Introduction
Fibrocytes are bone marrow-derived
collagen-expressing cells and constitute approximately 0.5% of the
peripheral blood leukocyte population (1,2).
They express markers of hematopoietic cells (CD34), leukocytes
(CD11b, CD13, and CD45), and extracellular matrices (collagens I
and III, fibronectin) (1–4). A number of studies have suggested
their potential role in fibrotic diseases in various organs,
including skin, lung, liver, and kidney (5–9).
Previous studies have also identified fibrocytes in tumor tissues
(10,11), but their role in tumor progression
has not yet been discussed. We and others previously demonstrated
that fibrocytes have the ability to produce several growth factors,
including platelet-derived growth factor (PDGF), fibroblast growth
factor (FGF), and vascular endothelial growth factor (VEGF)
(12–14). Furthermore, we recently found that
fibrocytes had accumulated in the tumor tissues of lung
adenocarcinoma (LUAD) patients after treatment with the anti-VEGF
antibody bevacizumab, and the accumulated fibrocytes mediated the
acquisition of resistance to anti-angiogenic agents by producing
FGF-2 in murine models (15).
Based on these findings, we suspected that
tumor-infiltrating fibrocytes might influence the growth and
progression of tumor cells and that regulating the accumulation of
such fibrocytes in the tumor microenvironment might be a novel
therapeutic approach. In addition to our previous study, which
demonstrated that fibrocytes accumulated in the tumor
microenvironment via the CXCL12/CXCR4 axis (15), several chemotactic factors, such as
CCL2, CCL5, CCL11, and CCL24, and PDGFs, have also been shown to
induce the migration of fibrocytes in pulmonary fibrosis and/or
asthma (16–18).
In the present study, to determine the key
therapeutic target regulating the migration of fibrocytes into the
tumor microenvironment, we examined the expression of
representative chemotactic factors and the number of
tumor-infiltrating fibrocytes in surgically resected tumor tissues
from lung cancer patients. We also analyzed the significance of
these factors for the prognosis of these patients.
Materials and methods
Patients' samples
Patients who underwent surgical resection from 2011
to 2012 in Tokushima University Hospital and whose resected tumor
tissues were available for analyses were included in this study.
Paraffin-embedded sections of tumor tissues surgically resected
from 52 lung cancer patients were used (Table I). The sections were taken from 23
adenocarcinoma (LUAD) and 29 squamous cell carcinoma (LUSQ)
patients. They were analyzed to evaluate the chemokine expression
on cancer cells and number of fibrocytes. All patients received
follow-up. The protocol was approved by the Institutional Review
Board (IRB) of Tokushima University Hospital (no. 2471).
 | Table I.Baseline characteristics of patients
with lung cancer. |
Table I.
Baseline characteristics of patients
with lung cancer.
| Factors | No. of
patients | (%) |
|---|
| No. of
patients | 52 | 100 |
| Sex (%) |
|
|
|
Male | 35 | 67.3 |
|
Female | 17 | 32.7 |
| Age (%) |
|
|
|
<75 | 29 | 55.8 |
|
≥75 | 23 | 44.2 |
| Smoking (%) |
|
|
|
Non-smoker | 16 | 30.8 |
|
Smoker | 36 | 69.2 |
| pT factor (%) |
|
|
| T1 | 28 | 53.9 |
| T2 | 21 | 40.4 |
| T3 | 2 | 3.8 |
| T4 | 1 | 1.9 |
| pN factor (%) |
|
|
| N0 | 46 | 88.5 |
| N1 | 5 | 9.6 |
| N2 | 1 | 1.9 |
| pStage (%) |
|
|
| I | 43 | 82.7 |
| II | 6 | 11.5 |
|
III | 3 | 5.8 |
| Histology (%) |
|
|
| Ad | 23 | 44.2 |
| Sq | 29 | 55.8 |
Isolation of human fibrocytes
Human fibrocytes were isolated from the peripheral
blood of healthy volunteers and patients with lung cancer as
previously reported (13,14,17).
Mononuclear cells were isolated from the peripheral blood of
healthy volunteers using Ficoll density centrifugation. The
isolated cells were cultured in DMEM supplemented with 20% fetal
bovine serum (FBS), penicillin, and streptomycin on bovine
fibronectin (R&D Systems)-coated 150 mm cell culture dishes (BD
Pharmingen). The medium was changed twice a week. After seven days,
the medium was aspirated, and cells were washed with sterile
phosphate-buffered saline (PBS) three times. The adherent cells
were harvested using 0.125% trypsin. Greater than 90% of the
adherent cells prepared using this method consisted of fibrocytes
as determined by the expression of CD45, collagen I, and CXCR4.
Informed consent was obtained from all volunteers, and the protocol
was approved by the IRB of Tokushima University Hospital (no.
2838).
Immunohistochemical staining
Fibrocytes in the clinical specimens were identified
using anti-CD45 and anti-fibroblast-specific protein 1 (FSP-1)
antibody as previously described (15,19).
Paraffin-embedded tissues (4 µm thick) were stained with a rabbit
anti-FSP-1 polyclonal antibody (1:2,000 dilution, D9F9D; Cell
Signaling Technology) and mouse anti-human CD45 monoclonal antibody
(1:100 dilution, 135-4C5; Novus Biotechnology). These sections were
re-incubated with appropriate secondary antibodies conjugated with
peroxidase or alkaline phosphatase (ready to use; Nichirei).
Immunoreactivity was detected using the DAB Liquid System or new
fuchsin (Nichirei), and samples were counterstained with
hematoxylin. We evaluated fibrocytes as double-positive cells for
both FSP-1 and CD45 by counting the average number in 6 high-power
fields at ×400 magnification among the tumor stromal cells
(Fig. 1B). To evaluate the
expression of CXCR4 on tumor-infiltrated fibrocytes, we performed
double staining for FSP-1 and CXCR4 using a mouse anti-CXCR4
antibody (1:25 dilution, 44716; R&D Systems).
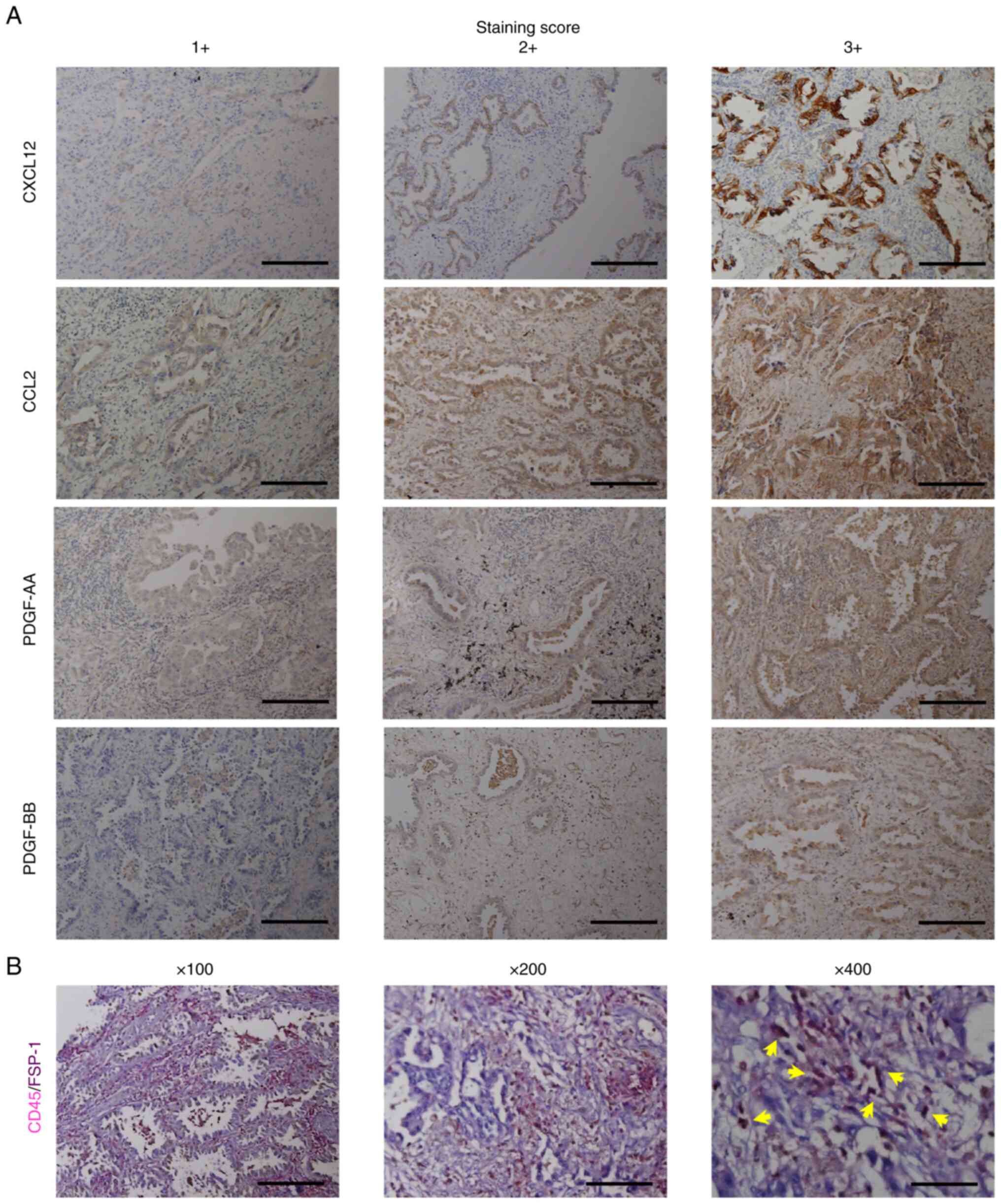 | Figure 1.Immunohistochemical staining of
CXCL12, CCL2, PDGF-AA and PDGF-BB, and fibrocytes in tumor tissues
of lung cancer and their scoring. (A) Immunohistochemical staining
of CXCL12, CCL2, PDGF-AA and PDGF-BB, and their scoring.
Magnification, ×100; scale bar, 200 µm. (B) Immunohistochemical
staining of tumor-infiltrating fibrocytes with antibodies for CD45
and FSP-1. Arrows indicate double-stained fibrocytes. Magnification
left to right, ×100, ×200, ×400; scale bar, 200 µm (×100), 100 µm
(×200) and 50 µm (×400). CXCL12, C-X-C motif chemokine 12; PDGF,
platelet-derived growth factor; FSP-1, anti-fibroblast-specific
protein 1. |
To detect the chemokines in lung cancer cells,
immunohistochemistry was performed using Leica Bond-Max (Leica) and
Bond Polymer Refine Detection (DS9800; Leica) with the following
antibodies: CCL12/SDF-1 (P-159X) (1:50 dilution, sc-74271; Santa
Cruz Biotechnology), anti-CCL2/MCP-1 antibody (1:200 dilution,
ab9669; Abcam, Cambridge, UK), anti-PDGF-AA polyclonal antibody
(1:50 dilution, PAB3678; Abnova), and anti-PDGF-BB antibody (1:500
dilution, ab23914; Abcam). The expression of chemokines in lung
cancer was determined by evaluation at ×200 magnification. Images
were acquired by Keyence BZ-9000 microscopy (Keyence). The
intensity of staining was scored as 0 (absent), 1+ (low intensity),
2+ (intermediate intensity), or 3+ (high intensity) (Fig. 1A).
The evaluation was performed individually by two
researchers in a blinded fashion.
Migration assays
Migration of fibrocytes was assayed as previously
described (13,17). Recombinant human CXCL12/SDF-1
(350-NS-010/CF; R&D Systems), CCL2/MCP-1 (279-MC-010; R&D
Systems), PDGF-AA, or PDGF-BB (221-AA-010, 220-BB-010; R&D
Systems) was used as a chemoattractant. These chemoattractants were
added to the well of a BD Falcon TC companion Plate (24-well
plate). Immediately afterwards, 1×105 fibrocytes/well
were added to the chambers in the well with DMEM containing 0.1%
FBS or chemokines through 8-µm filters. We incubated the cells for
6 h in a cell culture incubator. After a 6-h incubation, the cells
that had migrated to the bottom surface of the filter were stained
using Diff-Quik reagents I and II (Baxter), and were counted in 6
randomly selected fields on each filter under a microscope at ×200
magnification. All assays were performed in triplicate. Migration
was assessed by counting the number of cells in four high-power
fields with a light microscope.
FACS analyses
To examine the expression of cell surface receptors
of fibrocytes, fibrocytes isolated from peripheral blood were
stained with PE Mouse IgG1, κ Isotype Ctrl (FC) antibody (#400113;
Biolegend), PE CD184 (CXCR4) antibody (#306505; Biolegend), PE
anti-human CD192 (CCR2) antibody (#357205; Biolegend), PE
anti-human CD140a (PDGFRα) antibody, (#323505; Biolegend), and PE
anti-human CD140b (PDGFRβ) antibody (#323605; Biolegend). The
stained cells were analyzed by flow cytometry using BD LSRFortessa
(BD Bioscience) for acquisition and the FlowJo software program
(Treestar Inc.) for analyses (17).
Statistical analyses
The end of the follow-up period was defined as
either the date of patient mortality or the patient's last date of
contact. The overall survival was defined as the duration from the
date of the diagnosis to the date of last contact or patient
mortality. We performed Welch's t-test, a one-way analysis of
variance, Tukey's multiple-comparison post-hoc test, and a linear
regression analysis for the statistical analyses using the GraphPad
PRISM software program (5.01; GraphPad Software, Inc.) and R
Commander plug-in EZR (1.55; Saitama Medical Center, Jichi Medical
University, Saitama, Japan). The survival was estimated using the
Kaplan-Meier method, and the log-rank test was used to assess
differences in survival distributions among the groups. The data
were expressed as mean ± standard deviation, and P-values of
<0.05 were considered to indicate significance.
Results
The number of fibrocytes was
positively correlated with the expression level of CXCL12 in lung
cancer specimens
We examined the expressions of chemotactic factors
for fibrocytes with tumor tissues from 52 patients with lung
cancers (LUAD: 23, LUSQ: 29) who underwent surgical resection
between 2011 and 2012 at Tokushima University Hospital (Table I). These patients consisted of 35
males and 17 females, with a mean age of 71.2 years old (range
46–85) years old at the time of surgical resection. They were
pathologically diagnosed as follows: Stage I in 43 patients
(82.7%); Stage II in 6 patients (11.5%); and Stage III in 3
patients (5.8%).
To investigate correlations between the expression
of chemokines and the accumulation of fibrocytes in lung cancer
tissues, immunostaining for chemokines (CXCL12, CCL2, PDGF-AA,
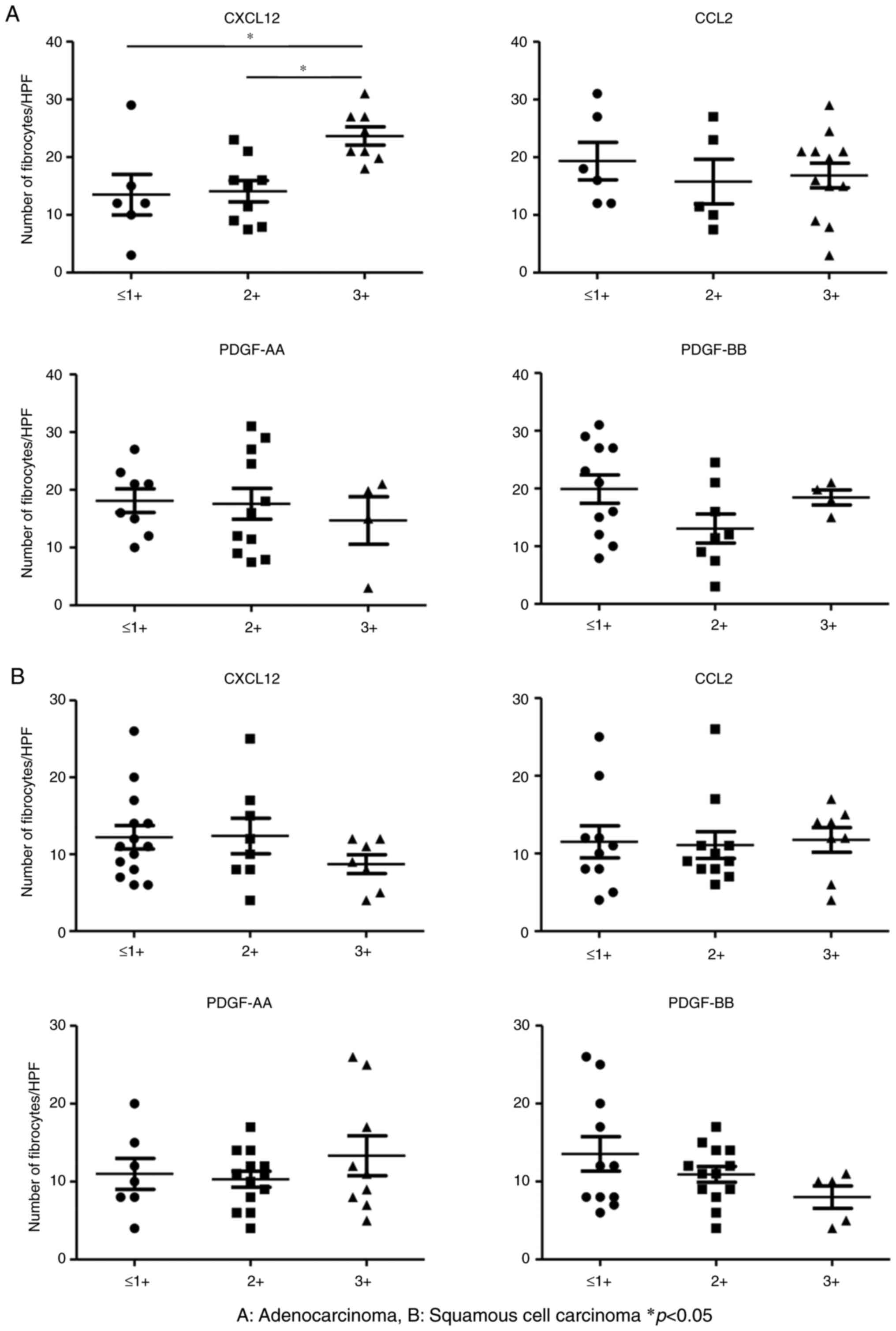
PDGF-BB) and fibrocytes was performed in serial sections (Fig. 1A and B). These chemokines were
strongly detected in the cytoplasm of tumor cells, but not in
interstitial areas; however, fibrocytes were mainly observed in the
peritumoral areas (Fig. 1B)
(14). In LUAD, fibrocytes showed
greater accumulation in the group with a high CXCL12 expression
than in the groups with a low or intermediate CXCL12 expression
(P<0.05) (Fig. 2A). No such
association was seen in CCL2, PDGF-AA, and PDGF-BB.
Conversely, we did not find any correlations among
the expression level of any chemotactic factor and the number of
fibrocytes in LUSQ (Fig. 2B).
The survival of patients was
negatively correlated with the expression level of CXCL12 in lung
cancer specimens
We analyzed the impact of the expression of
chemotactic factors for fibrocytes on the overall survival (OS) of
patients. In this analysis, the patients were divided into two
groups showing a high (score: 3+) and low (score: 0~2+) expression.
A high expression of CXCL12 was observed in 34.8% of LUAD and 24.1%
of LUSQ patients (Table II).
Furthermore, a high expression of CCL2 was found in 52.2% of LUAD
and 27.6% of LUSQ patients. However, a high expression of PDGF-AA
was more common in LUSQ than in LUAD (31.0% vs. 17.4%,
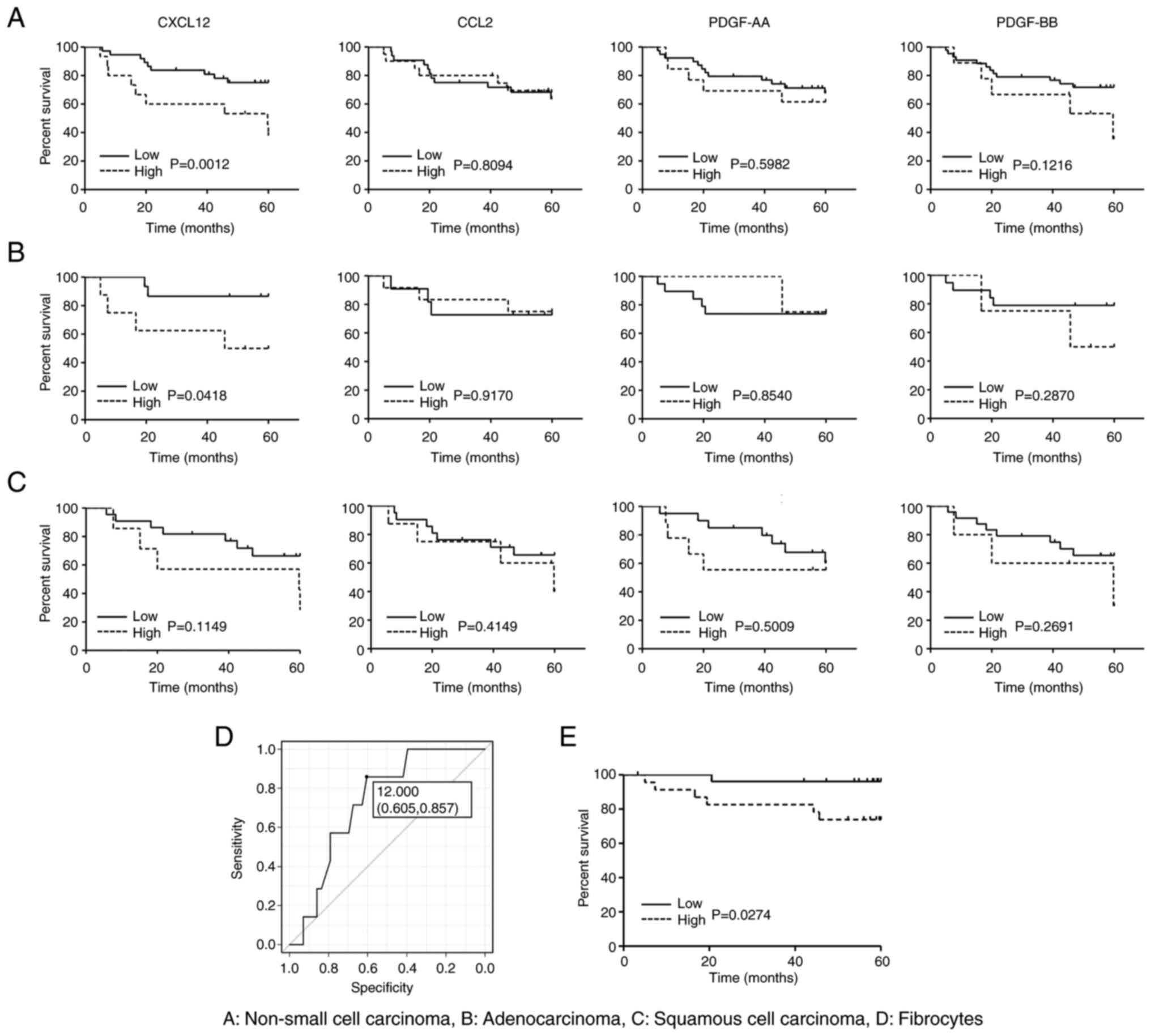
respectively) patients. As shown in Fig. 3A, the OS of NSCLC patients with a
high expression of CXCL12 was significantly worse than that of
patients with a low expression. However, there was no significant
difference in the OS between patients with a high and low
expression of CCL2, PDGF-AA, or PDGF-BB (Fig. 3A). Interestingly, a similar
difference was found in patients with LUAD, but not in those with
LUSQ (Fig. 3B and C). We also
evaluated the impact of age on the expression of chemotactic
factors, however, but no marked differences were seen between
elderly patients (≥75 years old) and others (data not shown).
 | Table II.Expression of CXCL12, CCL2, PDGF-AA
and PDGF-BB in lung cancer tissues. |
Table II.
Expression of CXCL12, CCL2, PDGF-AA
and PDGF-BB in lung cancer tissues.
|
| NSCLC | Ad | Sq |
|---|
|
|
|
|
|
|---|
| Chemotactic
factor | Low (0–2+) | High (3+) | Low (0–2+) | High (3+) | Low (0–2+) | High (3+) |
|---|
| CXCL12 | 37 (71.1) | 15 (28.9) | 15 (65.2) | 8 (34.8) | 22 (75.9) | 7 (24.1) |
| CCL2 | 32 (61.5) | 20 (38.5) | 11 (47.8) | 12 (52.2) | 21 (72.4) | 8 (27.6) |
| PDGF-AA | 39 (75.0) | 13 (25.0) | 19 (82.6) | 4 (17.4) | 20 (69.0) | 9 (31.0) |
| PDGF-BB | 43 (82.7) | 9 (17.3) | 19 (82.6) | 4 (17.4) | 24 (82.8) | 5 (17.2) |
The number of fibrocytes was
negatively correlated with the survival of LUAD patients
We also examined the correlations between the number
of fibrocytes and the OS of patients with lung cancer. To increase
the number of patients with LUAD, we combined the findings of our
present study and our previous data (19). As a result, we further included a
further 27 patients with stage I–III lung adenocarcinoma, resulting
in 50 patients in total (Table
SI). The patients with LUAD were divided into 2 groups by
employing a cut-off value (12.000), yielding 0.724 as the area
under the curve (AUC) (Fig. 3D).
Under this condition, we found that the patient survival of the
high-fibrocyte group was significantly worse than that of the
low-fibrocyte group (P-value=0.0274) (Fig. 3E). There was no correlation between
the number of fibrocytes and any clinical factors in LUAD (Table SI). In addition, there was no
correlation between the number of fibrocytes and the OS of patients
with NSCLC or LUSQ (data not shown).
Analysis of receptor expression for
chemotactic factors and the migration capacity of fibrocytes in
patients with lung cancer
To clarify the role of chemotactic factors in
fibrocyte migration in patients with lung cancer, we examined the
expression of receptors for chemotactic factors by flow cytometry.
As shown in Fig. 4A, the
expression of CXCR4, which is a receptor for CXCL12, was
consistently detected in healthy donors as well as patients with
LUAD. In contrast, the receptors for other chemokines (CCL2,
PDGF-AA, and PDGF-BB) were not consistently detected and the level
of expression was lower than that of CXCR4 (Fig. 4A). In the analysis to determine the
effects of chemokines on the migration of fibrocytes derived from
healthy donor PBMCs, CXCL12 consistently induced their migration
in vitro, but the effects of CCL2, PDGF-AA, and PDGF-BB were
varied among donors (Fig. 4B).
These results suggest the importance of the CXCL12-CCR4 axis over
the CCL2-CCR2 or PDGFs-PDGFRs axis in the migration of fibrocytes
into the tumor microenvironment. We finally evaluated the
expression of CXCR4 on tumor-infiltrated fibrocytes by double
staining for FSP-1 and CXCR4 and confirmed that the FSP-1-positive
fibrocytes definitely expressed CXCR4 (Fig. 4C).
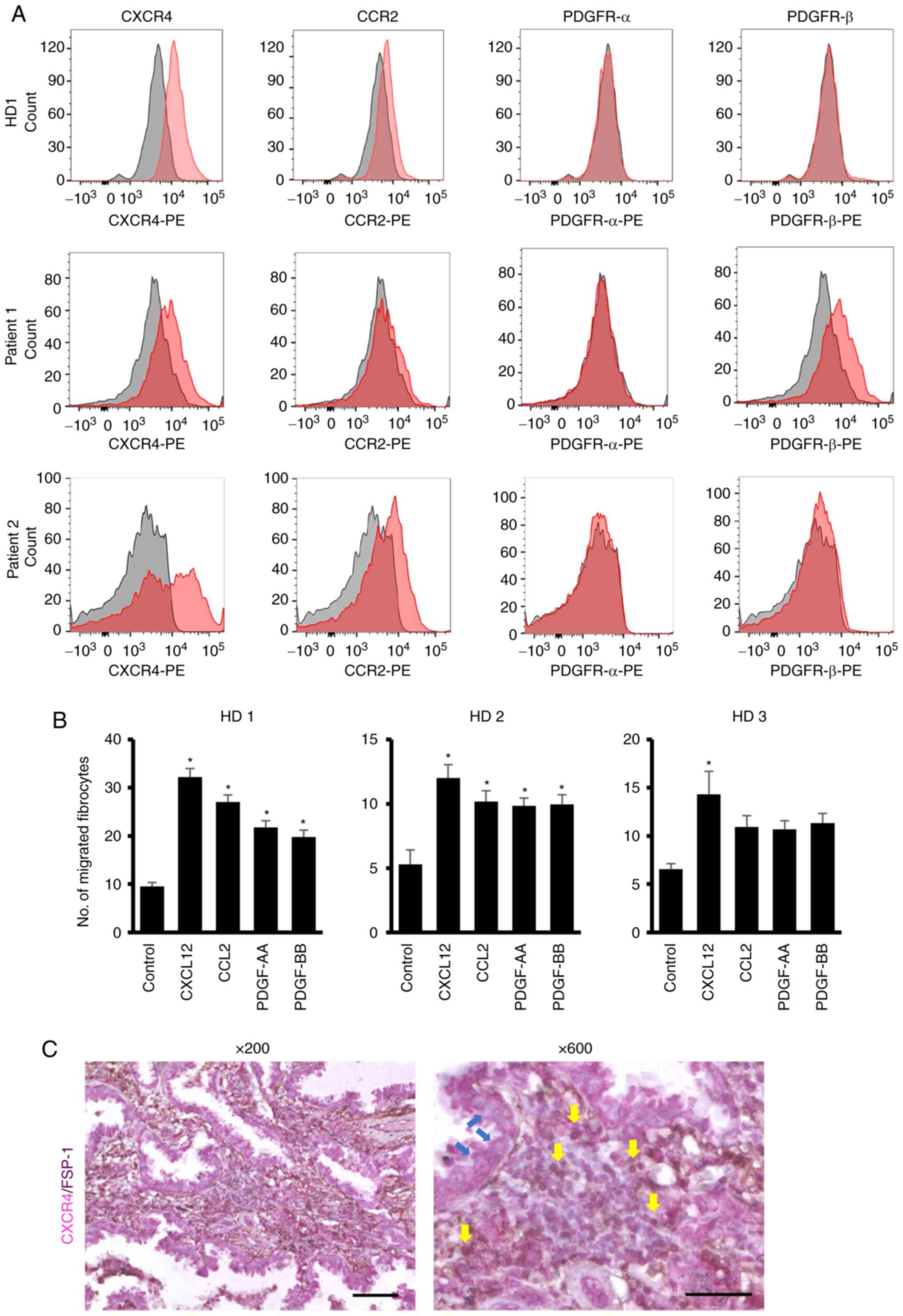 | Figure 4.Expression of CXCR4, CCR2 and PDGF
receptors in fibrocytes and their migration in response to their
ligands. (A) Expression levels of CXCR4, CCR2 and PDGF receptors in
fibrocytes generated from PBMC from HD and patients with LUAD. The
expression of each receptor was examined by flow cytometry. (B)
Migration of fibrocytes in response to CXCL12, CCL2, PDGF-AA and
PDGF-BB. The fibrocytes were generated from three HD. The migration
assay was performed with the optimal dose (100 ng/ml) of CXCL12,
CCL2, PDGF-AA and PDGF-BB. (C) Expression of CXCR4 on
tumor-infiltrated fibrocytes. The FSP-1-positive fibrocytes
definitely expressed CXCR4 in the tumor tissues from a patient with
LUAD. Yellow arrows indicate double-stained fibrocytes. Blue arrows
indicate cancer cells. Magnification left to right: ×200 and ×600;
scale bar 100 µm (×200) and 50 µm (×600). *P<0.05 vs.
control. HD, healthy donors; CXCR4, C-X-C chemokine receptor type
4; PDGF, platelet-derived growth factor; PBMC, peripheral blood
mononuclear cells; LUAD, lung adenocarcinoma; FSP-1,
anti-fibroblast-specific protein 1. |
Discussion
In the present study, we demonstrated that the
expression of CXCL12 in tumor cells was associated with the number
of tumor-infiltrating fibrocytes in LUAD patients. However, CCL2,
PDGF-AA, and PDGF-BB were not correlated with the accumulation of
fibrocytes. These results suggest that inhibiting the CXCL12/CXCR4
axis may be an effective strategy for regulating the number of
fibrocytes in LUAD.
Recently, substantial attention has been paid to the
roles of fibrocytes in tumor progression (20,21).
As fibrocytes can produce multiple factors that affect the function
of cancer cells, tumor-infiltrating fibrocytes have been thought to
play a role in tumor progression (1,3,13,14,20).
However, few studies have examined the relationships between
fibrocytes and tumors. We recently demonstrated that fibrocytes
have the ability to promote the stem cell-like property of tumor
cells by secreting the soluble factors, such as osteopontin, and
aid in the tumorigenesis of lung cancer cells (19). Based on these results, regulating
tumor progression by controlling the accumulation of fibrocytes
into tumors may be possible. Thus, it is necessary to clarify the
chemotactic factors of fibrocytes active in tumor tissues. While
several chemotactic factors, such as CXCL12, CCL2, CCL5, CCL11,
CCL24, and PDGFs, have been reported to play roles in the
recruitment of circulating fibrocytes, we focused on the
expressions of CXCL12, CCL2, PDGF-AA, and PDGF-BB in the present
study, as those factors have also been reported to be expressed in
lung cancer tumor tissues and associated with the prognosis of
patients (22–30).
The present study found that the expression of
CXCL12 in tumor tissues was associated with a poor prognosis of
patients with lung cancer. Similar results have been reported in
several studies of lung cancer (22–25).
Furthermore, it was also reported that the intra-tumoral expression
of CXCL12 was much higher than its expression in the serum
(31), suggesting the importance
of its expression in the tumor microenvironment. In addition, we
found that the number of tumor-infiltrating fibrocytes was
associated with the expression level of CXCL12 in tumors, but not
CCL2, PDGF-AA, or PDGF-BB in tumor, indicating that fibrocytes were
migrated into the tumor microenvironment via the CXCL12/CXCR4 axis.
We also demonstrated that the density of tumor-infiltrating
fibrocytes was correlated with the prognosis of LUAD patients. To
our knowledge, only one report has demonstrated the correlation of
circulating fibrocytes and the prognosis of the patients with lung
cancer (32), so our finding
supports the novel rationale in the utility of fibrocytes as a
prognostic factor in the patients with LUAD. However, additional
studies are required to explore the detailed relationship of CXCL12
expression and the survival of patients with lung cancer and to
determine the correlation of serum CXCL12 levels with the density
of tumor-infiltrating fibrocytes or the patient prognosis. In the
present study and two previous reports, the correlation was mainly
found in LUAD patients, but not LUSQ patients (22,24);
however, this point should also be explored further for
clarification.
Conversely, no correlation was noted among the
expression of CCL2 or PDGFs and the survival of patients with lung
cancer or the number of tumor-infiltrating fibrocytes. In previous
studies, the association of the expression of CCL2 or PDGFs in
tumor tissues and the prognosis of patients was considered
controversial in lung cancer (26–30).
On examining the expression of receptors for these chemotactic
factors in fibrocytes, the high and consistent expression of CXCR4,
which is the receptor for CXCL12, but not that of CCR2 and PDGFRs,
was observed in specimens from healthy subjects and patients with
LUAD. The pattern of receptor expression may affect the migration
of fibrocytes in vivo, as the migration of fibrocytes was
strongly induced by CXCL12.
Several limitations associated with the present
study warrant mention. First, the patients analyzed in this study
were biased toward having early-stage disease, given that
surgically resected tumor specimens and not trans-bronchial biopsy
specimens were used. Therefore, additional analyses will be needed
to determine whether or not similar findings are observed in
patients with stage III or IV disease. Second, the expression of
chemotactic factors in tumor tissues was examined based on
immunohistochemistry, a semi-quantitative method. Third, the
expression was evaluated in tumor cells but not in stromal cells,
as the expression was mainly found in tumor cells (although PDGF-AA
was to a lesser extent expressed in stromal cells in some
patients). Fourth, the influence of the CXCL12/CXCR4 autocrine
pathway in cancer cells was not evaluated, although the importance
of this pathway in the pathogenesis of lung cancer was already
reported in a previous study (33). In the immunohistochemical analyses
described above, we observed a clear positive signal on the cancer
cells themselves (Fig. 4C). Based
on these findings, we suspect that the impact of CXCL12 on
fibrocytes would be more significant than that on cancer cells.
However, we cannot deny the impacts of the CXCR4/CXCL12 autocrine
pathway on the correlation of the CXCL12 expression and the
prognosis of the patients with LUAD.
In summary, our findings suggest that the CXCL12 may
be an important chemotactic factor for tumor-infiltrating
fibrocytes in LUAD, regardless of the patient age. Thus, blockade
of the CXCL12/CXCR4 axis may be a therapeutic strategy for LUAD
through inhibition of the migration of fibrocytes into tumors.
Supplementary Material
Supporting Data
Acknowledgements
We thank Ms. Tomoko Oka and Ms. Akie Tanabe
(Department of Respiratory Medicine and Rheumatology, Graduate
School of Biomedical Sciences, Tokushima University, Tokushima,
Japan) for their technical assistance.
Funding
This work was partly supported by the Japan Society for the
Promotion of Science (JSPS) KAKENHI (grant no. JP16H0530910) and a
grant from the Ministry of Health, Labour and Welfare, the Study
Group on Diffuse Pulmonary Disorders and Scientific
Research/Research on Intractable Diseases (grant no.
0000025921).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YN conceptualized and designed the study. MT, AM,
AS, HOgi, TA, HG, SS, AA, KH and RO performed the flow cytometry,
migration assay and statistical analyses. MT, HOga and HT performed
the histological analyses with the surgically resected tumor
specimens. MT, AM, HOgi and YN confirmed the authenticity of all
the raw data, and participated in writing and editing. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The Institutional Review Board of Tokushima
University Hospital approved this study (approval nos. 2471 and
2838). Regarding the lung cancer patients who provided the tumor
specimens for the analyses, the need for written informed consent
was waived by the Ethics Committee of Tokushima University, because
of its retrospective nature.
Patient consent for publication
Written informed consent was obtained from both
patients and healthy donors who provided their peripheral
blood.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
PDGF
|
platelet-derived growth factor
|
|
FGF
|
fibroblast growth factor
|
|
VEGF
|
vascular endothelial growth factor
|
|
LUAD
|
lung adenocarcinoma
|
|
LUSQ
|
lung squamous cell carcinoma
|
|
PBS
|
phosphate-buffered saline
|
|
FSP-1
|
fibroblast-specific protein 1
|
References
|
1
|
Reilkoff RA, Bucala R and Herzog EL:
Fibrocytes: Emerging effector cells in chronic inflammation. Nat
Rev Immunol. 11:427–435. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bucala R: Fibrocytes at 20 years. Mol Med.
21 (Suppl 1):S3–S5. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abe R, Donnelly SC, Peng T, Bucala R and
Metz CN: Peripheral blood fibrocytes: Differentiation pathway and
migration to wound sites. J Immunol. 166:7556–7562. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pilling D, Fan T, Huang D, Kaul B and
Gomer RH: Identification of markers that distinguish
monocyte-derived fibrocytes from monocytes, macrophages, and
fibroblasts. PLoS One. 4:e74752009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hashimoto N, Jin H, Liu T, Chensue SW and
Phan SH: Bone marrow-derived progenitor cells in pulmonary
fibrosis. J Clin Invest. 113:243–252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Phillips RJ, Burdick MD, Hong K, Lutz MA,
Murray LA, Xue YY, Belperio JA, Keane MP and Strieter RM:
Circulating fibrocytes traffic to the lungs in response to CXCL12
and mediate fibrosis. J Clin Invest. 114:438–446. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dupin I, Contin-Bordes C and Berger P:
Fibrocytes in asthma and chronic obstructive pulmonary disease:
Variations on the same theme. Am J Respir Cell Mol Biol.
58:288–298. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakai N, Wada T, Yokoyama H, Lipp M, Ueha
S, Matsushima K and Kaneko S: Secondary lymphoid tissue chemokine
(SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis.
Proc Natl Acad Sci USA. 103:14098–14103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kisseleva T, Uchinami H, Feirt N,
Quintana-Bustamante O, Segovia JC, Schwabe RF and Brenner DA: Bone
marrow-derived fibrocytes participate in pathogenesis of liver
fibrosis. J Hepatol. 45:429–438. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suster S and Fisher C: Immunoreactivity
for the human hematopoietic progenitor cell antigen (CD34) in
lipomatous tumors. Am J Surg Pathol. 21:195–200. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barth PJ, Ebrahimsade S, Hellinger A, Moll
R and Ramaswamy A: CD34+ fibrocytes in neoplastic and inflammatory
pancreatic lesions. Virchows Arch. 440:128–133. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hartlapp I, Abe R, Saeed RW, Peng T,
Voelter W, Bucala R and Metz CN: Fibrocytes induce an angiogenic
phenotype in cultured endothelial cells and promote angiogenesis in
vivo. FASEB J. 15:2215–2224. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sato S, Shinohara S, Hayashi S, Morizumi
S, Abe S, Okazaki H, Chen Y, Goto H, Aono Y, Ogawa H, et al:
Anti-fibrotic efficacy of nintedanib in pulmonary fibrosis via the
inhibition of fibrocyte activity. Respir Res. 18:1722017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abe S, Sato S, Aono Y, Azuma M, Kishi M,
Koyama K, Takahashi N, Kagawa K, Kawano H and Nishioka Y:
Functional analysis of human fibrocytes derived from monocytes
reveals their profibrotic phenotype through paracrine effects. J
Med Invest. 67:102–112. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mitsuhashi A, Goto H, Saijo A, Trung VT,
Aono Y, Ogino H, Kuramoto T, Tabata S, Uehara H, Izumi K, et al:
Fibrocyte-like cells mediate acquired resistance to anti-angiogenic
therapy with bevacizumab. Nat Commun. 6:87922015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moore BB, Murray L, Das A, Wilke CA,
Herrygers AB and Toews GB: The role of CCL12 in the recruitment of
fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol.
35:175–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aono Y, Kishi M, Yokota Y, Azuma M,
Kinoshita K, Takezaki A, Sato S, Kawano H, Kishi J, Goto H, et al:
Role of platelet-derived growth factor/platelet-derived growth
factor receptor axis in the trafficking of circulating fibrocytes
in pulmonary fibrosis. Am J Respir Cell Mol Biol. 51:793–801. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Isgrò M, Bianchetti L, Marini MA, Bellini
A, Schmidt M and Mattoli S: The C-C motif chemokine ligands CCL5,
CCL11, and CCL24 induce the migration of circulating fibrocytes
from patients with severe asthma. Mucosal Immunol. 6:718–727. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saijo A, Goto H, Nakano M, Mitsuhashi A,
Aono Y, Hanibuchi M, Ogawa H, Uehara H, Kondo K and Nishioka Y:
Bone marrow-derived fibrocytes promote stem cell-like properties of
lung cancer cells. Cancer Lett. 421:17–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roife D, Fleming JB and Gomer RH:
Fibrocytes in the tumor microenvironment. Adv Exp Med Biol.
1224:79–85. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goto H and Nishioka Y: Fibrocytes: A novel
stromal cells to regulate resistance to anti-angiogenic therapy and
cancer progression. Int J Mol Sci. 19:982017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki M, Mohamed S, Nakajima T, Kubo R,
Tian L, Fujiwara T, Suzuki H, Nagato K, Chiyo M, Motohashi S, et
al: Aberrant methylation of CXCL12 in non-small cell lung cancer is
associated with an unfavorable prognosis. Int J Oncol. 33:113–119.
2008.PubMed/NCBI
|
|
23
|
Wagner PL, Hyjek E, Vazquez MF, Meherally
D, Liu YF, Chadwick PA, Rengifo T, Sica GL, Port JL, Lee PC, et al:
CXCL12 and CXCR4 in adenocarcinoma of the lung: Association with
metastasis and survival. J Thorac Cardiovasc Surg. 137:615–621.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sterlacci W, Saker S, Huber B, Fiegl M and
Tzankov A: Expression of the CXCR4 ligand SDF-1/CXCL12 is
prognostically important for adenocarcinoma and large cell
carcinoma of the lung. Virchows Arch. 468:463–471. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Katsura M, Shoji F, Okamoto T, Shimamatsu
S, Hirai F, Toyokawa G, Morodomi Y, Tagawa T, Oda Y and Maehara Y:
Correlation between CXCR4/CXCR7/CXCL12 chemokine axis expression
and prognosis in lymph-node-positive lung cancer patients. Cancer
Sci. 109:154–165. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Donnem T, Al-Saad S, Al-Shibli K, Andersen
S, Busund LT and Bremnes RM: Prognostic impact of platelet-derived
growth factors in non-small cell lung cancer tumor and stromal
cells. J Thorac Oncol. 3:963–970. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Donnem T, Al-Saad S, Al-Shibli K, Busund
LT and Bremnes RM: Co-expression of PDGF-B and VEGFR-3 strongly
correlates with lymph node metastasis and poor survival in
non-small-cell lung cancer. Ann Oncol. 21:223–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu J, Liu C, Qiu L, Li J, Zhang P and Sun
Y: Overexpression of both platelet-derived growth factor-BB and
vascular endothelial growth factor-C and its association with
lymphangiogenesis in primary human non-small cell lung cancer.
Diagn Pathol. 9:1282014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang XW, Qin X, Qin CY, Yin YL, Chen Y
and Zhu HL: Expression of monocyte chemoattractant protein-1 and CC
chemokine receptor 2 in non-small cell lung cancer and its
significance. Cancer Immunol Immunother. 62:563–570. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li L, Liu YD, Zhan YT, Zhu YH, Li Y, Xie D
and Guan XY: High levels of CCL2 or CCL4 in the tumor
microenvironment predict unfavorable survival in lung
adenocarcinoma. Thorac Cancer. 9:775–784. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wald O, Izhar U, Amir G, Kirshberg S,
Shlomai Z, Zamir G, Peled A and Shapira OM: Interaction between
neoplastic cells and cancer-associated fibroblasts through the
CXCL12/CXCR4 axis: Role in non-small cell lung cancer tumor
proliferation. J Thorac Cardiovasc Surg. 141:1503–1512. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Henrot P, Beaufils F, Thumerel M, Eyraud
E, Boudoussier A, Begueret H, Maurat E, Girodet PO, Marthan R,
Berger P, et al: Circulating fibrocytes as a new tool to predict
lung cancer progression after surgery? Eur Respir J.
58:21012212021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dai X, Mao Z, Huang J, Xie S and Zhang H:
The CXCL12/CXCR4 autocrine loop increases the metastatic potential
of non-small cell lung cancer in vitro. Oncol Lett.
5:277–282. 2013. View Article : Google Scholar : PubMed/NCBI
|