Introduction
Target therapy in adjuvant and metastatic settings
represents an unprecedented novelty in the history of cutaneous
melanoma treatment. In the last few years, the introduction of
immune checkpoint inhibitors (ICIs) and target therapy
significantly improved clinical outcome of metastatic melanoma
patients, leading to 5-years survival rates of 30% (1,2).
This benefit has been reached both in BRAF-mutated and BRAF-wild
type disease.
Dabrafenib (BRAF inhibitor, BRAFi) and trametinib
(MEK inhibitor, MEKi) have dramatically reduced risk of relapse and
disease progression in BRAF-mutated cutaneous melanoma and they
have been recently approved in adjuvant therapy for stage III
melanoma. In addition to specific molecular mutations targeting, an
influence on antitumor immunity and immune surveillance has been
described. In particular, several preclinical studies showed that
target therapy may modify tumor immune microenvironment, T cell
infiltration and T cell activity (3). BRAFi and MEKi's adverse events
include cutaneous side-effects, asthenia, nausea, pyrexia,
arthralgia, cardiovascular events, such as QT-prolongation or
decreased left ventricular ejection fraction and eye complications
are the most frequent (4).
We report here five cases of adult women affected by
stage III or IV melanoma, treated with BRAFi and MEKi. All patients
were admitted to our Hospital (Oncology Clinic, AOU Ospedali
Riuniti di Ancona, Ancona, Italy) and provided written informed
consent for the publication of their data. They experienced rare
adverse events, from which an immune-related etiopathogenesis can
be assumed.
Case report
Case 1
A forty-nine years-old woman was diagnosed with a
right back melanoma. In April 2019 she underwent surgery: a local
excision of the primary tumor was performed. Histological report
showed superficial spreading melanoma, Breslow thickness was 4.8 mm
and 15 mitoses mmq were found; neither ulceration nor regression
signs were described. Genomic DNA was isolated from formalin-fixed
paraffin-embedded (FFPE) tumor tissue and tumor molecular
characterization detected by Real-Time PCR (polymerase chain
reaction) showed a BRAF mutated melanoma (exon 15, V600E). In May
2019 the patient underwent a whole body (WB) CT-scan that did not
reveal distant metastases. A safety margins re-excision was
performed together with right axillary and inguinal sentinel lymph
node (SLN) biopsy: the latter was positive for melanoma metastasis.
Furthermore, a right inguinal-iliac-obturator dissection was
carried out, but no metastases were found. Finally, as part of an
expanded access program active at our Hospital, in June 2019 she
started an adjuvant therapy combining BRAFi/MEKi dabrafenib and
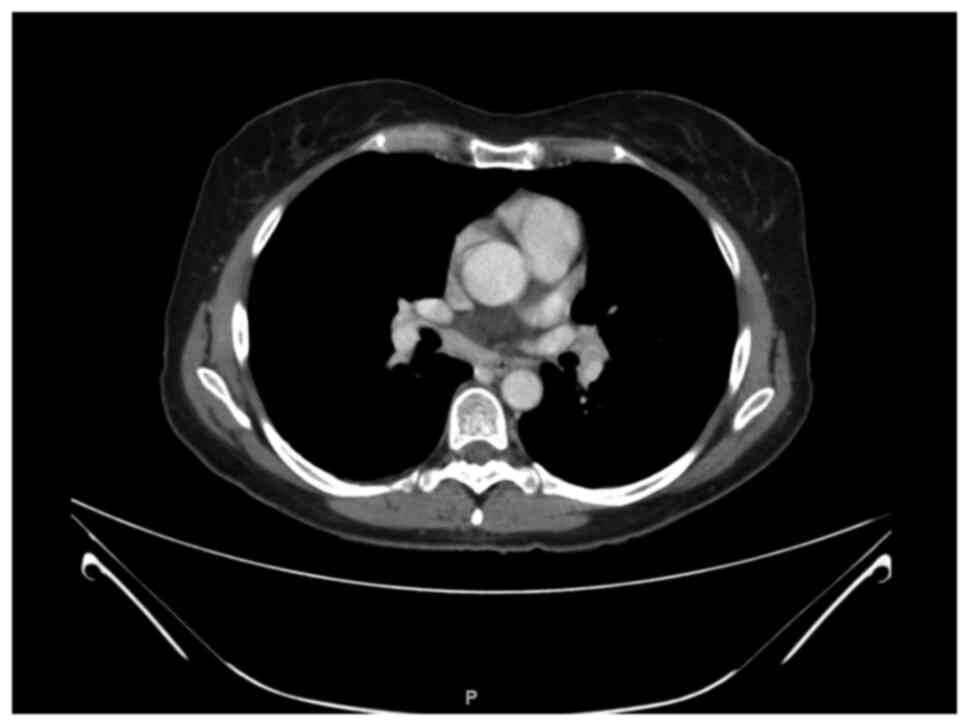
trametinib. In February 2020 a WB CT-scan found out mediastinal
lymphadenopathies (Fig. 1). A
bronchoscopy and fine needle aspiration were thus performed and the
pathological report revealed sarcoidosis. No medical treatment was
made and target therapy was continued without interruption. A
CT-scan in September 2020 did not show disease recurrence or signs
of sarcoidosis in mediastinal lymph nodes. She completed one
year-lasting adjuvant treatment in May 2020. To date, she is still
disease free.
Case 2
A fifty-nine years old woman was diagnosed with a
melanoma of the back and she underwent surgery in December 2020.
Histological diagnosis showed superficial spreading melanoma:
ulceration was present, Breslow thickness was 2.8 mm, and 20
mitoses/mq were found. No regression was detected and Tumor
Infiltrating lymphocytes (TILs) ‘non brisk’; surgical margins were
tumor-free. Genomic DNA was isolated from FFPE tumor tissue and
tumor molecular characterization detected by PCR displayed a BRAF
mutated melanoma (exon 15, V600E). A WB CT-scan showed no distant
metastases. In February 2021 she underwent surgical margins
radicalization, showing no residual disease. Bilateral axillary
sentinel lymph nodes were removed showing a 1.1 mm metastatic focus
in the right axilla and a 1.3 mm focus of infiltration in the left
axilla. Stage of disease, according to AJCC VIII edition, was pT3b
pN2a cM0 (IIIC). In April 2021 the patient started adjuvant therapy
with dabrafenib and trametinib. Since the beginning of treatment,
she experienced dyspnea. She performed a chest X-Ray highlighting
an elevation of diaphragm, that was bilateral but more evident on
the right side. An electromyography showed a right chronic phrenic
nerve neuropathy. A diagnosis of neuritis caused by BRAFi/MEKi was
made and in December 2021 she completed one-year adjuvant therapy
with dabrafenib and trametinib, without reporting further side
effects. Subsequent CT scans showed unchanged images about
diaphragm elevation.
Case 3
In July 2019 a 65 years old woman underwent surgery
for a pigmented lesion on the sole of the right foot with
histological diagnosis of acral lentiginous melanoma, with vertical
growth phase, V Clark level, 5.2 mm Breslow thickness, 18
mitoses/mmq, no TILs, with neoplastic vascular and endolymphatic
invasion. A sentinel lymph node was removed and showed multiple
melanoma microfoci in the subcapsular area, with the largest one of
0.1 mm. Molecular investigation (using the Real Time PCR method) on
FFPE tumor tissue further revealed a mutation of BRAF gene
(V600E). WB CT scan showed no distant metastases. According to AJCC
VIII edition, stage of disease was pT4b pN1a cM0 (IIIA). History
revealed that the patient was a strong smoker, suffering from
arterial hypertension receiving medical therapy. Her family history
included diagnosis of acral melanoma in a first-degree family
member.
After surgery, in November 2019, the patient started
an adjuvant therapy with BRAFi/MEKi combination (dabrafenib plus
trametinib). A CT scan performed in July 2020 highlighted
conglobate lymphadenopathies with partial necrotic contextual
aspects mainly in the right middle mediastinum, in the paratracheal
area involving the Barety loggia and up to the right
tracheo-bronchial corner with a maximum diameter of 2.5 cm. The
case was discussed in a multidisciplinary meeting. An EndoBronchial
UltraSound-guided TransBronchial Needle Aspiration (EBUS-TBNA) was
performed and histological examination of mediastinal
lymphadenopathies showed granulomatous lymphadenitis. At that time
the patient did not complain of cough or dyspnea.
Therefore the patient resumed treatment with
dabrafenib and trametinib and no steroid or medical therapy was
performed. She completed one year of adjuvant therapy in November
2020 and did not report any other toxicity. Chest CT-scan performed
in February and May 2021 showed a progressive reduction of
mediastinal lymphadenopathies.
Case 4
In November 2014, a 74-years old woman experienced a
painful swelling on the right axilla hollow. In December 2014 she
underwent agobiopsy with histological diagnosis of metastasis from
melanoma. A CT-scan showed subcutaneous metastases and
lymphadenopathies on axillary, supraclavicular and mediastinal
regions. Because of non-suspicious cutaneous lesion identified by
Dermatologist and the presence of V600E BRAF mutation on histologic
sample (detected with PCR and pyrosequencing on FFPE tumor tissue),
the patient began a target therapy with dabrafenib and trametinib
from February 2015 (within an expanded access program active at our
Hospital). In June 2015 and May 2017 the patient was subjected to
gamma-knife radiosurgery on cerebral lesions, located in the right
insular region and right deep parietal region.
CT-scan controls performed during treatment reported
a stable disease. In January 2019, the patient reported episodes of
diarrhea that required hospitalization in a Medicine Department
because of dehydration. Creatinine clearance was 12.76 ml/min upon
admission and gastrointestinal symptoms were associated with
electrolyte and acid-base disorders, leading to a clinical
diagnosis of prerenal acute kidney injury. Fecal cultures resulted
negative for Salmonella, Shigella and
Yersinia, as well as parasitological exams. Campylobacter
antigens were detected in fecal samples. A colonoscopy revealed no
macroscopic lesions on intestinal mucosa and random biopsies were
performed. The histologic examination showed microscopic features
of lymphocytic colitis.
A treatment with metronidazole was administered,
along with mesalazine and probiotics. Campylobacter infection has
been demonstrated to be correlated with lymphocytic colitis, and
the patient required both antibiotic and anti-inflammatory therapy.
Therapy with dabrafenib and trametinib was interrupted at the
beginning of gastrointestinal symptomatology, since January 2019.
Clinical conditions gradually improved during the hospitalization
and the patient was dismissed after renal function recovery.
In March 2019, the patient went to the Emergency
Department for recurrence of diarrhea and sickness: hematological
exams showed increase of creatininemia, hypocalcemia and
hypokalemia, associated with metabolic acidosis. Abdomen ultrasound
described diffuse distension of the loops of the small and large
intestine with hypermotility. Coproculture and Clostridium
difficile antigens research were negative, while urine culture
showed presence of colonies of Klebsiella pneumoniae and
Escherichia coli ESBL negative. Antibiotic therapy was
administered, along with fluid. In March 2019, the patient started
anti-inflammatory treatment with oral mesalazine and budesonide.
Therapy with dabrafenib and trametinib was suspended from January
2019 until recovery from acute symptoms, and it was resumed from
Aprile 2019 at reduced dosage (dabrafenib 75 mg 1 capsule twice
daily instead of 2 capsules twice daily; trametinib 2 mg
daily).
Since April 2019, the patient has not experienced
severe gastrointestinal symptoms and dabrafenib and trametinib were
administered at full dose along with anti-inflammatory treatment.
Budesonide was stopped in September 2019, while mesalazine was
administered for several months. The ongoing need to prolong
anti-inflammatory therapy (both steroidal and non-steroidal drugs)
leads to suppose that the infectious origin has not been the only
cause of the symptoms.
Radiological exams from the beginning of antitumoral
therapy showed stable disease on all metastatic lesions and
dabrafenib and trametinib treatment is currently ongoing at full
dose with no further toxicities.
Case 5
A 51-year old female patient underwent an excision
of cutaneous lesion on the left arm in 2010. Histological exam
revealed the presence of cutaneous melanoma, with the following
features: Clark level III invasion, 0.85 mm Breslow index, 2
mitoses/10 HPF, medium TILs (non-brisk), absence of neoplastic
vascular invasion (stage at diagnosis pT1b pN0 cM0). Furthermore,
V600E BRAF mutation involving the exon 15 was identified, by using
direct sequencing of PCR on FFPE tumor tissue. According to the
clinical guidelines, she started a clinical and radiological follow
up. After two years from diagnosis, CT-scan showed pathological
lymph nodes on the left axillary region, so a left axillary
lymphadenectomy was performed. The histological examination was
positive for metastasis of melanoma in 16 out of 25 lymph nodes. No
other pathological localizations were detected and a new follow up
based on the alternation between CT scan and PET examination was
started. In 2013 another left axillary lymphadenectomy was
performed based on 18-FDG PET-CT results and the histological
examination was positive for melanoma relapse in 2 out of 6 lymph
nodes.
CT-scan performed in May 2015 revealed pathological
lymphadenopathies on right axillary and left inguinal regions. No
visceral or cerebral disease was identified. The histological
examination confirmed relapse of melanoma in the lymph nodes
detected at the CT-scan.
Since June 2015, the patient started a first line
treatment with the combination of BRAFi and MEKi (dabrafenib plus
trametinib), resulting in a partial response of the disease during
the CT-scan control of October 2015 and reporting no
disturbances.
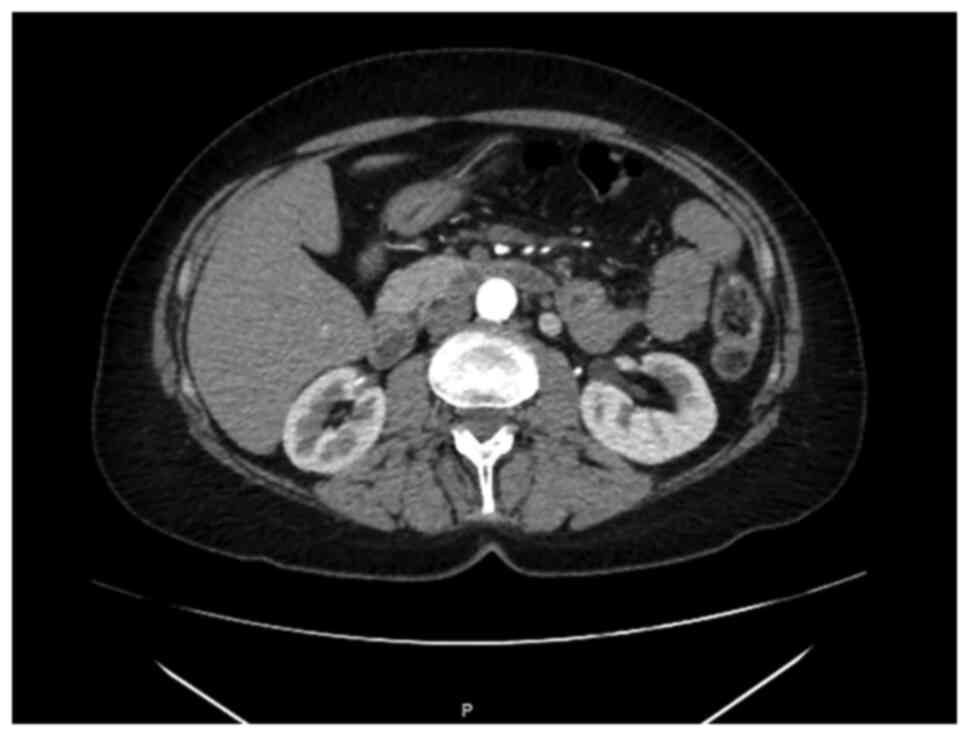
A CT-scan performed in October 2018 showed
thickening of mesenteric adipose tissue with some lymph nodes
suggesting a condition of mesenteric fibrosis (Fig. 2). At the clinical exam of December
2018, the patient reported mild abdominal pain for which she
performed abdominal ultrasound that confirmed the mesenteric
panniculitis shown in the previous CT-scan investigation. The
patient reported to assume occasional anti-inflammatory therapy
independently and continued treatment with dabrafenib and
trametinib without interruptions. In June 2022, the patient was
still assuming target therapy with optimal tolerance and
radiological evidence of stable disease.
Discussion
In this manuscript we reported a case series of
melanoma patients receiving BRAFi and MEKi, who experienced rare
adverse events not usually related to target therapy. Specifically,
in our clinical practice we have treated five adult women
experiencing one of the following conditions during treatment:
sarcoidosis, mesenteric panniculitis, lymphocytic colitis and
neuropathy of phrenic nerve (Table
I). Only two of five patients received a specific
anti-inflammatory treatment, with steroid and/or non-steroid
medications. In the other cases, symptoms or instrumental findings
resolved by themselves.
 | Table IClinical features of patients with
melanoma receiving dabrafenib and trametinib, that experienced
potential immune-related adverse events. |
Table I
Clinical features of patients with
melanoma receiving dabrafenib and trametinib, that experienced
potential immune-related adverse events.
| Case | Primary site | Stage at onset of
disease | Toxicity | Toxicity
treatment |
|---|
| 1 | Right back | IIIB | Sarcoidosis | No medical
treatment |
| 2 | Back | IIIC | Neuropathy of phrenic
nerve | Corticosteroids |
| 3 | Right foot | IIIA | Granulomatous
lymphadenitis | No medical
treatment |
| 4 | Unknown | IV | Lymphocytic
colitis | Metronidazole,
mesalazine, budesonide and probiotics |
| 5 | Left arm | IIIC | Mesenteric
panniculitis | Occasional
anti-inflammatory treatment |
To our knowledge, other Authors presented single
case reports of sarcoid-like reaction in melanoma patients treated
with a combination of BRAFi and MEKi. Lheure et al (5) described for the first time five cases
of sarcoidosis in melanoma patients treated with vemurafenib. In
other two reports (6,7), mediastinal lymphadenopathies coherent
with sarcoidosis localization occurred in a metastatic patient
receiving dabrafenib and trametinib or vemurafenib and cobimetinib.
In another case, a young female treated with adjuvant target
therapy received diagnosis of granulomatous synechizing uveitis and
cutaneous sarcoidosis located near a tattoo (8).
Ben-Betzalel et al (9) previously described 10 patients
treated with vemurafenib or vemurafenib and cobimetinib, who
experienced suspicious immune related events, including vitiligo,
uveitis, erythema nodosum and keratitis sicca. The Authors reported
an association between those toxicities and PFS (progression free
survival), revealing a more durable response to target therapy in
the described patients. The side effects we have encountered, with
the exception of sarcoidosis, are not yet described in literature
and represent novel toxicities due to BRAFi and MEKi treatment.
Clinical, radiological and histological findings of
our case series led us to hypothesize an immune-related
etiopathogenetic cause of target therapy toxicities. It is
interesting to note that all the cases we described concern women:
autoimmune diseases are notoriously more frequent in women and this
is explained by very different immunological responses between
women and men. Regardless of age, women are characterized by a
higher numbers of CD4+ T cells and CD4+/CD8+ T cell ratios,
compared to men, while androgens seem to have immunosuppressive
effects (10). In literature,
there are inconclusive results about the incidence of
immune-related events between women and men receiving ICIs, with
some of these studies showing no sex difference (11-13).
Sarcoidosis, mesenteric panniculitis and lymphocytic
colitis are caused by pro-inflammatory molecular mechanisms
(14-16)
and seem to be all related to an underlying chronic inflammatory
process.
Native and adaptive immune cells are potentially
involved in triggering BRAFi and MEKi toxicities. Indeed,
preclinical and clinical evidence showed that target therapies can
influence T-cells activation and modulate the immune system
activation within the tumor microenvironment.
Looking at the immune microenvironment of
BRAF-mutant melanoma cells, it has been demonstrated by Wang et
al (17) that CD4+
tumor-infiltrating lymphocytes is more represented in BRAF-mutant
tumors, in comparison to BRAF-wild type ones, both in primary and
metastatic sites. BRAF-mutant melanoma metastases showed an
increased enrichment of CD4+ T cells and B cells, while have a
decrease in CD8+ cells, when compared to BRAF-wild type tumor
tissues. So, the presence of BRAF-mutation in melanoma cells
characterized a distinct tumor and immune microenvironment, that
differs from BRAF-wild type melanoma.
In BRAF-mutant tumor cells, an upregulation of MAPK
signaling pathway that results in an ineffective immune response
and creates a microenvironment inhibitory to T cell functions is
known. Thus, BRAF-tumors are able to escape immune surveillance
(3). It has been further
demonstrated that BRAFi can increase IFNgamma expression of
intratumoral CD4+ TILs and decrease proportions of Treg and MDSCs
in BRAF-mutated melanoma mouse models (18,19).
An enhanced antitumor activity of T cells caused by BRAFi has been
revealed along with an up-regulation of melanocyte differentiation
antigens (MDA) and MHC expression on cancer cells, which makes
cells more susceptible to lymphocyte recognition (20). In addition BRAFi upregulate MHC
class I and CD70 molecules on APCs and increase CD8+ T cell
infiltration into tumor microenvironment (3,21),
enhancing T-cells cytotoxicity in BRAF-mutant and wild type
melanoma (22). Wilmott et
al (23) further reported an
increase of CD4+ and CD8+ T-cell infiltrate in tumors of patients
with BRAF-mutated metastatic melanoma.
On the other hand, MEKi increase the expression of
HLA class I and II on BRAF-mutated and BRAF-wild type melanoma
cells (24) and, as reported by
Vella et al (25), promotes
maturation of moDCs, through the inhibition of ERK phosphorylation.
In in vivo murine models, trametinib decreases a subset of
immunosuppression factors and increases CD4+ TILs levels. If
combined with anti-PD1 antibodies, it also increases CD8+
cells.
Ultimately, Kuske et al (3) affirmed that BRAFi and MEKi exert
their activity against melanoma cells also through modulation of
native and adaptive immune cells and can restore the immune
stimulatory microenvironment.
Both in vitro and in vivo, an
increased expression of melanoma differentiation antigens on
BRAF-mutated cells is stimulated by BRAFi (25), along with an enhanced expression of
melanoma antigens during the first week of treatment in melanoma
patients (26). MEKi suppress
PD-L1 expression on melanoma cells, which resulted more susceptible
to immune destruction (27). From
these results, it can be deduced that the synergy of dabrafenib and
trametinib improves antigen-specific T-cell recognition, results in
a pro-immunogenic effect on melanoma cells and potentially
strengthens immune response against cancer cells.
Whereas the activity of CD4+ and CD8+ lymphocytes is
responsible for immune-related adverse events in patients receiving
ICIs and there are growing evidences of the influence of dabrafenib
and trametinib on these cells, these drugs are likely to be
involved in the onset of such toxicities.
Moreover, investigations on exploratory biomarker
analyses on tissue samples of patients enrolled in COMBI-AD trial
(28) demonstrated that a subgroup
of BRAF-mutated melanoma patients characterized by an immune gene
signature experienced a very long relapse-free survival. This
benefit occurs both in the group receiving dabrafenib plus
trametinib as adjuvant therapy and in the group receiving placebo.
The immune gene signature corresponds to the high IFNgamma
signature subgroup, characterized by specific gene expression of
IFNG, CXCL9, CXCL10, CXCL11 and GBP1. Authors confirmed a
prognostic role of this immune gene signature in BRAF V600E stage
III melanoma patients, conferring them a relatively good clinical
outcome and establishing a link between BRAF mutation and immune
microenvironment.
Ultimately, we assumed that not only the long-term
efficacy but also the adverse events of BRAFi and MEKi might be
related to an immunomodulatory activity. In this perspective,
sarcoidosis, mesenteric panniculitis and lymphocytic colitis may be
interpreted as paradoxical effects caused by excessive stimulation
of the immune system by BRAFi and MEKi.
In conclusion, our study suggests that adverse
events reported by melanoma patients treated with target therapies
should not be underestimated, because of a possible role of immune
system activation in triggering onset of toxicities. Rare and
unexpected rare events, such as those we have described, can occur
and early recognition, appropriate diagnosis and specific medical
treatment allow a safe continuation of the therapy. Further
preclinical and clinical studies are needed to shed light on the
molecular mechanisms and intercellular interactions inside melanoma
and immune cells in patients treated with BRAFi and MEKi.
Acknowledgements
To analyze BRAF mutational status, tumor molecular
characterizations have been carried out on FFPE tumor samples by
commercial laboratories: In particular, Laboratory of Pathological
Anatomy, ‘Ospedale C. e G. Mazzoni’, (Ascoli Piceno, Italy) for
cases 1 and 2; Laboratory of Pathological Anatomy, ‘Ospedali
Riuniti di Ancona-Università Politecnica delle Marche’ (Ancona,
Italy) for cases 3 and 4; Laboratory of Pathological Anatomy,
‘Fondazione IRCCS Istituto Tumori di Milano’ (Milano, Italy) for
case 5.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FM conceived the present study, contributed to data
collection and analysis, and critically reviewed the manuscript.
VC, LS, NR, VL, AM and FT contributed to data collection and wrote
the manuscript. FM and VC contributed to interpretation of data and
confirm the authenticity of all the raw data. RB contributed to
conception of the manuscript and critically reviewed the data
collected. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All patients provided their written informed consent
for participation to this work.
Patient consent for publication
All patients provided their written consent for the
publication of their data and associated images.
Competing interests
RB is a consultant/advisory board member for Astra
Zeneca, Boehringer Ingelheim, Novartis, MSD, Otsuka, Eli-Lilly,
Roche. The other authors have no conflicts of interest to declare.
FM is a consultant/advisory board member for Novartis.
References
|
1
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph R, Weber JS, et al:
Five-year survival outcomes for patients with advanced melanoma
treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 30:582–588.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Robert C, Grob JJ, Stroyakovskiy D,
Karaszewska B, Hauschild A, Levchenko E, Chiarion Sileni V,
Schachter J, Garbe C, Bondarenko I, et al: Five-year outcomes with
dabrafenib plus trametinib in metastatic melanoma. N Engl J Med.
381:626–636. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kuske M, Westphal D, Wehner R, Schmitz M,
Beissert S, Praetorius C and Meier F: Immunomodulatory effects of
BRAF and MEK inhibitors: Implications for melanoma therapy.
Pharmacol Res. 136:151–159. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Welsh SJ and Corrie PG: Management of BRAF
and MEK inhibitor toxicities in patients with metastatic melanoma.
Ther Adv Med Oncol. 7:122–136. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lheure C, Kramkimel N, Franck N,
Laurent-Roussel S, Carlotti A, Queant A, Goldwasser F, Avril MF and
Dupin N: Sarcoidosis in patients treated with vemurafenib for
metastatic melanoma: A paradoxical autoimmune activation.
Dermatology. 231:378–384. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tijtgat J, Schwarze JK, Awada G, Neyns B
and Aspeslagh S: Sarcoid-like reaction in a BRAF V600E-mutated
metastatic melanoma patient during treatment with BRAF/MEK-targeted
therapy. Melanoma Res. 31:272–276. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gouveris P, Zouki DN, Sarris EG, Kolilekas
L, Tryfonopoulos D, Papaxoinis G and Demiri S: Melanoma and
sarcoidosis in patients receiving or not antineoplastic therapy.
Case Rep Oncol. 14:1059–1065. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Boutros A, Schiavi C, Cecchi F, Spagnolo
F, Guadagno A, Tanda ET, Giusti F, Murdaca G and Queirolo P: Case
report: Immune-related toxicity during adjuvant treatment with BRAF
Plus MEK inhibitors in a melanoma patient. Front Immunol.
11(579523)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ben-Betzalel G, Baruch EN, Boursi B,
Steinberg-Silman Y, Asher N, Shapira-Frommer R, Schachter J and
Markel G: Possible immune adverse events as predictors of durable
response to BRAF inhibitors in patients with BRAF V600-mutant
metastatic melanoma. Eur J Cancer. 101:229–235. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Klein SL and Flanagan KL: Sex differences
in immune responses. Nat Rev Immunol. 16:626–638. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Unger JM, Vaidya R, Albain KS, LeBlanc M,
Minasian LM, Gotay CC, Henry NL, Fisch MJ, Lee SM, Blanke CD and
Hershman DL: Sex differences in risk of severe adverse events in
patients receiving immunotherapy, targeted therapy, or chemotherapy
in cancer clinical trials. J Clin Oncol. 40:1474–1486.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
van der Kooij MK, Dekkers OM, Aarts MJB,
van den Berkmortel FWPJ, Boers-Sonderen MJ, de Groot JWB, Hospers
GAP, Piersma D, van Rijn RS, Suijkerbuijk KPM, et al: Sex-based
differences in treatment with immune checkpoint inhibition and
targeted therapy for advanced melanoma: A nationwide cohort study.
Cancers (Basel). 13(4639)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jing Y, Zhang Y, Wang J, Li K, Chen X,
Heng J, Gao Q, Ye Y, Zhang Z, Liu Y, et al: Association between sex
and immune-related adverse events during immune checkpoint
inhibitor therapy. J Natl Cancer Inst. 113:1396–1404.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Iannuzzi MC, Rybicki BA and Teirstein AS:
Sarcoidosis. N Engl J Med. 357:2153–2165. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hussein MRA and Abdelwahed SR: Mesenteric
panniculitis: An update. Expert Rev Gastroenterol Hepatol. 9:67–78.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Storr MA: Microscopic colitis:
Epidemiology, pathophysiology, diagnosis and current management-an
update 2013. ISRN Gastroenterol. 2013(352718)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang M, Zadeh S, Pizzolla A, Thia K,
Gyorki DE, McArthur GA, Scolyer RA, Long G, Wilmott JS, Andrews MC,
et al: Characterization of the treatment-naive immune
microenvironment in melanoma with BRAF mutation. J Immunother
Cancer. 10(e004095)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Steinberg SM, Zhang P, Malik BT, Boni A,
Shabaneh TB, Byrne KT, Mullins DW, Brinckerhoff CE, Ernstoff MS,
Bosenberg MW and Turk MJ: BRAF inhibition alleviates immune
suppression in murine autochthonous melanoma. Cancer Immunol Res.
2:1044–1050. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ho PC, Meeth KM, Tsui YC, Srivastava B,
Bosenberg MW and Kaech SM: Immune-based antitumor effects of BRAF
inhibitors rely on signaling by CD40L and IFNγ. Cancer Res.
74:3205–3217. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hu-Lieskovan S, Mok S, Homet Moreno B,
Tsoi J, Robert L, Goedert L, Pinheiro EM, Koya RC, Graeber TG,
Comin-Anduix B and Ribas A: Improved antitumor activity of
immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma.
Sci Transl Med. 7(279ra41)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jung T, Haist M, Kuske M, Grabbe S and
Bros M: Immunomodulatory properties of BRAF and MEK inhibitors used
for melanoma therapy-paradoxical ERK activation and beyond. Int J
Mol Sci. 22(9890)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ascierto PA and Dummer R: Immunological
effects of BRAF+MEK inhibition. Oncoimmunology.
7(e1468955)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wilmott JS, Long GV, Howle JR, Haydu LE,
Sharma RN, Thompson JF, Kefford RF, Hersey P and Scolyer RA:
Selective BRAF inhibitors induce marked T-cell infiltration into
human metastatic melanoma. Clin Cancer Res. 18:1386–1394.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu L, Mayes PA, Eastman S, Shi H,
Yadavilli S, Zhang T, Yang J, Seestaller-Wehr L, Zhang SY, Hopson
C, et al: The BRAF and MEK inhibitors dabrafenib and trametinib:
Effects on immune function and in combination with immunomodulatory
antibodies targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res.
21:1639–1651. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vella LJ, Andrews MC, Pasam A, Woods K,
Behren A and Cebon JS: The kinase inhibitors dabrafenib and
trametinib affect isolated immune cell populations. Oncoimmunology.
3(e946367)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Frederick DT, Piris A, Cogdill AP, Cooper
ZA, Lezcano C, Ferrone CR, Mitra D, Boni A, Newton LP, Liu C, et
al: BRAF inhibition is associated with enhanced melanoma antigen
expression and a more favorable tumor microenvironment in patients
with metastatic melanoma. Clin Cancer Res. 19:1225–1231.
2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jiang X, Zhou J, Giobbie-Hurder A, Wargo J
and Hodi FS: The activation of MAPK in melanoma cells resistant to
BRAF inhibition promotes PD-L1 expression that is reversible by MEK
and PI3K inhibition. Clin Cancer Res. 19:598–609. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dummer R, Brase JC, Garrett J, Campbell
CD, Gasal E, Squires M, Gusenleitner D, Santinami M, Atkinson V,
Mandalà M, et al: Adjuvant dabrafenib plus trametinib versus
placebo in patients with resected, BRAFV600-mutant,
stage III melanoma (COMBI-AD): Exploratory biomarker analyses from
a randomised, phase 3 trial. Lancet Oncol. 21:358–372.
2020.PubMed/NCBI View Article : Google Scholar
|