodontol. 35:1059–1065. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gomes MS, Hugo FN, Hilgert JB, Sant'Ana
Filho M, Padilha DM, Simonsick EM, Ferrucci L and Reynolds MA:
Apical periodontitis and incident cardiovascular events in the
baltimore longitudinal study of ageing. Int Endod J. 49:334–342.
2016. View Article : Google Scholar
|
|
6
|
Kierdorf U, Olsen MT, Kahle P, Ludolphy C
and Kierdorf H: Dental pulp exposure, periapical inflammation and
suppurative osteomyelitis of the jaws in juvenile Baltic grey seals
(Halichoerus grypus grypus) from the late 19th century. PLoS One.
14:e02154012019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cintra LTA, Gomes MS, da Silva CC, Faria
FD, Benetti F, Cosme-Silva L, Samuel RO, Pinheiro TN, Estrela C,
González AC and Segura-Egea JJ: Evolution of endodontic medicine: A
critical narrative review of the interrelationship between
endodontics and systemic pathological conditions. Odontology.
109:741–769. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Kolltveit KM, Tronstad L and Olsen
I: Systemic diseases caused by oral infection. Clin Microbiol Rev.
13:547–558. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Savarrio L, Mackenzie D, Riggio M,
Saunders WP and Bagg J: Detection of bacteraemias during
non-surgicalroot canal treatment. J Dent. 33:293–303. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baumgartner JC, Heggers JP and Harrison
JW: The incidence of bacteremias related to endodontic procedures.
I. Nonsurgical endodontics. J Endod. 2:135–140. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Debelian GJ, Olsen I and Tronstad L:
Bacteremia in conjunction with endodontic therapy. Endod Dent
Traumatol. 11:142–149. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gomes MS, Blattner TC, Sant'Ana Filho M,
Grecca FS, Hugo FN, Fouad AF and Reynolds MA: Can apical
periodontitis modify systemic levels of inflammatory markers? A
systematic review and meta-analysis. J Endod. 39:1205–1217. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Samuel RO, Gomes-Filho JE, Azuma MM,
Sumida DH, de Oliveira SHP, Chiba FY, Bomfim SRM, Ciarlini PC,
Narciso LG and Cintra LTA: Endodontic infections increase leukocyte
and lymphocyte levels in the blood. Clin Oral Investig.
22:1395–1401. 2018. View Article : Google Scholar
|
|
14
|
Poornima L, Ravishankar P, Abbott PV,
Subbiya A and PradeepKumar AR: Impact of root canal treatment on
high-sensitivity C-reactive protein levels in systemically healthy
adults with apical periodontitis-a preliminary prospective,
longitudinal interventional study. Int Endod J. 54:501–508. 2021.
View Article : Google Scholar
|
|
15
|
Chen Y, Liu S and Leng SX: Chronic
low-grade inflammatory phenotype (CLIP) and senescent immune
dysregulation. Clin Ther. 41:400–409. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan LT, Zhong S, Naqvi AR, Self-Fordham
J, Nares S, Bair E and Khan AA: MicroRNAs: New insights into the
pathogenesis of endodontic periapical disease. J Endod.
39:1498–1503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sasaki H, Hirai K, Martins CM, Furusho H,
Battaglino R and Hashimoto K: Interrelationship between periapical
lesion and systemic metabolic disorders. Curr Pharm Des.
22:2204–2215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Segura-Egea JJ, Martín-González J and
Castellanos-Cosano L: Endodontic medicine: Connections between
apical periodontitis and systemic diseases. Int Endod J.
48:933–951. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khalighinejad N, Aminoshariae MR,
Aminoshariae A, Kulild JC, Mickel A and Fouad AF: Association
between systemic diseases and apical periodontitis. J Endod.
42:1427–1434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cotti E and Mercuro G: Apical
periodontitis and cardiovascular diseases: Previous findings and
ongoing research. Int Endod J. 48:926–932. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
American Diabetes Association: Diagnosis
and classification of diabetes mellitus. Diabetes Care. 36(Suppl
1): S67–S74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: Estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho NH, Shaw JE, Karuranga S, Huang Y, da
Rocha Fernandes JD, Ohlrogge AW and Malanda B: IDF diabetes atlas:
Global estimates of diabetes prevalence for 2017 and projections
for 2045. Diabetes Res Clin Pract. 138:271–281. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Velasco-Ortega E, Delgado-Ruiz RA and
López-López J: Dentistry and diabetes: The influence of diabetes in
oral diseases and dental treatments. J Diabetes Res.
2016:60731902016. View Article : Google Scholar
|
|
25
|
Haag DG, Peres KG, Balasubramanian M and
Brennan DS: Oral conditions and health-related quality of life: A
systematic review. J Dent Res. 96:864–874. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rohani B: Oral manifestations in patients
with diabetes mellitus. World J Diabetes. 10:485–489. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Segura-Egea JJ, Cabanillas-Balsera D,
Jiménez-Sánchez MC and Martín-González J: Endodontics and diabetes:
Association versus causation. Int Endod J. 52:790–802. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tibúrcio-Machado CDS, Bello MDC, Maier J,
Wolle CFB and Bier CAS: Influence of diabetes in the development of
apical periodontitis: A critical literature review of human
studies. J Endod. 43:370–376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yip N, Liu C, Wu D and Fouad AF: The
association of apical periodontitis and type 2 diabetes mellitus: A
large hospital network cross-sectional case-controlled study. J Am
Dent Assoc. 152:434–443. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pérez-Losada FDL, Estrugo-Devesa A,
Castellanos-Cosano L, Segura-Egea JJ, López-López J and
Velasco-Ortega E: Apical periodontitis and diabetes mellitus type
2: A systematic review and meta-analysis. J Clin Med. 9:5402020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Segura-Egea JJ, Jiménez-Pinzón A,
Ríos-Santos JV, Velasco-Ortega E, Cisneros-Cabello R and
Poyato-Ferrera M: High prevalence of apical periodontitis amongst
type 2 diabetic patients. Int Endod J. 38:564–569. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smadi L: Apical periodontitis and
endodontic treatment in patients with type II diabetes mellitus:
Comparative cross-sectional survey. J Contemp Dent Pract.
18:358–362. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sánchez-Domínguez B, López-López J,
Jané-Salas E, Castellanos-Cosano L, Velasco-Ortega E and
Segura-Egea JJ: Glycated hemoglobin levels and prevalence of apical
periodontitis in type 2 diabetic patients. J Endod. 41:601–606.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saleh W, Xue W and Katz J: Diabetes
mellitus and periapical abscess: A cross-sectional study. J Endod.
46:1605–1609. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sisli SN: Evaluation of the relationship
between type II diabetes mellitus and the prevalence of apical
periodontitis in root-filled teeth using cone beam computed
tomography: An observational cross-sectional study. Med Princ
Pract. 28:533–538. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mesgarani A, Haghanifar S, Eshkevari N,
Ehsani M, Khafri S, Nafarzade S and Damankesh Z: Frequency of
odontogenic periradicular lesions in diabetic patients. Caspian J
Intern Med. 5:22–25. 2014.PubMed/NCBI
|
|
37
|
Kohsaka T, Kumazawa M, Yamasaki M and
Nakamura H: Periapical lesions in rats with streptozotocin-induced
diabetes. J Endod. 22:418–421. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Al-Nazhan SA, Alsaeed SA, Al-Attas HA,
Dohaithem AJ, Al-Serhan MS and Al-Maflehi NS: Prevalence of apical
periodontitis and quality of root canal treatment in an adult Saudi
population. Saudi Med J. 38:413–421. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Falk H, Hugoson A and Thorstensson H:
Number of teeth, prevalence of caries and periapical lesions in
insulin-dependent diabetics. Scand J Dent Res. 97:198–206.
1989.PubMed/NCBI
|
|
40
|
Alghofaily M, Tordik P, Romberg E,
Martinho F and Fouad AF: Healing of apical periodontitis after
nonsurgical root canal treatment: The role of statin intake. J
Endod. 44:1355–1360. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
De la Torre-Luna R, Domínguez-Pérez RA,
Guillén-Nepita AL, Ayala-Herrera JL, Martínez-Martínez RE,
Romero-Ayala ME, Pérez-Serrano RM and Vázquez-Garcidueñas MS:
Prevalence of Candida albicans in primary endodontic infections
associated with a higher frequency of apical periodontitis in type
two diabetes mellitus patients. Eur J Clin Microbiol Infect Dis.
39:131–138. 2020. View Article : Google Scholar
|
|
42
|
Graves DT, Corrêa JD and Silva TA: The
oral microbiota is modified by systemic diseases. J Dent Res.
98:148–156. 2019. View Article : Google Scholar :
|
|
43
|
Samuel RO, Ervolino E, de Azevedo Queiroz
ÍO, Azuma MM, Ferreira GT and Cintra LTA: Th1/Th2/Th17/Treg balance
in apical periodontitis of normoglycemic and diabetic rats. J
Endod. 45:1009–1015. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cintra LTA, da Silva Facundo AC, Prieto
AKC, Sumida DH, Narciso LG, Mogami Bomfim SR, Oliveira e Silva C,
Dezan-Júnior E and Gomes-Filho JE: Blood profile and histology in
oral infections associated with diabetes. J Endod. 40:1139–1144.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Azuma MM, Gomes-Filho JE, Prieto AKC,
Samuel RO, de Lima VMF, Sumida DH, Ervolino E and Cintra LTA:
Diabetes increases interleukin-17 levels in periapical, hepatic,
and renal tissues in rats. Arch Oral Biol. 83:230–235. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hasiakos S, Gwack Y, Kang M and Nishimura
I: Calcium signaling in T cells and chronic inflammatory disorders
of the oral cavity. J Dent Res. 100:693–699. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu Y, Yang Y, Wang L, Chen Y, Han X, Sun
L, Chen H and Chen Q: Effect of Bifidobacterium on osteoclasts:
TNF-α/NF-κB inflammatory signal pathway-mediated mechanism. Front
Endocrinol (Lausanne). 14:11092962023. View Article : Google Scholar
|
|
48
|
Iwama A, Nishigaki N, Nakamura K, Imaizumi
I, Shibata N, Yamasaki M, Nakamura H, Kameyama Y and Kapila Y: The
effect of high sugar intake on the development of periradicular
lesions in rats with type 2 diabetes. J Dent Res. 82:322–325. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cintra LTA, Samuel RO, Azuma MM, Ribeiro
CP, Narciso LG, de Lima VM, Sumida DH, Coclete GA, Dezan-Júnior E
and Gomes-Filho JE: Apical periodontitis and periodontal disease
increase serum IL-17 levels in normoglycemic and diabetic rats.
Clin Oral Investig. 18:2123–2128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bahrambeigi S, Yousefi B, Rahimi M and
Shafiei-Irannejad V: Metformin; an old antidiabetic drug with new
potentials in bone disorders. Biomed Pharmacother. 109:1593–1601.
2019. View Article : Google Scholar
|
|
51
|
Zhu M, Zhao Z, Xu HHK, Dai Z, Yu K, Xiao
L, Schneider A, Weir MD, Oates TW, Bai Y and Zhang K: Effects of
metformin delivery via biomaterials on bone and dental tissue
engineering. Int J Mol Sci. 23:159052022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang S, Xia Y, Ma T, Weir MD, Ren K,
Reynolds MA, Shu Y, Cheng L, Schneider A and Xu HHK: Novel
metformin-containing resin promotes odontogenic differentiation and
mineral synthesis of dental pulp stem cells. Drug Deliv Transl Res.
9:85–96. 2019. View Article : Google Scholar
|
|
53
|
Zhang R, Liang Q, Kang W and Ge S:
Metformin facilitates the proliferation, migration, and osteogenic
differentiation of periodontal ligament stem cells in vitro. Cell
Biol Int. 44:70–79. 2020. View Article : Google Scholar
|
|
54
|
Liu L, Zhang C, Hu Y and Peng B:
Protective effect of metformin on periapical lesions in rats by
decreasing the ratio of receptor activator of nuclear factor kappa
B ligand/osteoprotegerin. J Endod. 38:943–947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lai EHH, Yang CN, Lin SK, Wang HW, Kok SH,
Hong CY, Su IH, Yang H and Chang JZC: Metformin ameliorates
periapical lesions through suppression of hypoxia-induced apoptosis
of osteoblasts. J Endod. 44:1817–1825. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang HW, Lai EHH, Yang CN, Lin SK, Hong
CY, Yang H, Chang JZC and Kok SH: Intracanal metformin promotes
healing of apical periodontitis via suppressing inducible nitric
oxide synthase expression and monocyte recruitment. J Endod.
46:65–73. 2020. View Article : Google Scholar
|
|
57
|
Sarmento EB, Gomes CC, Pires FR, Pinto LC,
Antunes LAA and Armada L: Immunoexpression of bone resorption
biomarkers in apical periodontitis in diabetics and
normoglycaemics. Int Endod J. 53:1025–1032. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang CH, Chueh LH, Chen SC, Feng YC, Hsiao
CK and Chiang CP: Impact of diabetes mellitus, hypertension, and
coronary artery disease on tooth extraction after nonsurgical
endodontic treatment. J Endod. 37:1–5. 2011. View Article : Google Scholar
|
|
59
|
Mindiola MJ, Mickel AK, Sami C, Jones JJ,
Lalumandier JA and Nelson SS: Endodontic treatment in an American
Indian population: A 10-year retrospective study. J Endod.
32:828–832. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lima SMF, Grisi DC, Kogawa EM, Franco OL,
Peixoto VC, Gonçalves-Júnior JF, Arruda MP and Rezende TM: Diabetes
mellitus and inflammatory pulpal and periapical disease: A review.
Int Endod J. 46:700–709. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Uğur Aydın Z, Ocak MG, Bayrak S, Göller
Bulut D and Orhan K: The effect of type 2 diabetes mellitus on
changes in the fractal dimension of periapical lesion in teeth
after root canal treatment: A fractal analysis study. Int Endod J.
54:181–189. 2021. View Article : Google Scholar
|
|
62
|
Rudranaik S, Nayak M and Babshet M:
Periapical healing outcome following single visit endodontic
treatment in patients with type 2 diabetes mellitus. J Clin Exp
Dent. 8:e498–e504. 2016.PubMed/NCBI
|
|
63
|
Martinho JP, Coelho A, Oliveiros B, Pires
S, Abrantes AM, Paulo S, Carvalho AC, Carrilho E, Paula A, Carvalho
L, et al: Impairment of the angiogenic process may contribute to
lower success rate of root canal treatments in diabetes mellitus.
Int Endod J. 54:1687–1698. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Britto LR, Katz J, Guelmann M and Heft M:
Periradicular radiographic assessment in diabetic and control
individuals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
96:449–452. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fouad AF and Burleson J: The effect of
diabetes mellitus on endodontic treatment outcome: Data from an
electronic patient record. J Am Dent Assoc. 134(43-51): 117–118.
2003. View Article : Google Scholar
|
|
66
|
Laukkanen E, Vehkalahti MM and Kotiranta
AK: Impact of systemic diseases and tooth-based factors on outcome
of root canal treatment. Int Endod J. 52:1417–1426. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Arya S, Duhan J, Tewari S, Sangwan P,
Ghalaut V and Aggarwal S: Healing of apical periodontitis after
nonsurgical treatment in patients with type 2 diabetes. J Endod.
43:1623–1627. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Limeira FIR, Arantes DC, de Souza Oliveira
C, de Melo DP, Magalhães CS and Bento PM: Root canal treatment and
apical periodontitis in a brazilian population with type 1 diabetes
mellitus: A cross-sectional paired study. J Endod. 46:756–762.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Doyle SL, Hodges JS, Pesun IJ, Baisden MK
and Bowles WR: Factors affecting outcomes for single-tooth implants
and endodontic restorations. J Endod. 33:399–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Marotta PS, Fontes TV, Armada L, Lima KC,
Rôças IN and Siqueira JF Jr: Type 2 diabetes mellitus and the
prevalence of apical periodontitis and endodontic treatment in an
adult Brazilian population. J Endod. 38:297–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ng YL, Mann V and Gulabivala K: A
prospective study of the factors affecting outcomes of nonsurgical
root canal treatment: Part 1: Periapical health. Int Endod J.
44:583–609. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bloomgarden ZT: Inflammation and insulin
resistance. Diabetes Care. 26:1619–1623. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Cintra LTA, Samuel RO, Facundo ACS, Prieto
AK, Sumida DH, Bomfim SR, Souza JC, Dezan-Júnior E and Gomes-Filho
JE: Relationships between oral infections and blood glucose
concentrations or HbA1c levels in normal and diabetic rats. Int
Endod J. 47:228–237. 2014. View Article : Google Scholar
|
|
74
|
Astolphi RD, Curbete MM, Colombo NH,
Shirakashi DJ, Chiba FY, Prieto AK, Cintra LT, Bomfim SR, Ervolino
E and Sumida DH: Periapical lesions decrease insulin signal and
cause insulin resistance. J Endod. 39:648–652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Astolphi RD, Curbete MM, Chiba FY, Cintra
LT, Ervolino E, da Mota MS, Antoniali C, Garbin CA and Sumida DH:
Periapical lesions decrease insulin signaling in rat skeletal
muscle. J Endod. 41:1305–1310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pereira RF, de Oliveira da Mota MS, de
Lima Coutinho Mattera MS, Tsosura TV, Chiba FY, Garbin CA, Ervolino
E, Cintra LT, Okamoto MM, Machado UF and Sumida DH: Periapical
lesions decrease Akt serine phosphorylation and plasma membrane
GLUT4 content in rat skeletal muscle. Clin Oral Investig.
20:1625–1630. 2016. View Article : Google Scholar
|
|
77
|
Felipe Pereira R, Willian Lattari Tessarin
G, Yamamoto Chiba F, Sara de Lima Coutinho Mattera M, Gomes Pereira
A, Verônica Saori Tsosura T, Gustavo Balera Brito V, Akira Fujii,
de Oliveira R, Ervolino E, Helena Penha, de Oliveira S, et al:
Apical periodontitis promotes insulin resistance and alters
adaptive immunity markers in rats. Saudi Dent J. 33:979–986. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tsosura TVS, Chiba FY, Mattera MSLC,
Pereira RF, Cintra LTA, Conti LC, Santos RMD, Mateus JHP, Garbin
CAS and Sumida DH: Maternal apical periodontitis is associated with
insulin resistance in adult offspring. Int Endod J. 52:1040–1050.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ferreira LL: Diabetic rats present high
mean platelet count in the presence of oral infections. Braz Dent
J. 28:548–551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Prieto AKC, Gomes-Filho JE, Azuma MM,
Sivieri-Araújo G, Narciso LG, Souza JC, Ciarlini PC and Cintra LTA:
Influence of apical periodontitis on stress oxidative parameters in
diabetic rats. J Endod. 43:1651–1656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Barcelos RCS, Rosa HZ, Roversi K,
Tibúrcio-Machado CDS, Inchaki PT, Burger ME and Bier CAS: Apical
periodontitis induces changes on oxidative stress parameters and
increases Na+/K+-ATPase activity in adult
rats. Arch Oral Biol. 118:1048492020. View Article : Google Scholar
|
|
82
|
Schulze A, Schönauer M and Busse M: Sudden
improvement of insulin sensitivity related to an endodontic
treatment. J Periodontol. 78:2380–2384. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Stys LPA, Böttcher DE, Scarparo RK,
Gonçalves Waltrick SB, de Figueiredo JAP, Gomes MS and Campos MM:
Serum levels of inflammatory markers and HbA1c in patients with
type 2 diabetes and apical periodontitis: Preliminary findings.
Aust Endod J. 48:105–115. 2022. View Article : Google Scholar
|
|
84
|
No authors listed. Consensus development
conference: Diagnosis, prophylaxis, and treatment of osteoporosis.
Am J Med. 94:646–650. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
NIH Consensus Development Panel on
Osteoporosis Prevention, Diagnosis, and Therapy: Osteoporosis
prevention, diagnosis, and therapy. JAMA. 285:785–795. 2001.
View Article : Google Scholar
|
|
86
|
Gilles JA, Carnes DL, Dallas MR, Holt SC
and Bonewald LF: Oral bone loss is increased in ovariectomized
rats. J Endod. 23:419–422. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
López-López J, Castellanos-Cosano L,
Estrugo-Devesa A, Gómez-Vaquero C, Velasco-Ortega E and Segura-Egea
JJ: Radiolucent periapical lesions and bone mineral density in
post-menopausal women. Gerodontology. 32:195–201. 2015. View Article : Google Scholar
|
|
88
|
Tounta TS: Diagnosis of osteoporosis in
dental patients. J Frailty Sarcopenia Falls. 2:21–27. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Payne JB, Reinhardt RA, Nummikoski PV and
Patil KD: Longitudinal alveolar bone loss in postmenopausal
osteoporotic/osteopenic women. Osteoporos Int. 10:34–40. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Brennan RM, Genco RJ, Hovey KM, Trevisan M
and Wactawski-Wende J: Clinical attachment loss, systemic bone
density, and subgingival calculus in postmenopausal women. J
Periodontol. 78:2104–2111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Dervis E: Oral implications of
osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
100:349–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Katz J and Rotstein I: Prevalence of
periapical lesions in patients with osteoporosis. J Endod.
47:234–238. 2021. View Article : Google Scholar
|
|
93
|
Wayama MT, Yoshimura H, Ohba S, Yoshida H,
Matsuda S, Kobayashi J, Kobayashi M, Gomes Filho JE and Sano K:
Diminished progression of periapical lesions with zoledronic acid
in ovariectomized rats. J Endod. 41:2002–2007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ikeda M, Karakawa A, Takizawa H, Azetsu Y,
Sakai N, Chatani M, Suzuki N and Takami M: Effects of anti-receptor
activator of nuclear factor kappa B ligand antibody and zoledronic
acid on periapical lesion development in mice. J Endod. 48:632–640.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Xiong H, Peng B, Wei L, Zhang X and Wang
L: Effect of an estrogen-deficient state and alendronate therapy on
bone loss resulting from experimental periapical lesions in rats. J
Endod. 33:1304–1308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Silva RAB, Sousa-Pereira AP, Lucisano MP,
Romualdo PC, Paula-Silva FWG, Consolaro A, Silva LAB and
Nelson-Filho P: Alendronate inhibits osteocyte apoptosis and
inflammation via IL-6, inhibiting bone resorption in periapical
lesions of ovariectomized rats. Int Endod J. 53:84–96. 2020.
View Article : Google Scholar
|
|
97
|
Guan X, Guan Y, Shi C, Zhu X, He Y, Wei Z,
Yang J and Hou T: Estrogen deficiency aggravates apical
periodontitis by regulating NLRP3/caspase-1/IL-1β axis. Am J Transl
Res. 12:660–671. 2020.
|
|
98
|
Zhang X, Peng B, Fan M, Bian Z and Chen Z:
The effect of estrogen deficiency on receptor activator of nuclear
factor kappa B ligand and osteoprotegerin synthesis in periapical
lesions induced in rats. J Endod. 33:1053–1056. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Romualdo PC, Lucisano MP, Paula-Silva FWG,
Leoni GB, Sousa-Neto MD, Silva RAB, Silva LAB and Nelson-Filho P:
Ovariectomy exacerbates apical periodontitis in rats with an
increase in expression of proinflammatory cytokines and matrix
metalloproteinases. J Endod. 44:780–785. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Brasil SC, Santos RMM, Fernandes A, Alves
FR, Pires FR, Siqueira JF Jr and Armada L: Influence of oestrogen
deficiency on the development of apical periodontitis. Int Endod J.
50:161–166. 2017. View Article : Google Scholar
|
|
101
|
Lucisano MP, da Silva RAB, de Sousa
Pereira AP, Romualdo PC, Feres M, de Queiroz AM, Nelson-Filho P and
da Silva LAB: Alteration of the oral microbiota may be a
responsible factor, along with estrogen deficiency, by the
development of larger periapical lesions. Clin Oral Investig.
25:3651–3662. 2021. View Article : Google Scholar
|
|
102
|
Gomes-Filho JE, Wayama MT, Dornelles RCM,
Ervolino E, Yamanari GH, Lodi CS, Sivieri-Araújo G, Dezan-Júnior E
and Cintra LT: Raloxifene modulates regulators of
osteoclastogenesis and angiogenesis in an oestrogen deficiency
periapical lesion model. Int Endod J. 48:1059–1068. 2015.
View Article : Google Scholar
|
|
103
|
Qian H, Guan X and Bian Z: FSH aggravates
bone loss in ovariectomised rats with experimental periapical
periodontitis. Mol Med Rep. 14:2997–3006. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Liu S, Cheng Y, Xu W and Bian Z:
Protective effects of follicle-stimulating hormone inhibitor on
alveolar bone loss resulting from experimental periapical lesions
in ovariectomized rats. J Endod. 36:658–663. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Braz-Silva PH, Bergamini ML, Mardegan AP,
De Rosa CS, Hasseus B and Jonasson P: Inflammatory profile of
chronic apical periodontitis: A literature review. Acta Odontol
Scand. 77:173–180. 2019. View Article : Google Scholar
|
|
106
|
Matsuo T, Ebisu S, Shimabukuro Y, Ohtake T
and Okada H: Quantitative analysis of immunocompetent cells in
human periapical lesions: Correlations with clinical findings of
the involved teeth. J Endod. 18:497–500. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Guerrero-Gironés J, Ros-Valverde A,
Pecci-Lloret MP, Rodríguez-Lozano FJ and Pecci-Lloret MR:
Association between pulpal-periapical pathology and autoimmune
diseases: A systematic review. J Clin Med. 10:48862021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Karataş E, Kul A and Tepecik E:
Association of ankylosing spondylitis with radiographically and
clinically diagnosed apical periodontitis: A cross-sectional study.
Dent Med Probl. 57:171–175. 2020. View Article : Google Scholar
|
|
109
|
Barta Z: Apical periodontitis in patients
with inflammatory bowel disease: A puppet master? Inflamm Bowel
Dis. 26:280–282. 2020. View Article : Google Scholar
|
|
110
|
Nakamura K, Yamasaki M, Nishigaki N, Iwama
A, Imaizumi I, Nakamura H and Kameyama Y: Effect of
methotrexate-induced neutropenia on pulpal inflammation in rats. J
Endod. 28:287–290. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yoshinari N, Kameyama Y, Aoyama Y,
Nishiyama H and Noguchi T: Effect of long-term methotrexate-induced
neutropenia on experimental periodontal lesion in rats. J
Periodontal Res. 29:393–400. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Yamasaki M, Kumazawa M, Kohsaka T and
Nakamura H: Effect of methotrexate-induced neutropenia on rat
periapical lesion. Oral Surg Oral Med Oral Pathol. 77:655–661.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Waterman PA Jr, Torabinejad M, McMillan PJ
and Kettering JD: Development of periradicular lesions in
immunosuppressed rats. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 85:720–725. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Teixeira FB, Gomes BP, Ferraz CC,
Souza-Filho FJ and Zaia AA: Radiographic analysis of the
development of periapical lesions in normal rats,
sialoadenectomized rats and sialoadenectomized-immunosuppressed
rats. Endod Dent Traumatol. 16:154–157. 2000. View Article : Google Scholar
|
|
115
|
Wasserman A: Rheumatoid arthritis: Common
questions about diagnosis and management. Am Fam Physician.
97:455–462. 2018.PubMed/NCBI
|
|
116
|
Karataş E, Kul A and Tepecik E:
Association between rheumatoid arthritis and apical periodontitis:
A cross-sectional study. Eur Endod J. 5:155–158. 2020.
|
|
117
|
Rotstein I and Katz J: Prevalence of
periapical abscesses in patients with rheumatoid arthritis. A cross
sectional study. Am J Dent. 34:211–214. 2021.PubMed/NCBI
|
|
118
|
Oh WM, Hwang IN, Son HH and Hwang YC:
Rapid periapical bone destruction during endodontic treatment of a
patient with rheumatoid arthritis. J Endod. 34:1261–1263. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Jalali P, Glickman GN, Schneiderman ED and
Schweitzer JL: Prevalence of periapical rarefying osteitis in
patients with rheumatoid arthritis. J Endod. 43:1093–1096. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Cotti E, Schirru E, Acquas E and Usai P:
An overview on biologic medications and their possible role in
apical periodontitis. J Endod. 40:1902–1911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Malmström M: Immunoglobulin clases IgG,
IgM, IgA and complement component C3 in dental periapical lesions
of patients with rheumatoid disease. Scand J Rheumatol. 4:57–64.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Malmström M and Calonius PE: Teeth loss
and the inflammation of teeth-supporting tissues in rheumatoid
disease. Scand J Rheumatol. 4:49–55. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Malmström M and Jokinen EJ: Free
rheumatoid factor in dental periapical lesions and gingivae of
patients with rheumatoid disease. Scand J Rheumatol. 4:121–124.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Malmström M and Natvig JB: IgG rheumatoid
factor in dental periapical lesions of patients with rheumatoid
disease. Scand J Rheumatol. 4:177–185. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Malmström M and Husby G: Occurrence of
amyloid in the teeth-supporting tissues of patients with rheumatoid
diseases. An immunohistochemical study. Scand J Rheumatol.
4:186–192. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ishii K, Hatori K, Takeichi O, Makino K,
Himi K, Komiya H and Ogiso B: Expression of the Forkhead box
transcription factor Foxo3a in human periapical granulomas. J Oral
Sci. 60:479–483. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Vergani D and Mieli-Vergani G: Autoimmune
manifestations in viral hepatitis. Semin Immunopathol. 35:73–85.
2013. View Article : Google Scholar
|
|
128
|
Bjørklund G, Pivin M, Hangan T,
Yurkovskaya O and Pivina L: Autoimmune polyendocrine syndrome type
1: Clinical manifestations, pathogenetic features, and management
approach. Autoimmun Rev. 21:1031352022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Joyce E, Glasner P, Ranganathan S and
Swiatecka-Urban A: Tubulointerstitial nephritis: Diagnosis,
treatment, and monitoring. Pediatr Nephrol. 32:577–587. 2017.
View Article : Google Scholar
|
|
130
|
Cohen G: Immune dysfunction in Uremia
2020. Toxins (Basel). 12. pp. 4392020, View Article : Google Scholar
|
|
131
|
Grønkjær LL and Vilstrup H: Oral health in
patients with liver cirrhosis. Eur J Gastroenterol Hepatol.
27:834–839. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Coates EA, Brennan D, Logan RM, Goss AN,
Scopacasa B, Spencer AJ and Gorkic E: Hepatitis C infection and
associated oral health problems. Aust Dent J. 45:108–114. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Lins L, Bittencourt PL, Evangelista MA,
Lins R, Codes L, Cavalcanti AR, Paraná R and Bastos J: Oral health
profile of cirrhotic patients awaiting liver transplantation in the
Brazilian Northeast. Transplant Proc. 43:1319–1321. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Barbero P, Garzino Demo MG, Milanesio M
and Ottobrelli A: The dental assessment of the patient waiting for
a liver transplant. Minerva Stomatol. 45:431–439. 1996.In Italian.
PubMed/NCBI
|
|
135
|
Guggenheimer J, Eghtesad B and Stock DJ:
Dental management of the (solid) organ transplant patient. Oral
Surg Oral Med Oral Pathol Oral Radiol Endod. 95:383–389. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Helenius-Hietala J, Meurman JH,
Höckerstedt K, Lindqvist C and Isoniemi H: Effect of the aetiology
and severity of liver disease on oral health and dental treatment
prior to transplantation. Transpl Int. 25:158–165. 2012. View Article : Google Scholar
|
|
137
|
Castellanos-Cosano L, Machuca-Portillo G,
Segura-Sampedro JJ, Torres-Lagares D, López-López J, Velasco-Ortega
E and Segura-Egea JJ: Prevalence of apical periodontitis and
frequency of root canal treatments in liver transplant candidates.
Med Oral Patol Oral Cir Bucal. 18:e773–e779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Grønkjær LL, Holmstrup P, Schou S,
Schwartz K, Kongstad J, Jepsen P and Vilstrup H: Presence and
consequence of tooth periapical radiolucency in patients with
cirrhosis. Hepat Med. 8:97–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Cantiga-Silva C, Estrela C, Segura-Egea
JJ, Azevedo JP, de Oliveira PHC, Cardoso CBM, Pinheiro TN, Ervolino
E, Sivieri-Araújo G and Cintra LTA: Inflammatory profile of apical
periodontitis associated with liver fibrosis in rats: Histological
and immunohistochemical analysis. Int Endod J. 54:1353–1361. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Echavarría-García AC, Pozos-Guillén A,
Tejeda-Nava F, Flores Arriaga JC and Garrocho-Rangel A: Oral
management of children with Henoch-Schönlein purpura and associated
glomerulonephritis: A scoping review. Eur J Paediatr Dent.
19:134–138. 2018.
|
|
141
|
Inoue CN, Nagasaka T, Matsutani S,
Ishidoya M, Homma R and Chiba Y: Efficacy of early dental and ENT
therapy in preventing nephropathy in pediatric Henoch-Schönlein
purpura. Clin Rheumatol. 27:1489–1496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Al-Wahadni A and Al-Omari MA: Dental
diseases in a Jordanian population on renal dialysis. Quintessence
Int. 34:343–347. 2003.PubMed/NCBI
|
|
143
|
Souza CM, Braosi APR, Luczyszyn SM,
Casagrande RW, Pecoits-Filho R, Riella MC, Ignácio SA and
Trevilatto PC: Oral health in Brazilian patients with chronic renal
disease. Rev Med Chil. 136:741–746. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Bayraktar G, Kurtulus I, Duraduryan A,
Cintan S, Kazancioglu R, Yildiz A, Bural C, Bozfakioglu S, Besler
M, Trablus S and Issever H: Dental and periodontal findings in
hemodialysis patients. Oral Dis. 13:393–397. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Sobrado Marinho JS, Tomás Carmona I,
Loureiro A, Limeres Posse J, García Caballero L and Diz Dios P:
Oral health status in patients with moderate-severe and terminal
renal failure. Med Oral Patol Oral Cir Bucal. 12:E305–E310.
2007.PubMed/NCBI
|
|
146
|
Buhlin K, Bárány P, Heimbürger O,
Stenvinkel P and Gustafsson A: Oral health and pro-inflammatory
status in end-stage renal disease patients. Oral Health Prev Dent.
5:235–244. 2007.PubMed/NCBI
|
|
147
|
Khalighinejad N, Aminoshariae A, Kulild
JC, Sahly K and Mickel A: Association of end-stage renal disease
with radiographically and clinically diagnosed apical
periodontitis: A hospital-based study. J Endod. 43:1438–1441. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Caly WR and Strauss E: A prospective study
of bacterial infections in patients with cirrhosis. J Hepatol.
18:353–358. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Nair PN: Apical periodontitis: A dynamic
encounter between root canal infection and host response.
Periodontol. 2000(13): 121–148. 1997. View Article : Google Scholar
|
|
150
|
Mizutani K, Mikami R, Gohda T, Gotoh H,
Aoyama N, Matsuura T, Kido D, Takeda K, Izumi Y, Sasaki Y and Iwata
T: Poor oral hygiene and dental caries predict high mortality rate
in hemodialysis: A 3-year cohort study. Sci Rep. 10:218722020.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Olsen I and Yamazaki K: Can oral bacteria
affect the microbiome of the gut? J Oral Microbiol. 11:15864222019.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Ogura Y, Suzuki S, Shirakawa T, Masuda M,
Nakamura H, Iijima K and Yoshikawa N: Haemophilus parainfluenzae
antigen and antibody in children with IgA nephropathy and
Henoch-Schönlein nephritis. Am J Kidney Dis. 36:47–52. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Kojima A, Nakano K, Wada K, Takahashi H,
Katayama K, Yoneda M, Higurashi T, Nomura R, Hokamura K, Muranaka
Y, et al: Infection of specific strains of Streptococcus mutans,
oral bacteria, confers a risk of ulcerative colitis. Sci Rep.
2:3322012. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Tahmassebi JF and Paterson SA: Development
of acute Henoch-Schönlein purpura subsequent to endodontic
treatment. Int J Paediatr Dent. 17:217–222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Papageorgiou SN, Hagner M, Nogueira AVB,
Franke A, Jäger A and Deschner J: Inflammatory bowel disease and
oral health: Systematic review and a meta-analysis. J Clin
Periodontol. 44:382–393. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Baumgart DC and Sandborn WJ: Crohn's
disease. Lancet. 380:1590–1605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Kalmar JR: Crohn's disease: Orofacial
considerations and disease pathogenesis. Periodontol. 2000(6):
101–115. 1994. View Article : Google Scholar
|
|
158
|
She YY, Kong XB, Ge YP, Liu ZY, Chen JY,
Jiang JW, Jiang HB and Fang SL: Periodontitis and inflammatory
bowel disease: A meta-analysis. BMC Oral Health. 20:672020.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zhang Y, Qiao D, Chen R, Zhu F, Gong J and
Yan F: The association between periodontitis and inflammatory bowel
disease: A systematic review and meta-analysis. Biomed Res Int.
2021:66924202021.PubMed/NCBI
|
|
160
|
Tan CXW, Brand HS, Kalender B, De Boer
NKH, Forouzanfar T and de Visscher JGAM: Dental and periodontal
disease in patients with inflammatory bowel disease. Clin Oral
Investig. 25:5273–5280. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Piras V, Usai P, Mezzena S, Susnik M, Ideo
F, Schirru E and Cotti E: Prevalence of apical periodontitis in
patients with inflammatory bowel diseases: A retrospective clinical
study. J Endod. 43:389–394. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Poyato-Borrego M, Segura-Egea JJ,
Martín-González J, Jiménez-Sánchez MC, Cabanillas-Balsera D,
Areal-Quecuty V and Segura-Sampedro JJ: Prevalence of endodontic
infection in patients with Crohn’s disease and ulcerative colitis.
Med Oral Patol Oral Cir Bucal. 26:e208–e215. 2021. View Article : Google Scholar
|
|
163
|
Poyato-Borrego M, Segura-Sampedro JJ,
Martín-González J, Torres-Domínguez Y, Velasco-Ortega E and
Segura-Egea JJ: High prevalence of apical periodontitis in patients
with inflammatory bowel disease: An age- and gender-matched
case-control study. Inflamm Bowel Dis. 26:273–279. 2020. View Article : Google Scholar
|
|
164
|
Johannsen A, Fored MC, Håkansson J, Ekbom
A and Gustafsson A: Consumption of dental treatment in patients
with inflammatory bowel disease, a register study. PLoS One.
10:e01340012015. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Segura-Sampedro JJ, Jiménez-Giménez C,
Jane-Salas E, Cabanillas-Balsera D, Martín-González J, Segura-Egea
JJ and López-López J: Periapical and endodontic status of patients
with inflammatory bowel disease: Age- and sex-matched case-control
study. Int Endod J. 55:748–757. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Lu Y, Li X, Liu S, Zhang Y and Zhang D:
Toll-like receptors and inflammatory bowel disease. Front Immunol.
9:722018. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Ng YL, Mann V, Rahbaran S, Lewsey J and
Gulabivala K: Outcome of primary root canal treatment: systematic
review of the literature-part 2. Influence of clinical factors. Int
Endod J. 41:6–31. 2008. View Article : Google Scholar
|
|
168
|
Bamias G, Martin C, Mishina M, Ross WG,
Rivera-Nieves J, Marini M and Cominelli F: Proinflammatory effects
of TH2 cytokines in a murine model of chronic small intestinal
inflammation. Gastroenterology. 128:654–666. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Fukada SY, Silva TA, Garlet GP, Rosa AL,
da Silva JS and Cunha FQ: Factors involved in the T helper type 1
and type 2 cell commitment and osteoclast regulation in
inflammatory apical diseases. Oral Microbiol Immunol. 24:25–31.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Stashenko P, Wang CY, Tani-Ishii N and Yu
SM: Pathogenesis of induced rat periapical lesions. Oral Surg Oral
Med Oral Pathol. 78:494–502. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Wei L, Xu M and Xiong H: An update of
knowledge on the regulatory role of Treg cells in apical
periodontitis. Oral Dis. 27:1356–1365. 2021. View Article : Google Scholar
|
|
172
|
Van Dyke TE, Dowell VR Jr, Offenbacher S,
Snyder W and Hersh T: Potential role of microorganisms isolated
from periodontal lesions in the pathogenesis of inflammatory bowel
disease. Infect Immun. 53:671–677. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Chi AC, Neville BW, Krayer JW and
Gonsalves WC: Oral manifestations of systemic disease. Am Fam
Physician. 82:1381–1388. 2010.PubMed/NCBI
|
|
174
|
Rodriguez Herrero E, Boon N, Pauwels M,
Bernaerts K, Slomka V, Quirynen M and Teughels W: Necrotrophic
growth of periodontopathogens is a novel virulence factor in oral
biofilms. Sci Rep. 7:11072017. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Read E, Curtis MA and Neves JF: The role
of oral bacteria in inflammatory bowel disease. Nat Rev
Gastroenterol Hepatol. 18:731–742. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Tavares CO, Rost FL, Silva RBM, Dagnino
AP, Adami B, Schirmer H, de Figueiredo JAP, Souto AA, Maito FDM and
Campos MM: Cross talk between apical periodontitis and metabolic
disorders: Experimental evidence on the role of intestinal
adipokines and akkermansia muciniphila. J Endod. 45:174–180. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Carding S, Verbeke K, Vipond DT, Corfe BM
and Owen LJ: Dysbiosis of the gut microbiota in disease. Microb
Ecol Health Dis. 26:261912015.PubMed/NCBI
|
|
178
|
Gan G, Lu B, Zhang R, Luo Y, Chen S, Lei
H, Li Y, Cai Z and Huang X: Chronic apical periodontitis
exacerbates atherosclerosis in apolipoprotein E-deficient mice and
leads to changes in the diversity of gut microbiota. Int Endod J.
55:152–163. 2022. View Article : Google Scholar
|
|
179
|
Ideo F, Niazi S, Mezzena S, Mannocci F and
Cotti E: Prevalence of apical periodontitis in patients with
autoimmune diseases under immunomodulators: A retrospective cohort
study. J Endod. 48:722–729. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Ardizzone S and Bianchi Porro G: Biologic
therapy for inflammatory bowel disease. Drugs. 65:2253–2286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Cotti E, Mezzena S, Schirru E, Ottonello
O, Mura M, Ideo F, Susnik M and Usai P: Healing of apical
periodontitis in patients with inflammatory bowel diseases and
under anti-tumor necrosis factor alpha therapy. J Endod.
44:1777–1782. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Ritchlin CT, Haas-Smith SA, Li P, Hicks DG
and Schwarz EM: Mechanisms of TNF-alpha- and RANKL-mediated
osteoclastogenesis and bone resorption in psoriatic arthritis. J
Clin Invest. 111:821–831. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Joseph P, Leong D, McKee M, Anand SS,
Schwalm JD, Teo K, Mente A and Yusuf S: Reducing the global burden
of cardiovascular disease, part 1: The epidemiology and risk
factors. Circ Res. 121:677–694. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Andersson C and Vasan RS: Epidemiology of
cardiovascular disease in young individuals. Nat Rev Cardiol.
15:230–240. 2018. View Article : Google Scholar
|
|
185
|
Prabhakaran D, Jeemon P and Roy A:
Cardiovascular diseases in india: current epidemiology and future
directions. Circulation. 133:1605–1620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Cotti E, Dessì C, Piras A and Mercuro G:
Can a chronic dental infection be considered a cause of
cardiovascular disease? A review of the literature. Int J Cardiol.
148:4–10. 2011. View Article : Google Scholar
|
|
187
|
Berlin-Broner Y, Febbraio M and Levin L:
Association between apical periodontitis and cardiovascular
diseases: A systematic review of the literature. Int Endod J.
50:847–859. 2017. View Article : Google Scholar
|
|
188
|
González Navarro B, Pintó Sala X and Jané
Salas E: Relationship between cardiovascular disease and dental
pathology. Systematic review. Med Clin (Barc). 149:211–216. 2017.In
English, Spanish. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Koletsi D, Iliadi A, Tzanetakis GN,
Vavuranakis M and Eliades T: Cardiovascular disease and chronic
endodontic infection. Is there an association? A systematic review
and meta-analysis. Int J Environ Res Public Health. 18:91112021.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Garg P and Chaman C: Apical
periodontitis-is it accountable for cardiovascular diseases? J Clin
Diagn Res. 10:ZE08–ZE12. 2016.PubMed/NCBI
|
|
191
|
Jakovljevic A, Duncan HF, Nagendrababu V,
Jacimovic J, Milasin J and Dummer PMH: Association between
cardiovascular diseases and apical periodontitis: An umbrella
review. Int Endod J. 53:1374–1386. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Aloutaibi YA, Alkarim AS, Qumri EM,
Almansour LA and Alghamdi FT: Chronic endodontic infections and
cardiovascular diseases: Does the evidence support an independent
association? Cureus. 13:e198642021.
|
|
193
|
Jiménez-Sánchez MC, Cabanillas-Balsera D,
Areal-Quecuty V, Velasco-Ortega E, Martín-González J and
Segura-Egea JJ: Cardiovascular diseases and apical periodontitis:
Association not always implies causality. Med Oral Patol Oral Cir
Bucal. 25:e652–e659. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Noites R, Teixeira M, Cavero-Redondo I,
Alvarez-Bueno C and Ribeiro F: Apical periodontitis and
cardiovascular disease in adults: A systematic review with
meta-analysis. Rev Cardiovasc Med. 23:1002022. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Leão TSS, Tomasi GH, Conzatti LP, Marrone
LCP, Reynolds MA and Gomes MS: Oral inflammatory burden and carotid
atherosclerosis among stroke patients. J Endod. 48:597–605. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Garrido M, Cárdenas AM, Astorga J, Quinlan
F, Valdés M, Chaparro A, Carvajal P, Pussinen P, Huamán-Chipana P,
Jalil JE and Hernández M: Elevated systemic inflammatory burden and
cardiovascular risk in young adults with endodontic apical lesions.
J Endod. 45:111–115. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Conti LC, Segura-Egea JJ, Cardoso CBM,
Benetti F, Azuma MM, Oliveira PHC, Bomfim SRM and Cintra LTA:
Relationship between apical periodontitis and atherosclerosis in
rats: Lipid profile and histological study. Int Endod J.
53:1387–1397. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Caplan DJ: Chronic apical periodontitis is
more common in subjects with coronary artery disease. J Evid Based
Dent Pract. 14:149–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Caplan DJ, Chasen JB, Krall EA, Cai J,
Kang S, Garcia RI, Offenbacher S and Beck JD: Lesions of endodontic
origin and risk of coronary heart disease. J Dent Res. 85:9962006.
View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Liljestrand JM, Mäntylä P, Paju S, Buhlin
K, Kopra KA, Persson GR, Hernandez M, Nieminen MS, Sinisalo J,
Tjäderhane L and Pussinen PJ: Association of endodontic lesions
with coronary artery disease. J Dent Res. 95:1358–1365. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Costa THR, de Figueiredo Neto JA, de
Oliveira AE, Lopes e Maia Mde F and de Almeida AL: Association
between chronic apical periodontitis and coronary artery disease. J
Endod. 40:164–167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Paloma de Oliveira B, Câmara AC and Aguiar
CM: Prevalence of asymptomatic apical periodontitis and its
association with coronary artery disease in a Brazilian
subpopulation. Acta Stomatol Croat. 51:106–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Milojevic Samanovic A, Jakovljevic V,
Vasovic M, Mitrovic S, Rankovic M, Mihajlovic K, Bolevich S and
Zivkovic V: Cardiac, biochemical and histopathological analysis
reveals impaired heart function in hypertensive rats with apical
periodontitis. Int Endod J. 54:1581–1596. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Katz J and Rotstein I: Prevalence of
periapical abscesses in patients with hypertension: A
cross-sectional study of a large hospital population. J Endod.
47:1070–1074. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Petersen J, Glaßl EM, Nasseri P, Crismani
A, Luger AK, Schoenherr E, Bertl K and Glodny B: The association of
chronic apical periodontitis and endodontic therapy with
atherosclerosis. Clin Oral Investig. 18:1813–1823. 2014. View Article : Google Scholar :
|
|
206
|
Vidal F, Fontes TV, Marques TVF and
Gonçalves LS: Association between apical periodontitis lesions and
plasmatic levels of C-reactive protein, interleukin 6 and
fibrinogen in hypertensive patients. Int Endod J. 49:1107–1115.
2016. View Article : Google Scholar
|
|
207
|
Rashmi N, Galhotra V, Goel P, Rajguru JP,
Jha SK and Kulkarni K: Assessment of C-reactive proteins,
cytokines, and plasma protein levels in hypertensive patients with
apical periodontitis. J Contemp Dent Pract. 18:516–521. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Messing M, Souza LCD, Cavalla F, Kookal
KK, Rizzo G, Walji M, Silva R and Letra A: Investigating potential
correlations between endodontic pathology and cardiovascular
diseases using epidemiological and genetic approaches. J Endod.
45:104–110. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Sebring D, Buhlin K, Norhammar A, Rydén L,
Jonasson P; EndoReCo; Lund H and Kvist T: Endodontic inflammatory
disease: A risk indicator for a first myocardial infarction. Int
Endod J. 55:6–17. 2022. View Article : Google Scholar
|
|
210
|
Bordagaray MJ, Fernández A, Garrido M,
Astorga J, Hoare A and Hernández M: Systemic and extraradicular
bacterial translocation in apical periodontitis. Front Cell Infect
Microbiol. 11:6499252021. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Jiménez C, Garrido M, Pussinen P,
Bordagaray MJ, Fernández A, Vega C, Chaparro A, Hoare A and
Hernández M: Systemic burden and cardiovascular risk to
Porphyromonas species in apical periodontitis. Clin Oral Investig.
26:993–1001. 2022. View Article : Google Scholar
|
|
212
|
Kazanci E, Oguz KK, Gurgey A and Topçu M:
Streptococcus oralis as a risk factor for middle cerebral artery
thrombosis. J Child Neurol. 20:611–613. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Theofilis P, Sagris M, Oikonomou E,
Antonopoulos AS, Siasos G, Tsioufis C and Tousoulis D: Inflammatory
mechanisms contributing to endothelial dysfunction. Biomedicines.
9:7812021. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Cotti E, Dessì C, Piras A, Flore G, Deidda
M, Madeddu C, Zedda A, Longu G and Mercuro G: Association of
endodontic infection with detection of an initial lesion to the
cardiovascular system. J Endod. 37:1624–1629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Bergandi L, Giuggia B, Alovisi M, Comba A,
Silvagno F, Maule M, Aldieri E, Scotti N, Scacciatella P, Conrotto
F, et al: Endothelial dysfunction marker variation in young adults
with chronic apical periodontitis before and after endodontic
treatment. J Endod. 45:500–506. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Chauhan N, Mittal S, Tewari S, Sen J and
Laller K: Association of apical periodontitis with cardiovascular
disease via noninvasive assessment of endothelial function and
subclinical atherosclerosis. J Endod. 45:681–690. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Hernández-Ríos P, Pussinen PJ, Vernal R
and Hernández M: Oxidative stress in the local and systemic events
of apical periodontitis. Front Physiol. 8:8692017. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Chapple IL: Reactive oxygen species and
antioxidants in inflammatory diseases. J Clin Periodontol.
24:287–296. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Tucker MA, Fox BM, Seigler N,
Rodriguez-Miguelez P, Looney J, Thomas J, McKie KT, Forseen C,
Davison GW and Harris RA: Endothelial dysfunction in cystic
fibrosis: Role of oxidative stress. Oxid Med Cell Longev.
2019:16296382019. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Brilhante Wolle CF, de Aguiar Zollmann L,
Etges A, Vitalis GS, Leite CE and Campos MM: Effects of the
antioxidant agent tempol on periapical lesions in rats with
doxorubicin-induced cardiomyopathy. J Endod. 38:191–195. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Vailhé B, Dietl J, Kapp M, Toth B and Arck
P: Increased blood vessel density in decidua parietalis is
associated with spontaneous human first trimester abortion. Hum
Reprod. 14:1628–1634. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Zygmunt M, Herr F, Münstedt K, Lang U and
Liang OD: Angiogenesis and vasculogenesis in pregnancy. Eur J
Obstet Gynecol Reprod Biol. 110(Suppl 1): pp. S10–S18. 2003,
View Article : Google Scholar
|
|
223
|
Plaisier M: Decidualisation and
angiogenesis. Best Pract Res Clin Obstet Gynaecol. 25:259–271.
2011. View Article : Google Scholar
|
|
224
|
Bobetsis YA, Graziani F, Gürsoy M and
Madianos PN: Periodontal disease and adverse pregnancy outcomes.
Periodontol. 2000(83): 154–174. 2020. View Article : Google Scholar
|
|
225
|
Harjunmaa U, Järnstedt J, Alho L, Dewey
KG, Cheung YB, Deitchler M, Ashorn U, Maleta K, Klein NJ and Ashorn
P: Association between maternal dental periapical infections and
pregnancy outcomes: results from a cross-sectional study in Malawi.
Trop Med Int Health. 20:1549–1558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Leal ASM, de Oliveira AEF, Brito LMO,
Lopes FF, Rodrigues VP, Lima KF and de Araújo Martins IC:
Association between chronic apical periodontitis and
low-birth-weight preterm births. J Endod. 41:353–357. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Khalighinejad N, Aminoshariae A, Kulild JC
and Mickel A: Apical periodontitis, a predictor variable for
preeclampsia: A case-control study. J Endod. 43:1611–1614. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Bain JL, Lester SR, Henry WD, Naftel JP
and Johnson RB: Effects of induced periapical abscesses on rat
pregnancy outcomes. Arch Oral Biol. 54:162–171. 2009. View Article : Google Scholar
|
|
229
|
Bain JL, Lester SR, Henry WD, Pongetti JL,
Blackman ME and Johnson RB: Association between maternal periapical
lesions and brain inflammation in rat pups. Arch Oral Biol.
58:266–271. 2013. View Article : Google Scholar
|
|
230
|
Kc K, Shakya S and Zhang H: Gestational
diabetes mellitus and macrosomia: A literature review. Ann Nutr
Metab. 66(Suppl 2): S14–S20. 2015. View Article : Google Scholar
|
|
231
|
Shah H, Nisar N, Hassan A and Butt S:
Association between maternal chronic apical periodontitis (CAP) and
low birth weight preterm birth (LBWPT). J Pak Med Assoc.
72:436–439. 2022.PubMed/NCBI
|
|
232
|
Orstavik D, Kerekes K and Eriksen HM: The
periapical index: A scoring system for radiographic assessment of
apical periodontitis. Endod Dent Traumatol. 2:20–34. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Ao M, Miyauchi M, Furusho H, Inubushi T,
Kitagawa M, Nagasaki A, Sakamoto S, Kozai K and Takata T: Dental
Infection of Porphyromonas gingivalis induces preterm birth in
mice. PLoS One. 10:e01372492015. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Doyle RM, Alber DG, Jones HE, Harris K,
Fitzgerald F, Peebles D and Klein N: Term and preterm labour are
associated with distinct microbial community structures in
placental membranes which are independent of mode of delivery.
Placenta. 35:1099–1101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Lin D, Smith MA, Champagne C, Elter J,
Beck J and Offenbacher S: Porphyromonas gingivalis infection during
pregnancy increases maternal tumor necrosis factor alpha,
suppresses maternal interleukin-10, and enhances fetal growth
restriction and resorption in mice. Infect Immun. 71:5156–5162.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Robertson SA, Care AS and Skinner RJ:
Interleukin 10 regulates inflammatory cytokine synthesis to protect
against lipopolysaccharide-induced abortion and fetal growth
restriction in mice. Biol Reprod. 76:738–748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Coultas L, Chawengsaksophak K and Rossant
J: Endothelial cells and VEGF in vascular development. Nature.
438:937–945. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Awasthi S and Pandey M: Association of
TLR4 and TNF-a gene polymorphisms and TLR4 mRNA levels in preterm
birth in a Northern Indian population. Indian Pediatr. 56:202–204.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Jakovljevic A, Sljivancanin Jakovljevic T,
Duncan HF, Nagendrababu V, Jacimovic J, Aminoshariae A, Milasin J
and Dummer PMH: The association between apical periodontitis and
adverse pregnancy outcomes: A systematic review. Int Endod J.
54:1527–1537. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Regier DA, Farmer ME, Rae DS, Locke BZ,
Keith SJ, Judd LL and Goodwin FK: Comorbidity of mental disorders
with alcohol and other drug abuse. Results from the epidemiologic
catchment area (ECA) study. JAMA. 264:2511–2518. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Sundararajan S, Muthukumar S and Rao SR:
Relationship between depression and chronic periodontitis. J Indian
Soc Periodontol. 19:294–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Chen P, Yao H, Su W, He Y, Cheng K, Wang
Y, Peng W and Li P: Sleep deprivation worsened oral ulcers and
delayed healing process in an experimental rat model. Life Sci.
232:1165942019. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Lavigne GJ, Khoury S, Abe S, Yamaguchi T
and Raphael K: Bruxism physiology and pathology: An overview for
clinicians. J Oral Rehabil. 35:476–494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Maes M: Evidence for an immune response in
major depression: A review and hypothesis. Prog
Neuropsychopharmacol Biol Psychiatry. 19:11–38. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Haug SR and Marthinussen MC: Acute dental
pain and salivary biomarkers for stress and inflammation in
patients with pulpal or periapical inflammation. J Oral Facial Pain
Headache. 33:227–233. 2019. View Article : Google Scholar
|
|
246
|
Wang YX, Kang XN, Cao Y, Zheng DX, Lu YM,
Pang CF, Wang Z, Cheng B and Peng Y: Porphyromonas gingivalis
induces depression via downregulating p75NTR-mediated BDNF
maturation in astrocytes. Brain Behav Immun. 81:523–534. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Martínez M, Martín-Hernández D, Virto L,
MacDowell KS, Montero E, González-Bris Á, Marín MJ, Ambrosio N,
Herrera D, Leza JC, et al: Periodontal diseases and depression: A
pre-clinical in vivo study. J Clin Periodontol. 48:503–527. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Martín-Hernández D, Martínez M,
Robledo-Montaña J, Muñoz-López M, Virto L, Ambrosio N, Marín MJ,
Montero E, Herrera D, Sanz M, et al: Neuroinflammation related to
the blood-brain barrier and sphingosine-1-phosphate in a
pre-clinical model of periodontal diseases and depression in rats.
J Clin Periodontol. 50:642–656. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Saei Ghare Naz M, Ramezani Tehrani F,
Behroozi Lak T, Mohammadzadeh F, Nasiri M, Kholosi Badr F and
Ozgoli G: Quality of life and emotional states of depression,
anxiety and stress in adolescents with polycystic ovary syndrome: A
cross-sectional study. Psychol Res Behav Manag. 13:203–209. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Montoro J, Mullol J, Jáuregui I, Dávila I,
Ferrer M, Bartra J, del Cuvillo A, Sastre J and Valero A: Stress
and allergy. J Investig Allergol Clin Immunol. 19(Suppl 1):
S40–S47. 2009.
|
|
251
|
Eisenhofer G, Lambie DG and Johnson RH:
Beta-adrenoceptor responsiveness and plasma catecholamines as
determinants of cardiovascular reactivity to mental stress. Clin
Sci (Lond). 69:483–492. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Haug SR and Heyeraas KJ: Modulation of
dental inflammation by the sympathetic nervous system. J Dent Res.
85:488–495. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Esteban MA, Rodríguez A, Ayala AG and
Meseguer J: Effects of high doses of cortisol on innate cellular
immune response of seabream (Sparus aurata L.). Gen Comp
Endocrinol. 137:89–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
do Nascimento IV, Rodrigues MIDQ, Isaias
PHC, Barros-Silva PG, Sousa FB, Nunes Alves APN and Mota MRL:
Chronic systemic corticosteroid therapy influences the development
of pulp necrosis and experimental apical periodontitis,
exacerbating the inflammatory process and bone resorption in rats.
Int Endod J. 55:646–659. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Gomes ESB, Farias LC, Silveira LH, Jesus
CÍ, Rocha RGD, Ramos GV, Magalhães HTAT, Brito-Júnior M, Santos
SHS, Jham BC, et al: Conditioned fear stress increases bone
resorption in apical periodontitislesions in Wistar male rats. Arch
Oral Biol. 97:35–41. 2019. View Article : Google Scholar
|
|
256
|
Minhoto GB, Khoury RD, Orozco EIF, Prado
RF and Valera MC: Effect of chronic unpredictable stress on the
progression of experimental apical periodontitis in rats. Int Endod
J. 54:1342–1352. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Khoury RD, Prado RFD, Matos FDS, Meireles
BR, Cardoso FGDR, Oliveira LD, Carvalho CAT and Valera MC: The
influence of adrenergic blockade in rats with apical periodontitis
under chronic stress conditions. Arch Oral Biol. 110:1045902020.
View Article : Google Scholar
|
|
258
|
Van Someren EJW: Brain mechanisms of
insomnia: New perspectives on causes and consequences. Physiol Rev.
101:995–1046. 2021. View Article : Google Scholar
|
|
259
|
Scott J, Kallestad H, Vedaa O, Sivertsen B
and Etain B: Sleep disturbances and first onset of major mental
disorders in adolescence and early adulthood: A systematic review
and meta-analysis. Sleep Med Rev. 57:1014292021. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Park JS, Jeong Y, Jung J, Ryu JJ, Lim HK,
Jung SK and Song IS: Shift work sleep disorder is closely
associated with an increased risk for periodontal disease. J Clin
Periodontol. 48:1066–1075. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Fang HF, Miao NF, Chen CD, Sithole T and
Chung MH: Risk of cancer in patients with insomnia, parasomnia, and
obstructive sleep apnea: A nationwide nested case-control study. J
Cancer. 6:1140–1147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Chen LL: Advances in circadian rhythms in
oral maxillofacial tissues and oral-related diseases. Zhonghua Kou
Qiang Yi Xue Za Zhi. 57:481–489. 2022.In Chinese. PubMed/NCBI
|
|
263
|
Lange T, Dimitrov S and Born J: Effects of
sleep and circadian rhythm on the human immune system. Ann N Y Acad
Sci. 1193:48–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Chu FCS, Leung WK, Tsang PCS, Chow TW and
Samaranayake LP: Identification of cultivable microorganisms from
root canals with apical periodontitis following two-visit
endodontic treatment with antibiotics/steroid or calcium hydroxide
dressings. J Endod. 32:17–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Brzezinski A: Melatonin in humans. N Engl
J Med. 336:186–195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Reiter RJ, Rosales-Corral SA, Liu XY,
Acuna-Castroviejo D, Escames G and Tan DX: Melatonin in the oral
cavity: Physiological and pathological implications. J Periodontal
Res. 50:9–17. 2015. View Article : Google Scholar
|
|
267
|
Tavares BS, Tsosura TVS, Mattera MSLC,
Santelli JO, Belardi BE, Chiba FY, Cintra LTA, Silva CC and
Matsushita DH: Effects of melatonin on insulin signaling and
inflammatory pathways of rats with apical periodontitis. Int Endod
J. 54:926–940. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Sarıtekin E, Üreyen Kaya B, Aşcı H and
Özmen Ö: Anti-inflammatory and antiresorptive functions of
melatonin on experimentally induced periapical lesions. Int Endod
J. 52:1466–1478. 2019. View Article : Google Scholar
|