Introduction
Aphasia is the inability to reproduce or understand
speech and occurs in 21-38% of patients immediately following a
stroke (1-4).
Furthermore, when considering recovery in the first weeks following
a stroke, chronic deficits persist in ~20% of patients 6 months
later (5,6). Therefore, speech and language therapy
(SLAT) remains the ideal treatment option for patients with chronic
aphasia (7). However, whether
intensive speech therapy is useful or not remains controversial
(8,9).
Non-invasive brain stimulation methods, such as
transcranial direct current stimulation (tDCS), have exhibited
clinical benefits in improving the efficacy of regular SLAT in
aphasia rehabilitation conditions (10). However, there are some technical
limitations to these possible benefits of tDCS in different
clinical trials, such as the lack of randomization and the small
size of the samples (10-17).
Clinical studies have gradually used an alternative
treatment of experimental/traditional music during exercise to
manage post-stroke rehabilitation, with positive outcomes for
patients (18,19). In addition, music during exercise
increases desire, decreases cognition during exercise (20), and alleviates feelings of fatigue
(21). Nevertheless, data on the
mechanisms through which the renewal environment following a stroke
affects brain plasticity are limited. Music therapy, on the other
hand, is difficult to judge and predict in terms of its importance
(22).
Therefore, the present study systematically
investigated the effects of tDCS and traditional/experiential music
therapy (MT) on the recovery of patients with aphasia following a
stroke who were attending a regular stroke rehabilitation
program.
Patients and methods
Study design
The present retrospective cohort study investigated
the clinical effectiveness of tDCS in combination with MT for
improving aphasia following a stroke. All participants had suffered
a single stroke diagnosed by computed tomography perfusion (CTP).
In the present 5-year follow-up cohort study (from February, 2015
to February, 2020), 98 patients, with a mean age of 68.4±5 years
(55 males, 56.1%), who met the requirements, were divided into
three groups as follows: Group A, no MT or tDCS (only standard
treatment); group B, daily MT; group C, combined treatment with
daily MT and tDCS at a 1:1.21:1.28 ratio for the three groups,
respectively. The study was conducted at the University Hospital of
Larissa, Greece and was based on anonymized hospital records. The
Institutional Review Board (IRB) of the University of Larisa,
Greece/The School of Medicine/School of Health Sciences approved
the study (IRB no. 2492/19-01-2015, finalized by the 9th General
Assembly on 28/01/2015).
Furthermore, all the patients received the usual
care for a stroke, including medical care and rehabilitation. All
patients who suffered a cerebrovascular accident (CVA) underwent
neuropsychological assessment (including questionnaires and
cognitive tests) and a mini-mental test (mMT) to assess cognitive
deficits at baseline, during admission and at 6 months following
the CVA. CTP was performed at 3 to 6 days (control) and at 6
months, and CTP scores [cerebral blood flow (CBF)] were assessed.
The Barthel Index (BI) was used to assess impairment in daily
living activities. All cases were screened and assessed for
language ability before taking the Aachen Aphasia Test (AAT).
Speech presentation was assessed using four subscales: Repetition,
Token test, naming and comprehension. Writing (due to hemiparesis)
and spontaneous speech (as construct validity was not sufficient)
were not included as AAT subscales. Normally distributed t-scores
were the AAT scores averaged over the subscales. Patients were
scored three points for correctly naming target words; two points
for correctly or incorrectly pronouncing target words on the second
try, but with syntactically correct or phonologically related
expressions; and one point for any other expressions or omissions.
The average total score was expressed as a t-score based on these
scores. In a repeated measures design, language testing was
performed 1 day before (T0) and at 6 months after tDCS +/or MT
(T1). All reported alterations in language performance
were described using the interval between two test phases of the
AAT [Δ (Τ1-T0)] (Table I).
 | Table IAphasia test results. |
Table I
Aphasia test results.
| Parameters | T0
(baseline) | T1
(after 6 months) |
Δ(T1-T0) | P-value |
|---|
| Group A, n=28 | | | | |
|
Token test,
mean | 2.4 | 3.9 | 1.5 | 0.398 |
|
Repetition,
mean | 5.1 | 5.5 | 0.4 | 0.561 |
|
Naming,
mean | 5.3 | 11.2 | 5.9 | 0.080 |
|
Comprehension,
mean | 3 | 11 | 8 | 0.293 |
|
Sum, mean ±
SD | 3.9±2 | 8.6±3 | 3.9±2 | 0.293 |
| Group B, n=34 | | | | |
|
Token test,
mean | 2.1 | 7.3 | 5.2 | |
|
Repetition,
mean | 4.4 | 9.2 | 4.8 | |
|
Naming,
mean | 2.3 | 8.7 | 6.5 | |
|
Comprehension,
mean | 3.8 | 11.8 | 8 | |
|
Sum, mean ±
SD | 2.5±1 | 9.2±1 | 6.1±2 | |
| Group C, n=36 | | | | |
|
Token test,
mean | 2.5 | 10.5 | 8 | |
|
Repetition,
mean | 1.2 | 12 | 10.8 | |
|
Naming,
mean | 1.3 | 12 | 10.7 | |
|
Comprehension,
mean | 1.6 | 12 | 8.4 | |
|
Sum, mean ±
SD | 1.6±1 | 11.6±1 | 9.4±2 | |
|
AAT
t-scores, mean | 2.6±1 | 9.8±2 | 6.4±2 | 0.001a |
The severity of aphasia was categorized into
subgroups, as presented in Table
II (conduction aphasia: Good repetition of words/phrases;
difficulty in word-finding; use of generic word fillers (e.g.,
‘thing’) or circumlocutions; Broca's aphasia: High ability to
repeat; possible difficulty responding to questions spontaneously;
Wernicke's aphasia: capability of repeating words or phrases;
repeats questions instead of providing answers (echolalia);
substantial disability in both expressive and receptive language;
can interact by gestures, intonation, and facial expressions).
 | Table IISeverity of aphasia in the
subgroups. |
Table II
Severity of aphasia in the
subgroups.
| Parameters | Conduction | Wernicke's | Broca's | Global | Sum |
|---|
| T0
(baseline), n=98 | | | | | |
|
Group A, n
(%) | 3 (3.0) | 9 (9.1) | 14 (14.2) | 3 (3.0) | 28 |
|
Group B, n
(%) | 5 (5.1) | 7 (7.1) | 20 (20.4) | 2 (2.0) | 34 |
|
Group C, n
(%) | 2 (2.0) | 8 (8.1) | 22 (22.4) | 4 (4.0) | 36 |
|
All, n
(%) | 10 (10.2) | 24 (24.4) | 56 (57.1) | 9 (9.1) | 98 |
| T1
(after 6 months, n=36) | | | | | |
|
Group A, n
(%) | 1 (1.0) | 7 (7.1) | 11 (11.2) | 3 (3.0) | 22 |
|
Group B, n
(%) | 1 (1.0) | 1 (1.0) | 6 (6.1) | 2 (2.0) | 10 |
|
Group C, n
(%) | 0 | 0 | 2 (2.0) | 2 (2.0) | 4 |
|
All, n
(%) | 2 (2.0) | 8 (8.1) | 19 (9.1) | 7 (7.1) | 36 |
Inclusion and exclusion criteria
The inclusion criteria were as follows: Patients
with a first clinical onset of stroke 6 months prior; ab age
between 18-75 years; a BI score ≥50 (possible score of 100)
(23); modified Ashworth scale of
≤II; Greek-speaking; patients who were previously right-handed in
all daily activities and were not forced to switch hands as
children.
The exclusion criteria were the following: An
inability to participate in an entire testing session; pre-stroke
illiteracy; neuropsychological sequelae of brain injury likely to
impair test performance (e.g., severe memory or visual perceptual
impairment); no patients were diagnosed with other psychiatric or
neurological disorders or depression. Additionally, all patients
were native Greek speakers. The medical records of each patient
were used to determine any issues with their central nervous
system.
Procedures. MT
The patients in the exercise groups took part in a
music-based exercise program for 6 months. There were four 45-min
sessions per week. Patients in group C were subjected to a combined
treatment (tDCS 20 min/MT 20 min) for 40 min daily. Patients were
instructed to sit near a table and place their upper limbs on it.
The primary instructor used simple and precise instructions in a
verbal form in combination with continuous visuals. The number of
medical students was the same as the number of patients. This made
it possible to provide each patient with the assistance and care
they needed on a one-to-one basis. In order to pique the interest
and promote the involvement of the older patients,
experiential/traditional music that was popular when they were
young and was thus maintained in their minds for a long period of
time was selected.
The study session began with 5-min warm-up exercises
involving breathing and flexibility. The main part of the session
consisted of moderately difficult exercises to strengthen the upper
and lower limbs, as well as exercises to improve coordination and
balance, while sitting or standing. Finally, during a 5- to 10-min
cool-down period, the subjects moved slowly in a circle, while
holding their hands and listening to music.
tDCS. The tDCS procedure was performed after
the MT session for each patient. The neuroConn DC stimulator PLUS
class II (neuroCare Group GmbH) was used, and two electrodes
measuring 7x5 cm were placed and fixed on the head. The most common
electrode configuration was used, i.e., anodal tDCS over the left
inferior frontal gyrus (IFG) localized as F5, compared to sham
tDCS. The cathode was placed in the contralateral supraorbital area
localized as Fp2.
During each intervention, anodal tDCS was applied
over the left IFG (1 mA for 20 min; experimental condition), or
sham tDCS was applied over the same region (control condition) in
the combined therapy group (group C).
In the sham tDCS condition, stimulation commenced
with a 15-sec fade-in, just like in the experimental condition, but
stimulation was turned off for the control group. In addition, both
the patient and therapist were blinded to the stimulation
condition.
CTP. Two radiologists performed the DTP. CTP
parameters, i.e., CBF and cerebral blood volume (CBV) values were
recorded and assessed using two consecutive 10-mm slices focused at
the region of the basal ganglia and with the identical angulation
as the native CT. A power injector administered a bolus dose of 50
ml non-ionic contrast medium (Imeron 400, Bracco Imaging
Deutschland GmbH) through a central venous line at a flow rate of 4
ml/sec, followed by the administration of 30 ml of saline. A set of
40 images were collected at each slice level at a rate of two
frames per second, 4 sec after intravenous contrast was
administered (120 kV, 110 mAs, matrix 512x512). These values were
measured utilizing post-processing software (Perfusion CT,
Siemens), and the CTP color maps were evaluated for their quality
using a visual rating scale. A positive visual score was recorded
for side-to-side asymmetries or evident bilateral defects,
demonstrating a reduction in CBF, CBV and the mean transit time
(MTT), which were related to the central volume principle:
CBF=CBV/MTT (24). CBV was
calculated in milliliters of blood per 100 g of brain tissue and
determined as the volume of inbound blood for a specific brain
volume (25).
Outcome measures. Primary
endpoints
AAT Δ(T1-T0): The intermediate
period between two phases of the AAT study. Patients were assessed
1 day before (AAT0) and 6 months after therapy (AAT1). As shown in
Table II, rehabilitation was
defined as the improvement of aphasia in the subgroups.
Secondary outcomes. The mean values [mean=(V1
+ V0)/2] of i) CBF mean [mean CBF=(V1-CBF + V0-CBF)/2] from CTP
conducted 3-6 days (V0-CBF) and 6 months after admission (V1-CBF);
ii) mMT mean [mean mMT=(V1-mMT + V0-mMT)/2] were used to assess
cognitive deficits. BI mean [mean BI=(V1-BI + V0-BI)/2] was used to
measure performance in activities of daily living; first as a
pre-screening test at admission to the rehabilitation center
(baseline) (V0-mMT) (V0-BI), and 6 months after the CVA (V1-mMT)
(V1-BI).
Statistical analysis
Data are expressed as the mean ± SD. Data were
examined for regularity using the Shapiro-Wilk test and analyzed
using one-way ANOVA. Categorical data were analyzed using the
Chi-squared test or Fisher's exact test. The Bonferroni test was
used following one-way ANOVA. A multivariable analysis model was
used to assess variables significantly associated with the
univariate analysis. A P-value <0.05 was considered to indicate
a statistically significant difference. The discriminative ability
of significant variables was evaluated using the area under the
receiver operating characteristic curve (ROC). Statistical analyses
were executed using Statistical Product and Service Solutions
software, version 15 (SPSS Inc.).
Results
Baseline data
A total of 98 patients were enrolled in the present
study, and their baseline data are presented in Table III. Statistically significant
differences between groups were found for AAT
Δ(T1-T0) mean (P<0.05) (Table I), CBF mean in affected areas
(P=0.007), mMT mean (P<0.05) and BI mean (P=0.002) (Table III).
 | Table IIIBaseline characteristics of the study
participants. |
Table III
Baseline characteristics of the study
participants.
| Parameters | All patients, n=98
(100%) | Group A, n=28
(28.5%) | Group B, n=34
(34.6%) | Group C, n=36
(36.7%) | P-value |
|---|
| Age, years | 68.4±5 | 68.0±5 | 68.5±4 | 68.8±5 | 0.430 |
| Sex (male), n
(%) | 55 (56.1) | 13 (13.2) | 23 (23.4) | 19 (19.3) | 0.221 |
| CBF mean in
affected area, cm/sec | 15.1±4 | 12.7±2 | 16.5±4 | 15.6±5 | 0.007 |
| mMT mean | 25.0±1 | 23.7±1 | 26.1±1 | 25.0±1 | 0.001 |
| BI mean | 78.7±5 | 77.3±4 | 81.0±5 | 77.7±5 | 0.002 |
| Clinical
characteristics | | | | | |
|
Hemiparesis
(yes), n (%) | 73 (74.4) | 20 (20.4) | 26 (26.5) | 27 (27.5) | 0.902 |
|
Handedness | | | | | |
|
Right,
n (%) | 83 (84.6) | 26 (26.5) | 28 (28.5) | 29 (29.5) | 0.333 |
|
Left,
n (%) | 13 (13.2) | 2 (2.0) | 5 (5.1) | 6 (6.1) | |
|
Ambidextrous,
n (%) | 2 (2.0) | 0 (0) | 1 (1.0) | 1 (1.0) | |
|
CT
findings | | | | | |
|
Ischemic
or hemorrhage, (ischemic), n (%) | 35 (35.7) | 9 (9.1) | 12 (12.2) | 14 (14.2) | 0.858 |
|
Lesion
size, max diameter in cm | 29.9±5 | 29.4±4 | 29.7±5 | 30.5±5 | 0.758 |
|
Damage
location | | | | | |
|
MCA,
n (%) | 53 (54.0) | 13 (13.2) | 21 (21.4) | 19 (19.3) | 0.395 |
|
ACA,
n (%) | 38 (38.7) | 13 (13.2) | 12 (12.2) | 13 (13.2) | |
|
PCA,
n (%) | 7 (7.1) | 2 (2.0) | 1 (1.0) | 4 (4.0) | |
|
Type of
aphasia (after 6 months) | | | | | |
|
Conduction,
n (%) | 2 (2.0) | 1(10) | 1 (1.0) | 0 (0) | 0.533 |
|
Wernicke's,
n (%) | 8 (8.1) | 7 (7.1) | 1 (1.0) | 0 (0) | 0.002 |
|
Broca's,
n (%) | 19 (19.3) | 11 (11.2) | 6 (6.1) | 2 (2.0) | 0.003 |
|
Global,
n (%) | 7 (7.1) | 3 (3.0) | 2 (2.0) | 2 (2.0) | 0.692 |
|
Sum | 36 (36.7) | 22 (22.4) | 10 (10.2) | 4 (4.0) | |
Wernicke's, Broca's and global aphasias were the
only statistically significant aphasia types for recovery (P=0.001;
Table IV). The overall recovery
rate was 63.2% (62 of 98 patients). A higher recovery rate was
documented in group C (32.6%) compared with groups B (24.4%) and A
(6.1%), and the difference was statistically significant (P=0.001;
Table V).
 | Table IVOutcomes of the patients in the
present study. |
Table IV
Outcomes of the patients in the
present study.
| Parameters | All patients, n=98
(100%) | Recovery, n=62
(63.2%) | No recovery, n=36
(36.7%) | P-value |
|---|
| AAT
Δ(T1-T0) mean | 6.4±2 | 7.5±1.9 | 5.1±1.8 | 0.001 |
| CBF mean in
affected area, cm/sec | 15.1±4.5 | 15.6±4.6 | 14.4±4.3 | 0.353 |
| BI mean | 78.7±5.1 | 79.6±5.4 | 77.2±4.0 | 0.027 |
| mMT mean | 25.0±1.7 | 25.5±1.6 | 24.2±1.7 | 0.001 |
| Type of aphasia
(after 6 months, n=98) | | | | |
|
Conduction,
n (%) | 10 (10.2) | 8 (8.1) | 2 (2.0) | 0.061 |
|
Wernicke's,
n (%) | 24 (24.4) | 16 (16.3) | 8 (8.1) | 0.001 |
|
Broca's, n
(%) | 56 (57.1) | 37 (37.7) | 19 (19.3) | 0.001 |
|
Global, n
(%) | 9 (9.1) | 2 (2.0) | 7 (7.1) | 0.001 |
 | Table VUnivariate analysis for recovery. |
Table V
Univariate analysis for recovery.
| Parameters | Recovery, n=62
(63.2%) | No Recovery, n=36
(36.7%) | P-value |
|---|
| Age, years | 68.4±4 | 68.5±5 | 0.944 |
| Sex (male), n
(%) | 34 (34.6) | 21 (21.4) | 0.737 |
| Clinical
characteristics | | | |
|
Hemiparesis
(yes), n (%) | 46 (46.9) | 27 (27.5) | 0.930 |
| Handedness | | | |
|
Right, n
(%) | 50 (51.0) | 33 (33.6) | 0.282 |
|
Left, n
(%) | 10 (10.2) | 3 (3.0) | |
|
Ambidextrous,
n (%) | 2 (2.0) | 0 (0) | |
| CT findings | | | |
|
Ischemic or
hemorrhage, (ischemic), n (%) | 25 (25.5) | 10 (10.2) | 0.211 |
|
Lesion size,
max diameter in cm | 29.7±5 | 30.2±5 | 0.687 |
| Damage
location | | | |
|
• MCA, n
(%) | 32 (32.6) | 21 (21.4) | 0.781 |
|
• ACA, n
(%) | 25 (25.5) | 13 (13.2) | |
|
• PCA, n
(%) | 5 (5.1) | 2 (2.0) | |
| CBF mean in
affected area, cm/sec | 15.6±4 | 14.4±3 | 0.353 |
|
mMT
mean | 25.5±1 | 24.2±1 | 0.001 |
|
BI mean | 79.6±5 | 77.2±4 | 0.027 |
| Groups | | | |
|
Group A,
n(%) | 6 (6.1) | 22 (22.4) | 0.001 |
|
Group B, n
(%) | 24 (24.4) | 10 (10.2) | |
|
Group C, n
(%) | 32 (32.6) | 4 (4.0) | |
| Type of aphasia
(after 6 months, n=98) | | | |
|
Conduction,
n (%) | 8 (8.1) | 2 (2.0) | 0.061 |
|
Wernicke's,
n (%) | 16 (16.3) | 8 (8.1) | 0.001 |
|
Broca's, n
(%) | 37 (37.7) | 19 (19.3) | 0.001 |
|
Global, n
(%) | 2 (2.0) | 7 (7.1) | 0.001 |
Univariate analysis
Univariate analysis demonstrated that the mMT mean,
BI mean, Wernicke's, Broca's and global aphasia groups were
associated with recovery (P=0.001, 0.027, 0.001, 0.001 and 0.001,
respectively; Table V).
Multivariate analysis
Multivariate analysis revealed that only the
parameters, BI mean (OR, 0.013; 95% CI, 0.002-0.024; P=0.022),
groups (OR, 0.304; 95% CI, 0.232-0.376; P<0.05), and Wernicke's
(OR, -0.407; 95% CI, -0.621 to -0.192; P<0.05), Broca's (OR,
-0.814; 95% CI (-1.010 to -0.618; P<0.05) and global aphasia
(OR, -0.885; 95% CI, -1.157 to -0.614; P<0.05) were independent
predictors of improvement (Table
VI).
 | Table VIMultivariate analysis for
recovery. |
Table VI
Multivariate analysis for
recovery.
| Parameter | OR | 95% CI,
lower-upper | P-value |
|---|
| BI mean | 0.013 | 0.002-0.024 | 0.022 |
| mMT mean | 0.030 | -0.003 to
-0.064 | 0.074 |
| Groups, n (%) | 0.304 | 0.232-0.376 | 0.001 |
| Type of Aphasia
(after 6 months) | | | |
|
Wernicke's,
n (%) | -0.407 | -0.621 to
-0.192 | 0.001 |
|
Broca's, n
(%) | -0.814 | -1.010 to
-0.618 | 0.001 |
|
Global, n
(%) | -0.885 | -1.157 to
-0.614 | 0.001 |
ROC analysis
Following ROC analysis, AAT
Δ(T1-T0)/recovery was the most accurate
measure for discriminating recovery with a standard error of area
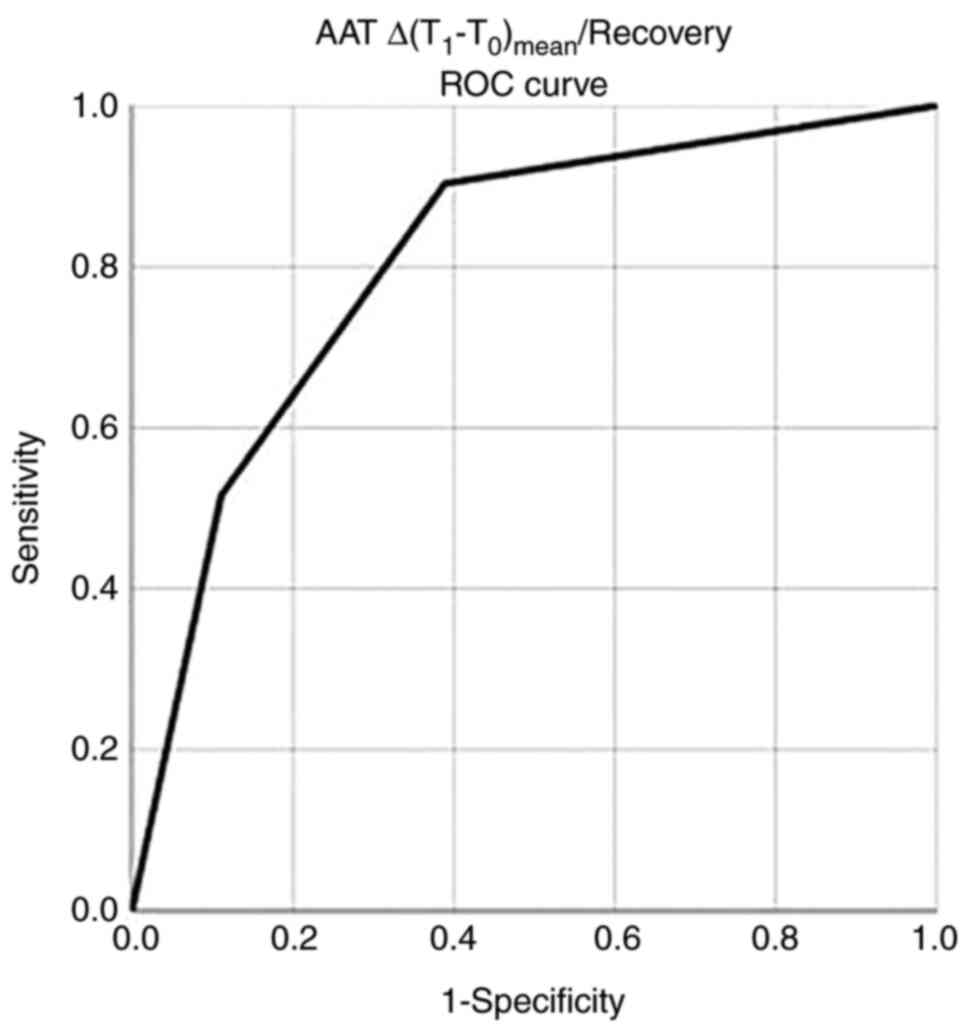
under the curve [AUC (SE)] of 0.807 (0.047), P<0.05, while an
AAT Δ(T1-T0)/recovery value of >7.7
exhibited a sensitivity of 90% and a specificity of 89% for
recovery (Fig. 1 and Table VII).
 | Table VIIROC analysis. |
Table VII
ROC analysis.
| Parameters | Area | Std. error | 95% CI,
lower-upper | P-value |
|---|
| AAT
Δ(T1-T0) mean/recovery | 0.807 | 0.047 | 0.715-0.900 | 0.001 |
| BI
mean/recovery | 0.615 | 0.057 | 0.503-0.727 | 0.058 |
| MMT
mean/recovery | 0.707 | 0.055 | 0.599-0.816 | 0.001 |
| Type of aphasia
(after 6 months) | | | | |
|
Wernicke's,
n (%) | 0.611 | 0.062 | 0.490-0.733 | 0.068 |
|
Broca's, n
(%) | 0.611 | 0.062 | 0.490-0.733 | 0.068 |
|
Global, n
(%) | 0.556 | 0.062 | 0.434-0.677 | 0.361 |
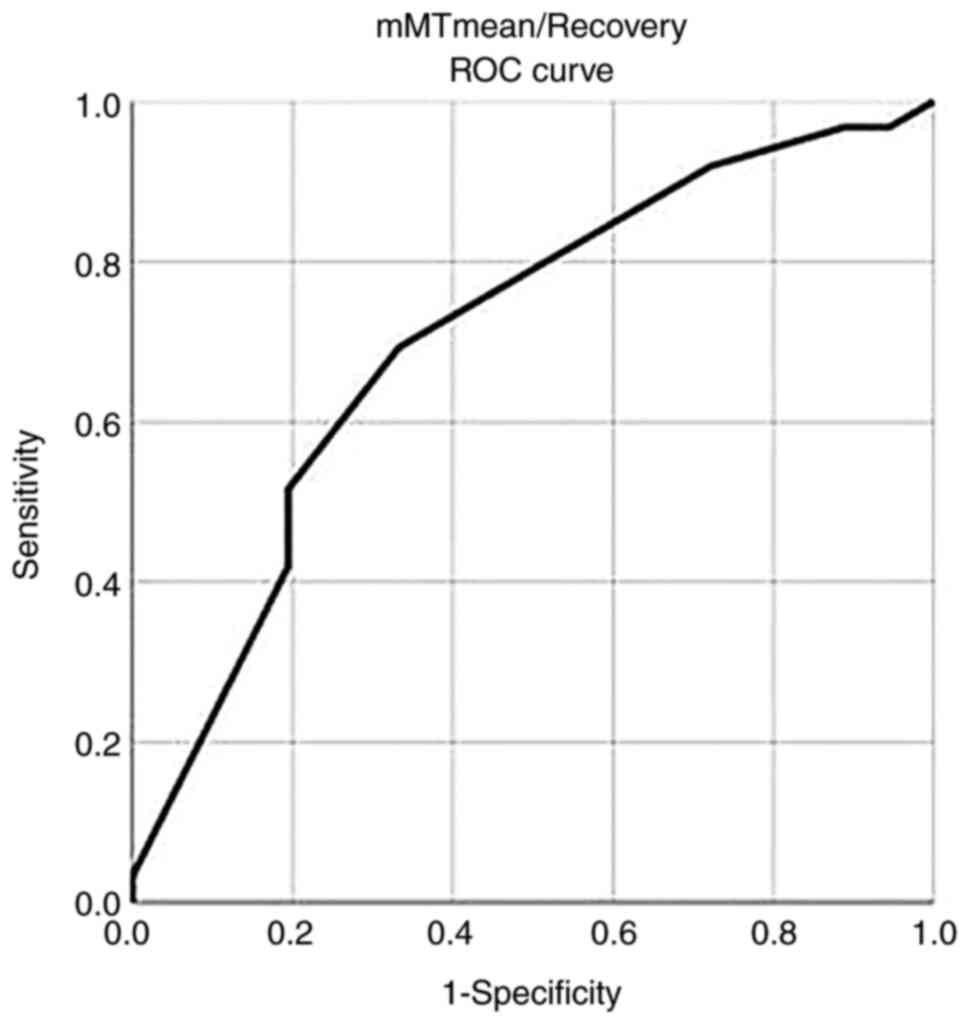
In addition, a mMT mean/recovery value >23.5
exhibited a sensitivity of 91.9% and a specificity of 67% for
recovery with an AUC (SE) of 0.707 (0.055) P=0.001 (Fig. 2 and Table VII).
Discussion
The present retrospective cohort study on patients
with post-stroke aphasia found that when MT and tDCS were added to
the exercise rehabilitation program, the patients in group C
(32.6%) recovered to a greater extent than those in groups B
(24.4%) and A (6.1%). In addition, the AAT
(T1-T0) mean was one of the most important
independent predictors of the recovery of patients with CVA who
completed a post-stroke rehabilitation program accompanied by MT
and tDCS (group C), and an AAT Δ(T1-T0) mean
>7.7 had better dispersion in predicting recovery. Moreover,
according to the results, patients with a mMT mean >23.5 could
also expect clinical recovery. Furthermore, the BI mean could also
predict recovery in patients post-stroke.
It is generally accepted that music activates
various neural networks in brain structures important for emotions,
cognition and motor functions (19,26,27).
In a number of studies on tDCS in the chronic phase, the use of
electrode configuration (anodal tDCS over the left IFG compared to
sham tDCS) was most commonly used in conjunction with
disorder-oriented aphasia treatment (28-31).
It appears to enhance the rate of language improvement (32).
It has been suggested that tDCS improves learning
via long-lasting synergism, i.e., long-term synaptic plasticity
(29). However, the debate on the
effect of tDCS on recovery from aphasia is still ongoing (33). The present study found that the
recovery rate was higher when the rehabilitation program was
conducted with MT and tDCS using one electrode configuration.
Although the present study demonstrated positive (albeit minor)
effects, the influence of different parameters is currently
unknown. Parameters, such as the type of aphasia or the
size/location of the lesion probably play a significant role in the
response to tDCS treatment.
The AAT is a reliable test for detecting aphasia. It
can categorize aphasia into four subgroups, also assessing the
speakers' language performance to provide more accurate information
about the severity of the disorder (34). In the present study, the AAT
Δ(T1-T0) mean was one of the most important
independent predictors of improvement in patients with CVA who
participated in a post-stroke rehabilitation program accompanied by
MT and tDCS. Indeed, a change in AAT >7.7 at T0=0 and T1=6
months predicted clinical improvement with 90% sensitivity and 89%
specificity.
In clinical practice, it is helpful to predict
recovery in patients who have suffered a stroke. However, as
predictive factors are variable, it is complex to assess the
prediction of improvement in aphasia (35). The most accurate components are the
severity of aphasia, lesion size and location (35). In the present study, the mean mMT
score was one of the main factors for improvement, and patients
with a score >23.5 can expect clinical improvement.
tDCS is a procedure that can alter brain functions
temporarily (36). Furthermore,
there is evidence to indicate that other brain regions (e.g., the
non-damaged right hemisphere) can also promote speech recovery
after stroke (36). However, it is
unclear whether different stimulation sites affect specific parts
of language function differently (37). Previous studies on healthy
participants have demonstrated significant and long-term gains in
cognitive and motor learning that were maintained for up to 12
months, and indicated that the effects of tDCS during the follow-up
period may be more robust in older participants than in younger
ones (38,39). In the present study, the long-term
effects of tDCS were maintained for up to 12 months due to the
advanced age of the patients.
The present study has some limitations. First, the
intervention duration was relatively brief. However, the intensity
and treatment duration used were consistent with studies (37-39)
that have demonstrated an effect of tDCS in chronic aphasia
following a stroke. Secondly, the present study was a single-center
study. For this reason, the positive effect of MT and tDCS on
recovery from aphasia following stroke cannot be generally assumed.
However, the results presented herein may serve as a basis for
future, more comprehensive clinical studies.
In conclusion, the present study aimed to elucidate
the synergistic effects of an exercise rehabilitation program, an
enriched acoustic environment, and tDCS leading to better recovery
from aphasia following a stroke. Although the present study
revealed positive (albeit minor) effects, the impact of different
parameters, such as the size/location of the lesion or the type of
aphasia, is likely to play a significant role in the response to MT
and the tDCS treatment. These encouraging results also suggest that
more non-invasive treatments for aphasia following a stroke need to
be tested in large, multicenter, double-blind, randomized control
trials.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GF, AAF and VEG conceptualized the study. VEG, AAF,
SC, PP, PS, NT, NM and KT made a substantial contribution to data
interpretation and analysis, and wrote and prepared the draft of
the manuscript. DAS, VEG and GF analyzed the data and provided
critical revisions. VEG and GF confirm the authenticity of all the
data. All authors contributed to manuscript revision and have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The Institutional Review Board (IRB) of University
of Thessaly, Greece/The School of Medicine/School of Health
Sciences approved the study (IRB no. 2492/19-01-2015, finalized by
the 9th General Assembly on 28/01/2015). The study was in line with
the Declaration of Helsinki (1995; as revised in Edinburgh 2000).
Due to the retrospective design of the study, a waiver for informed
consent was granted by the Institutional Review Board.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Managing Editor of the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this
article.
References
|
1
|
Inatomi Y, Yonehara T, Omiya S, Hashimoto
Y, Hirano T and Uchino M: Aphasia during the acute phase in
ischemic stroke. Cerebrovasc Dis. 25:316–323. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lackland DT, Roccella EJ, Deutsch AF,
Fornage M, George MG, Howard G, Kissela BM, Kittner SJ, Lichtman
JH, Lisabeth LD, et al: Factors influencing the decline in stroke
mortality: A statement from the American heart association/American
stroke association. Strok. 45:315–353. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lindsay MP, Norrving B, Sacco RL, Brainin
M, Hacke W, Martins S, Pandian J and Feigin V: World stroke
organization (WSO): Global stroke fact sheet 2019. Int J Stroke.
14:806–817. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Murray CJ, Vos T, Lozano R, Naghavi M,
Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S,
et al: Disability-adjusted life years (DALYs) for 291 diseases and
injuries in 21 regions, 1990-2010: A systematic analysis for the
global burden of disease study 2010. Lancet. 380:2197–223.
2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
El Hachioui H, Lingsma HF, van de
Sandt-Koenderman ME, Dippel DW, Koudstaal PJ and Visch-Brink EG:
Recovery of aphasia after stroke: A 1-year follow-up study. J
Neurol. 260:166–171. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Maas MB, Lev MH, Ay H, Singhal AB, Greer
DM, Smith WS, Harris GJ, Halpern EF, Koroshetz WJ and Furie KL: The
prognosis for aphasia in stroke. J Stroke Cerebrovasc Dis.
21:350–357. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Doogan C, Dignam J, Copland D and Leff A:
Aphasia recovery: When, how and who to treat? Curr Neurol Neurosci
Rep. 18(90)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Brady MC, Kelly H, Godwin J, Enderby P and
Campbell P: Speech and language therapy for aphasia following
stroke. Cochrane Database Syst Rev. 2016(CD000425)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Stahl B, Mohr B, Büscher V, Dreyer FR,
Lucchese G and Pulvermüller F: Efficacy of intensive aphasia
therapy in patients with chronic stroke: A randomised controlled
trial. J Neurol Neurosurg Psychiatry. 89:586–592. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Monti A, Ferrucci R, Fumagalli M, Mameli
F, Cogiamanian F, Ardolino G and Priori A: Transcranial direct
current stimulation (tDCS) and language. J Neurol Neurosurg
Psychiatry. 84:832–842. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Baker JM, Rorden C and Fridriksson J:
Using transcranial direct-current stimulation to treat stroke
patients with aphasia. Stroke. 41:1229–1236. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Flöel A, Meinzer M, Kirstein R, Nijhof S,
Deppe M, Knecht S and Breitenstein C: Short-term anomia training
and electrical brain stimulation. Stroke. 42:2065–2067.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fridriksson J, Richardson JD, Baker JM and
Rorden C: Transcranial direct current stimulation improves naming
reaction time in fluent aphasia: A double-blind, sham-controlled
study. Stroke. 42:819–821. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kang EK, Kim YK, Sohn HM, Cohen LG and
Paik NJ: Improved picture naming in aphasia patients treated with
cathodal tDCS to inhibit the right Broca's homologue area. Restor
Neurol Neurosci. 29:141–152. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Marangolo P, Fiori V, Calpagnano MA,
Campana S, Razzano C, Caltagirone C and Marini A: tDCS over the
left inferior frontal cortex improves speech production in aphasia.
Front Hum Neurosci. 7(539)2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Marangolo P, Fiori V, Campana S,
Calpagnano MA, Razzano C, Caltagirone C and Marini A: Something to
talk about: Enhancement of linguistic cohesion through tdCS in
chronic non fluent aphasia. Neuropsychologia. 53:246–256.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Monti A, Cogiamanian F, Marceglia S,
Ferrucci R, Mameli F, Mrakic-Sposta S, Vergari M, Zago S and Priori
A: Improved naming after transcranial direct current stimulation in
aphasia. J Neurol Neurosurg Psychiatry. 79:451–453. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Aravantinou-Fatorou K and Fotakopoulos G:
Efficacy of exercise rehabilitation program accompanied by
experiential music for recovery of aphasia in single
cerebrovascular accidents: A randomized controlled trial. Ir J Med
Sci. 190:771–778. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fotakopoulos G and Kotlia P: The value of
exercise rehabilitation program accompanied by experiential music
for recovery of cognitive and motor skills in stroke patients. J
Stroke Cerebrovasc Dis. 27:2932–2939. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Terry PC, Karageorghis CI, Curran ML,
Martin OV and Parsons-Smith RL: Effects of music in exercise and
sport: A meta-analytic review. Psychol Bull. 146:91–117.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Terry PC and Karageorghis CI:
Psychophysical effects of music in sport and exercise: An updated
theory, research, and application. In: Katsikitis M (ed).
Psychology bridging the Tasman: Science, culture, and practice.
Proceedings of the 2006 Joint Conference of the Australian
Psychology Society and the New Zealand Psychological Society.
Melboume, VIC. Australian Psychological Society, pp415-419, 2006.
https://www.piuvivi.com/docs/effetti-musica-sulla-psiche.pdf.
|
|
22
|
Särkämö T, Ripollés P, Vepsäläinen H,
Autti T, Silvennoinen HM, Salli E, Laitinen S, Forsblom A, Soinila
S and Rodríguez-Fornells A: Structural changes induced by daily
music listening in the recovering brain after middle cerebral
artery stroke: A voxel-based morphometry study. Front Hum Neurosci.
8(245)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sihvonen AJ, Särkämö T, Leo V, Tervaniemi
M, Altenmüller E and Soinila S: Music-based interventions in
neurological rehabilitation. Lancet Neurol. 16:648–660.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ripollés P, Rojo N, Grau-Sánchez J,
Amengual JL, Càmara E, Marco-Pallarés J, Juncadella M, Vaquero L,
Rubio F, Duarte E, et al: Music supported therapy promotes motor
plasticity in individuals with chronic stroke. Brain Imaging Behav.
10:1289–1307. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Salimpoor VN, Benovoy M, Larcher K, Dagher
A and Zatorre RJ: Anatomically distinct dopamine release during
anticipation and experience of peak emotion to music. Nat Neurosci.
14:257–262. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Särkämö T and Soto D: Music listening
after stroke: Beneficial effects and potential neural mechanisms.
Ann N Y Acad Sci. 1252:266–281. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sihvonen AJ, Leo V, Ripollés P, Lehtovaara
T, Ylönen A, Rajanaro P, Laitinen S, Forsblom A, Saunavaara J,
Autti T, et al: Vocal music enhances memory and language recovery
after stroke: Pooled results from two RCTs. Ann Clin Transl Neurol.
7:2272–2287. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Elsner B, Kugler J, Pohl M and Mehrholz J:
Transcranial direct current stimulation (tDCS) for improving
aphasia in patients with aphasia after stroke. Cochrane Database
Syst Rev. (CD009760)2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fritsch B, Reis J, Martinowich K, Schambra
HM, Ji Y, Cohen LG and Lu B: Direct current stimulation promotes
BDNF-dependent synaptic plasticity: Potential implications for
motor learning. Neuron. 66:198–204. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Polanowska KE, Leśniak MM, Seniów JB,
Czepiel W and Członkowska A: Anodal transcranial direct current
stimulation in early rehabilitation of patients with post-stroke
non-fluent aphasia: A randomized, double-blind, sham-controlled
pilot study. Restor Neurol Neurosci. 31:761–771. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Poreisz C, Boros K, Antal A and Paulus W:
Safety aspects of transcranial direct current stimulation
concerning healthy subjects and patients. Brain Res Bull.
72:208–214. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Spielmann K, van de Sandt-Koenderman WM,
Heijenbrok-Kal MH and Ribbers GM: Transcranial direct current
stimulation in post-stroke sub-acute aphasia: Study protocol for a
randomized controlled trial. Trials. 17(380)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Spielmann K, van de Sandt-Koenderman WME,
Heijenbrok-Kal MH and Ribbers GM: Transcranial direct current
stimulation does not improve language outcome in subacute
poststroke aphasia. Stroke. 49:1018–1020. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Miller N, Willmes K and De Bleser R: The
psychometric properties of the English language version of the
Aachen aphasia test (EAAT). Aphasiology. 14:683–722. 2000.
|
|
35
|
Plowman E, Hentz B and Ellis C Jr:
Post-stroke aphasia prognosis: A review of patient-related and
stroke-related factors. J Eval Clin Pract. 18:689–694.
2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Branscheidt M, Hoppe J, Zwitserlood P and
Liuzzi G: tDCS over the motor cortex improves lexical retrieval of
action words in poststroke aphasia. J Neurophysiol. 119:621–630.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hamilton RH, Chrysikou EG and Coslett B:
Mechanisms of aphasia recovery after stroke and the role of
noninvasive brain stimulation. Brain Lang. 118:40–50.
2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Dockery CA, Hueckel-Weng R, Birbaumer N
and Plewnia C: Enhancement of planning ability by transcranial
direct current stimulation. J Neurosci. 29:7271–7277.
2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Meinzer M, Darkow R, Lindenberg R and
Flöel A: Electrical stimulation of the motor cortex enhances
treatment outcome in post-stroke aphasia. Brain. 139:1152–1163.
2016.PubMed/NCBI View Article : Google Scholar
|