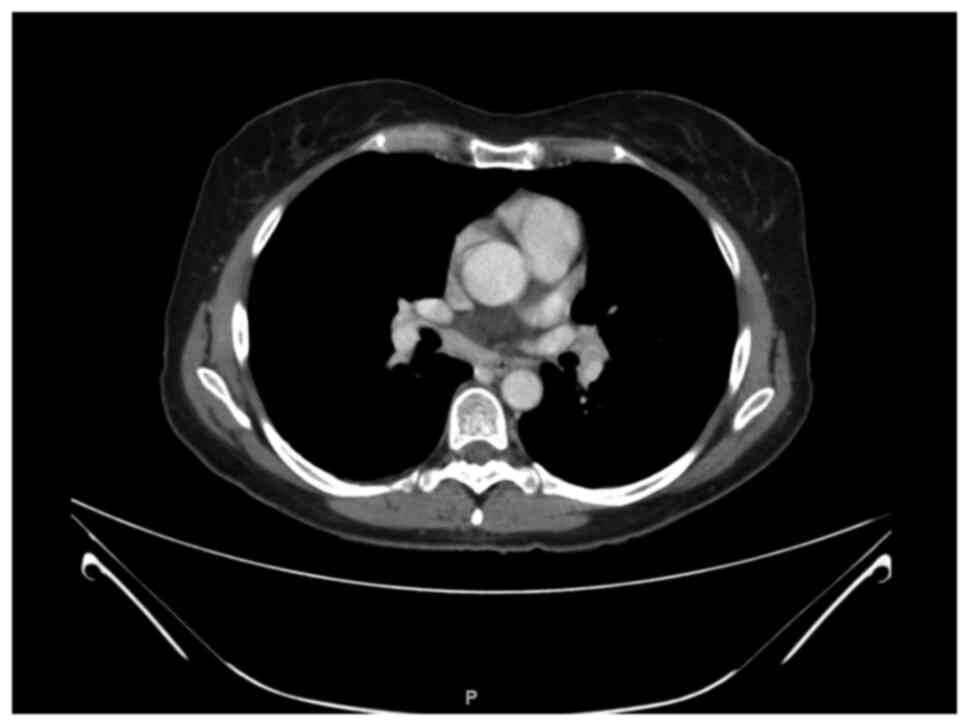
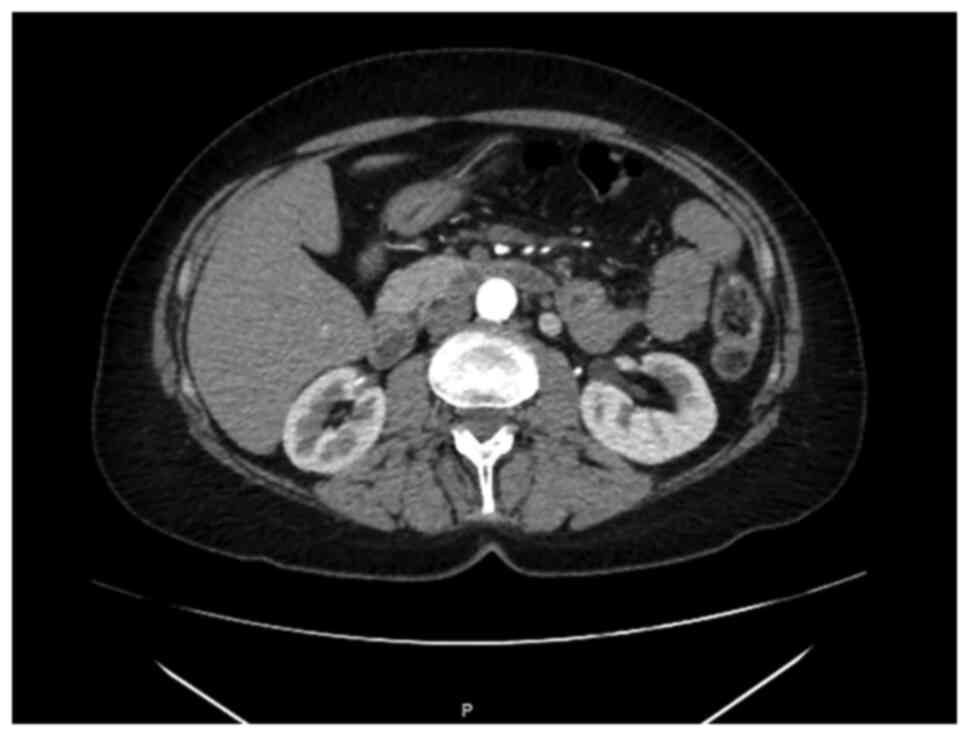
|
1
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph R, Weber JS, et al:
Five-year survival outcomes for patients with advanced melanoma
treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 30:582–588.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Robert C, Grob JJ, Stroyakovskiy D,
Karaszewska B, Hauschild A, Levchenko E, Chiarion Sileni V,
Schachter J, Garbe C, Bondarenko I, et al: Five-year outcomes with
dabrafenib plus trametinib in metastatic melanoma. N Engl J Med.
381:626–636. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kuske M, Westphal D, Wehner R, Schmitz M,
Beissert S, Praetorius C and Meier F: Immunomodulatory effects of
BRAF and MEK inhibitors: Implications for melanoma therapy.
Pharmacol Res. 136:151–159. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Welsh SJ and Corrie PG: Management of BRAF
and MEK inhibitor toxicities in patients with metastatic melanoma.
Ther Adv Med Oncol. 7:122–136. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lheure C, Kramkimel N, Franck N,
Laurent-Roussel S, Carlotti A, Queant A, Goldwasser F, Avril MF and
Dupin N: Sarcoidosis in patients treated with vemurafenib for
metastatic melanoma: A paradoxical autoimmune activation.
Dermatology. 231:378–384. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tijtgat J, Schwarze JK, Awada G, Neyns B
and Aspeslagh S: Sarcoid-like reaction in a BRAF V600E-mutated
metastatic melanoma patient during treatment with BRAF/MEK-targeted
therapy. Melanoma Res. 31:272–276. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gouveris P, Zouki DN, Sarris EG, Kolilekas
L, Tryfonopoulos D, Papaxoinis G and Demiri S: Melanoma and
sarcoidosis in patients receiving or not antineoplastic therapy.
Case Rep Oncol. 14:1059–1065. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Boutros A, Schiavi C, Cecchi F, Spagnolo
F, Guadagno A, Tanda ET, Giusti F, Murdaca G and Queirolo P: Case
report: Immune-related toxicity during adjuvant treatment with BRAF
Plus MEK inhibitors in a melanoma patient. Front Immunol.
11(579523)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ben-Betzalel G, Baruch EN, Boursi B,
Steinberg-Silman Y, Asher N, Shapira-Frommer R, Schachter J and
Markel G: Possible immune adverse events as predictors of durable
response to BRAF inhibitors in patients with BRAF V600-mutant
metastatic melanoma. Eur J Cancer. 101:229–235. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Klein SL and Flanagan KL: Sex differences
in immune responses. Nat Rev Immunol. 16:626–638. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Unger JM, Vaidya R, Albain KS, LeBlanc M,
Minasian LM, Gotay CC, Henry NL, Fisch MJ, Lee SM, Blanke CD and
Hershman DL: Sex differences in risk of severe adverse events in
patients receiving immunotherapy, targeted therapy, or chemotherapy
in cancer clinical trials. J Clin Oncol. 40:1474–1486.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
van der Kooij MK, Dekkers OM, Aarts MJB,
van den Berkmortel FWPJ, Boers-Sonderen MJ, de Groot JWB, Hospers
GAP, Piersma D, van Rijn RS, Suijkerbuijk KPM, et al: Sex-based
differences in treatment with immune checkpoint inhibition and
targeted therapy for advanced melanoma: A nationwide cohort study.
Cancers (Basel). 13(4639)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jing Y, Zhang Y, Wang J, Li K, Chen X,
Heng J, Gao Q, Ye Y, Zhang Z, Liu Y, et al: Association between sex
and immune-related adverse events during immune checkpoint
inhibitor therapy. J Natl Cancer Inst. 113:1396–1404.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Iannuzzi MC, Rybicki BA and Teirstein AS:
Sarcoidosis. N Engl J Med. 357:2153–2165. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hussein MRA and Abdelwahed SR: Mesenteric
panniculitis: An update. Expert Rev Gastroenterol Hepatol. 9:67–78.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Storr MA: Microscopic colitis:
Epidemiology, pathophysiology, diagnosis and current management-an
update 2013. ISRN Gastroenterol. 2013(352718)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang M, Zadeh S, Pizzolla A, Thia K,
Gyorki DE, McArthur GA, Scolyer RA, Long G, Wilmott JS, Andrews MC,
et al: Characterization of the treatment-naive immune
microenvironment in melanoma with BRAF mutation. J Immunother
Cancer. 10(e004095)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Steinberg SM, Zhang P, Malik BT, Boni A,
Shabaneh TB, Byrne KT, Mullins DW, Brinckerhoff CE, Ernstoff MS,
Bosenberg MW and Turk MJ: BRAF inhibition alleviates immune
suppression in murine autochthonous melanoma. Cancer Immunol Res.
2:1044–1050. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ho PC, Meeth KM, Tsui YC, Srivastava B,
Bosenberg MW and Kaech SM: Immune-based antitumor effects of BRAF
inhibitors rely on signaling by CD40L and IFNγ. Cancer Res.
74:3205–3217. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hu-Lieskovan S, Mok S, Homet Moreno B,
Tsoi J, Robert L, Goedert L, Pinheiro EM, Koya RC, Graeber TG,
Comin-Anduix B and Ribas A: Improved antitumor activity of
immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma.
Sci Transl Med. 7(279ra41)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jung T, Haist M, Kuske M, Grabbe S and
Bros M: Immunomodulatory properties of BRAF and MEK inhibitors used
for melanoma therapy-paradoxical ERK activation and beyond. Int J
Mol Sci. 22(9890)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ascierto PA and Dummer R: Immunological
effects of BRAF+MEK inhibition. Oncoimmunology.
7(e1468955)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wilmott JS, Long GV, Howle JR, Haydu LE,
Sharma RN, Thompson JF, Kefford RF, Hersey P and Scolyer RA:
Selective BRAF inhibitors induce marked T-cell infiltration into
human metastatic melanoma. Clin Cancer Res. 18:1386–1394.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu L, Mayes PA, Eastman S, Shi H,
Yadavilli S, Zhang T, Yang J, Seestaller-Wehr L, Zhang SY, Hopson
C, et al: The BRAF and MEK inhibitors dabrafenib and trametinib:
Effects on immune function and in combination with immunomodulatory
antibodies targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res.
21:1639–1651. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vella LJ, Andrews MC, Pasam A, Woods K,
Behren A and Cebon JS: The kinase inhibitors dabrafenib and
trametinib affect isolated immune cell populations. Oncoimmunology.
3(e946367)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Frederick DT, Piris A, Cogdill AP, Cooper
ZA, Lezcano C, Ferrone CR, Mitra D, Boni A, Newton LP, Liu C, et
al: BRAF inhibition is associated with enhanced melanoma antigen
expression and a more favorable tumor microenvironment in patients
with metastatic melanoma. Clin Cancer Res. 19:1225–1231.
2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jiang X, Zhou J, Giobbie-Hurder A, Wargo J
and Hodi FS: The activation of MAPK in melanoma cells resistant to
BRAF inhibition promotes PD-L1 expression that is reversible by MEK
and PI3K inhibition. Clin Cancer Res. 19:598–609. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dummer R, Brase JC, Garrett J, Campbell
CD, Gasal E, Squires M, Gusenleitner D, Santinami M, Atkinson V,
Mandalà M, et al: Adjuvant dabrafenib plus trametinib versus
placebo in patients with resected, BRAFV600-mutant,
stage III melanoma (COMBI-AD): Exploratory biomarker analyses from
a randomised, phase 3 trial. Lancet Oncol. 21:358–372.
2020.PubMed/NCBI View Article : Google Scholar
|