Introduction
Fulminant hepatitis is characterized by sudden,
severe liver dysfunction leading to coagulopathy and hepatic
encephalopathy (1). In their study,
Alam et al(2) reported the
natural course of fulminant hepatitis with most of the patients
developing fulminant hepatic failure within 2 weeks after the onset
of jaundice, resulting in a 73.1% rate in mortality in the study
population. Hepatocyte transplantation offers a potential
therapeutic option for the treatment of fulminant hepatitis.
Induced pluripotent stem (iPS) cells are an ideal source of
autologous hepatocytes as they potentially prevent the need for
immunosuppression prior to cell engraftment (3). However, the prompt differentiation of
iPS cells into hepatocytes is essential.
Strategies used for the direct differentiation of
iPS cells into hepatocytes have indicated the sequential
supplementation of cytokines and growth factors involved in the
embryonic development of the mammalian liver (3). Current strategies to generate
hepatocytes from iPS cells that mimic liver embryogenesis by adding
essential growth factors and simulators to serum-free culture
medium have yielded more hepatocytes and hepatocyte-like cells and
an increased homogeneity in the final cell population (3). Endodermal markers were previously
analyzed in human embryonic stem (ES) cells, cultured with growth
factors (4). Retinoic acid (RA),
nerve growth factor (NGF), hepatocyte growth factor (HGF) and
endodermal growth factor (EGF) are known to increase the expression
of α-fetoprotein (AFP), while dexamethasone (Dex) and ITS are used
to maintain the in vitro functions of hepatocytes (5). The use of activin A for the
differentiation of iPS cells into endodermal cells is
controversial. Activin A at a concentration of 100 ng/ml promotes
the differentiation of ES cells into endoderm, whereas activin A
maintains pluripotency, regulating the expression of Nanog
(6,7).
Transcription factors play an important role in
hepatocyte differentiation. Mice deficient in GATA-binding protein
(GATA) 6 died between embryonic Days 6.5 and 7.5 and exhibited a
specific defect in endodermal differentiation (8). The expression of Sry-related HMG box
(Sox) 17 is restricted to the nascent primitive endodermal
epithelium (9). The absence of
Sox17 leads to the premature delamination and migration of the
parietal endoderm. Forkhead box protein (Fox) A2 and GATA4 are the
first known proteins that bind to the albumin gene enhancer in
liver precursor cells in embryos (10). Disruption of the hematopoietically
expressed homeobox (HEX) resulted in embryonic lethality
attributable to insubstantial liver formation, making it essential
for liver organogenesis (11). The
CCAAT/enhancer binding protein (C/EBP) α promotes the
differentiation of hepatoblasts into mature hepatocytes (12,13).
Clones expressing the activating albumin promoter express C/EBPβ
(14). HNF4α is essential for the
hepatic specification of iPS cells (15). These data suggest that Sox17, GATA6,
FoxA2, GATA4, HEX, C/EBPα, C/EBPβ and HNF4α are expected to promote
the differentiation of iPS cells into hepatocytes.
Thus, the aim of this study was to analyze various
growth and transcription factors involved in hepatocyte
differentiation from iPS cells within 8 days.
Materials and methods
Cell culture
The iPS cell line (RIKEN Cell Bank, Tsukuba, Japan)
201B7 was cultured in ReproFF media (ReproCELL, Inc., Yokohama,
Japan) as a feeder-free culture (ReproCELL, Inc., Tokyo, Japan) in
dishes (Asahi Techno Glass, Funabashi, Japan) coated with Matrigel
(Becton-Dickinson, Franklin Lakes, NJ) and were kept in 5%
CO2 at 37°C in a humidified chamber. The cells were
collected with Accutase (Innovative Cell Technologies, Inc., San
Diego, CA, USA) for the experiments. 201B7 cells were cultured in
Dulbecco’s modified Eagle’s medium/F12-medium (Sigma-Aldrich Corp.,
St. Louis, MO, USA) supplemented with 20% of knockout serum
replacement (Life Technologies, Grand Island, NY, USA), 10% of
minimum essential amino acids (Life Technologies), 2 mM of
L-glutamine (Life Technologies), and 1 mM of 2-mercaptoethanol
(iPSm(-); Sigma-Aldrich Corp.). Basic fibroblast growth factor
(bFGF) (5 ng/ml; Wako Pure Chemical Industries, Ltd., Osaka,
Japan), bone morphogenic protein (BMP) 4 (20 ng/ml; Wako Pure
Chemical Industries, Ltd.), oncostatin M (20 ng/ml; Wako Pure
Chemical Industries), EGF (20 ng/ml; Wako Pure Chemical Industries,
Ltd.), NGF (100 ng/ml; R&D Systems, Inc., Minneapolis, MN),
transforming growth factor (TGF)-β (1 ng/ml; R&D Systems,
Inc.), RA (1 μM; Sigma-Aldrich Corp.), HGF (10 ng/ml; Sigma-Aldrich
Corp.), Dex (10-7 M; Wako Pure Chemical Industries, Ltd.), and
insulin, transferrin, selenium (ITS) (Wako Pure Chemical
Industries, Ltd.) were added in iPSm(-).
Reverse transcriptase and real-time
quantitative polymerase chain reaction
Total RNA (5 μg), isolated using Isogen (Nippon
Gene, Tokyo, Japan), was used for first-strand cDNA synthesis using
SuperScript III and oligo(dT), according to the manufacturer’s
instructions (Life Technologies). Polymerase chain reaction (PCR)
was performed using the GeneAmp® PCR System 9700 (Life
Technologies) and subjected to gel electrophoresis. Real-time
quantitative PCR was performed with Fast SYBR-Green Master mix
(Life Technologies) and analyzed using the MiniOpticon (Bio-Rad,
Hercules, CA, USA). The primer pairs used for RT-PCR were: GATA4,
5′-GAAAACGGAAGCCCAAGAACC and 5′-AGACATCGCACTGACTGAGAACG (NM_002052,
56°C, 218 bp); GATA6, 5′-TTCATCACGGCGGCTTGGATTGTC and
5′-GTGTTGTGGGGGAAGTATTTTTGC (NM_005257, 56°C, 299 bp); Sox17,
5′-CGCTTTCATGGTGTGGGCTAAGGACG and 5′-TAGTTGGGGTGGTCCTGCATGTGCTG
(NM_022454, 63°C, 186 bp); FoxA2, 5′-CCACCACCAACCCCACAAAATG and
5′-TGCAACACCGTCTCCCCAAAGT (NM_021784, 60°C, 294 bp); HEX,
5′-TTCTCCAACGACCAGACCATCG and 5′-TTTTATCGCCCTCAATGTCCAC (NM_002729,
56°C, 364 bp); C/EBPα, 5′-TGGAGACGCAGCAGAAGGTG and
5′-TCGGGAAGGAGGCAGGAAAC (U34070, 69°C, 538 bp); and C/EBPβ,
5′-AGACGCAGCACAAGGTCCTG and 5′-GAGAGGGGCAGAGGGAGAGC (X52560, 60°C,
421 bp). RT-PCR was performed with 30 cycles of 1 min of
denaturation, 1 min of annealing and 1 min of extension. The primer
pairs for real-time quantitative PCR of AFP, ribosomal protein L19
(RPL19), δ-like (Dlk)-1, GATA6, HNF4α and γ-glutamyl transpeptidase
(GTP) were 5′-ACA CAAAAAGCCCACTCCAG and 5′-GGTGCATACAGGAA GGGATG
(NM_005618, 147 bp), 5′-CGAATGCCAGA GAAGGTCAC and
5′-CCATGAGAATCCGCTTGTTT (157 bp), 5′-GGATGAGTGCGTCATAGCAA and
5′-CCT CCTCTTCAGCAGCATTC (121 bp), 5′-CCACTCGTGTC TGCTTTTGTGC and
5′-CCCTTCCCTTCCATCTTCT CTCAC (139 bp), 5′-CAACGGACAGATGTGTGAGTGG
and 5′-ATAACTTCCTGCTTGGTGATGGTC (NM_000457, 183 bp), and
5′-CCTCATCCTCAACATCCTCAAAGG and 5′-CACCTCAGTCACATCCACAAACTTG
(J04131, 163 bp), respectively. The primer pairs for the
quantitative PCR of Sox17 were the same as those for RT-PCR.
Real-time quantitative PCR was performed for 40 cycles, using 5 sec
for denaturation and 5 sec for annealing-extension.
Indocyanine green uptake study
Indocyanine green (ICG, 25 mg; Dai-ichi
Pharmaceutical, Co., Ltd., Tokyo, Japan) was dissolved in 5 ml of
water in a sterile vial and 20 ml were added to each medium at a
final concentration of 1 mg/ml (16). The ICG solution was added to the
cell culture and incubated at 37°C for 15 min (5). After the dish was rinsed with
phosphate-buffered saline (PBS), the ICG uptake was observed
through microscopy.
Results
Expression of transcription factors in
iPS and liver cells
RT-PCR was performed to identify the transcription
factors involved in hepatocyte differentiation (Fig. 1). GATA4, GATA6, Sox17, FoxA2, HEX
and C/EBPα were expressed in fetal or adult liver cells, but not in
iPS cells, whereas C/EBPβ was expressed in iPS cells as well as in
fetal and adult liver cells.
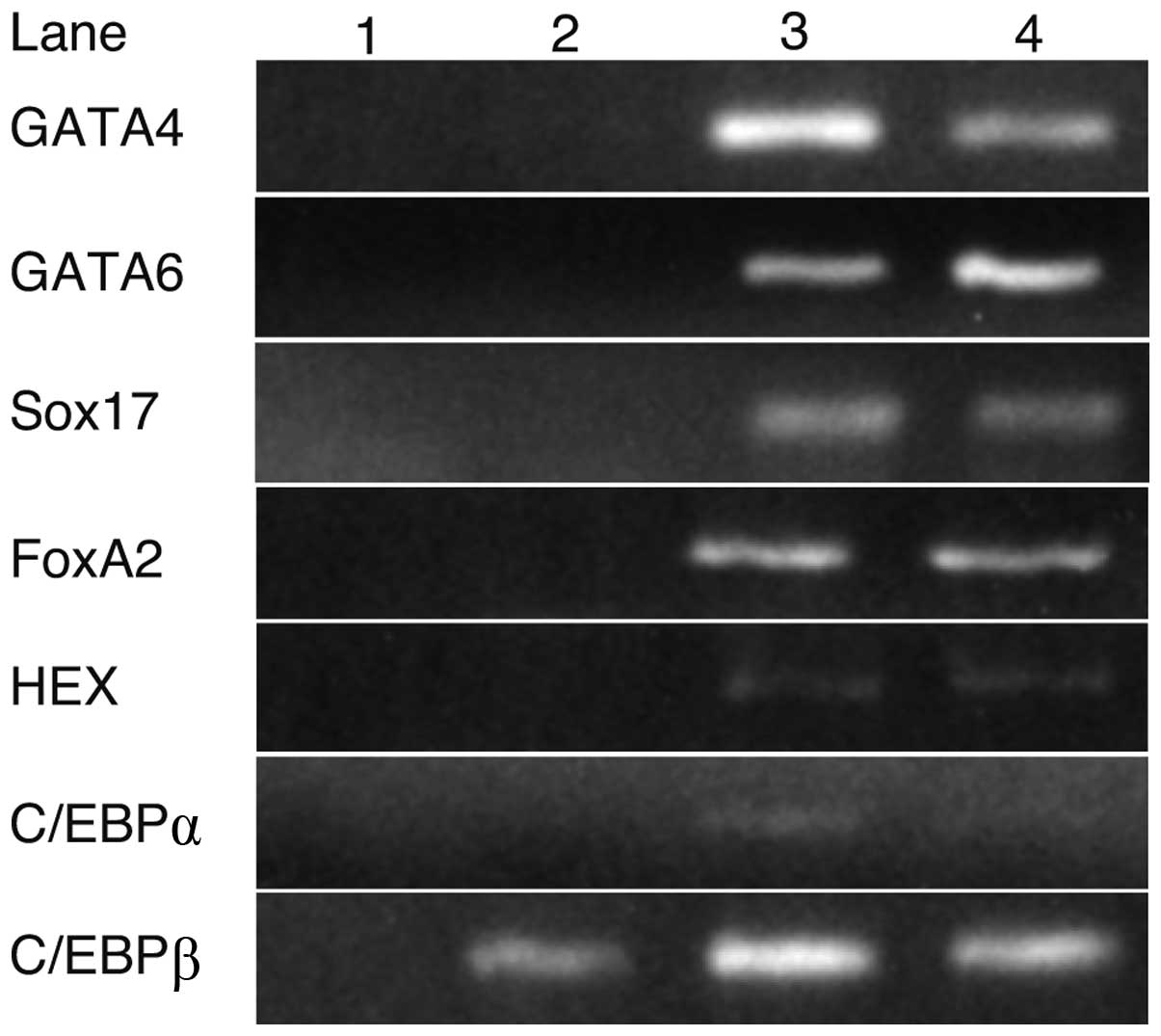 | Figure 1.The expression of transcription
factors in iPS and liver cells. RT-PCR analysis indicated that
GATA4, GATA6, Sox17, FoxA2, HEX and C/EBPα were not expressed in
iPS cells, whereas their expression was detected in fetal or adult
liver cells. C/EBPβ was expressed in iPS, fetal and adult liver
cells. Lanes: 1, H2O; 2, iPS cells; 3, fetal liver and
4, adult liver. |
RT-PCR analysis of transcription and
growth factors
Using RNA isolated from cells cultured with each
growth factor for 8 days, RT-PCR was performed to detect the growth
factors promoting iPS cells to express GATA4, GATA6, Sox17, FoxA2,
HEX and C/EBPα (Fig. 2). C/EBPβ was
not included in this experiment as it was expressed in iPS cells.
GATA6 and Sox17 were strongly expressed in iPS cells cultured with
EGF and oncostatin M, respectively. Sox7 and GATA6 were expressed
in cells cultured with RA. C/EBPα was weakly expressed with
oncostatin M. GATA4, FoxA2 and HEX were not expressed in iPS cells
using any of the growth factors. These results suggest that
oncostatin M, EGF and RA stimulated the expression of GATA6 and
Sox17 in iPS cells.
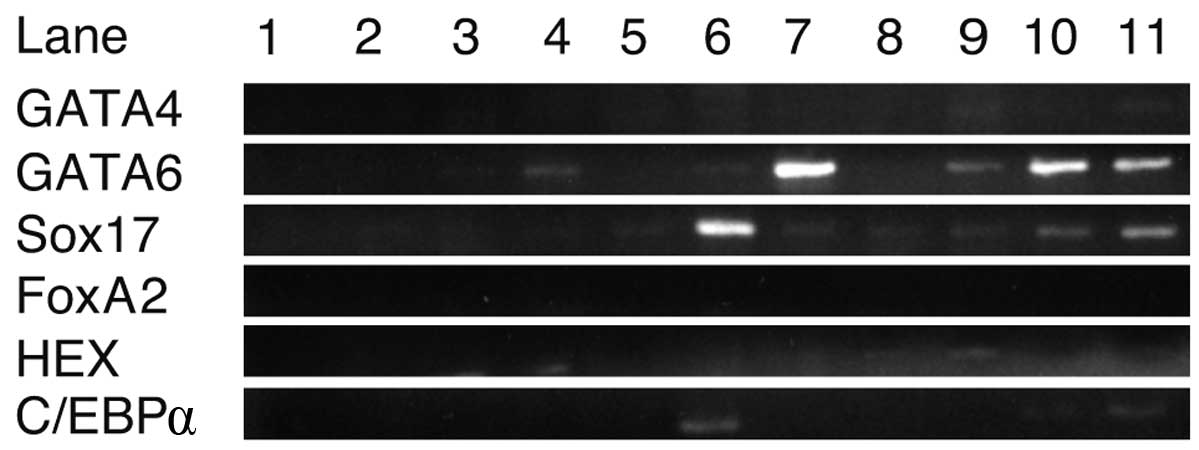 | Figure 2.RT-PCR analysis of transcription and
growth factors. Sox17 and GATA6 were strongly expressed in the
presence of oncostatin M and EGF, respectively. GATA4, FoxA2, HEX
and C/EBPα were not sufficiently expressed with either growth
factor. Lanes: 1, H2O; 2, ReproFF; 3, no growth factors;
4, basic fibroblast growth factor; 5, BMP4; 6, oncostatin M; 7,
epidermal growth factor; 8, nerve growth factor; 9, transforming
growth factor β; 10, retinoic acid and 11, hepatocyte growth
factor. |
Expression of GATA6, Sox17 and HNF4α in
the presence of growth factors
Real-time quantitative PCR conducted in cell
cultures with a combination of oncostatin M, EGF and RA showed an
increase in the expression of GATA6, Sox17 and HNF4α (Fig. 3). The expression of GATA6 and Sox17
showed the greatest increase during culture with a combination of
oncostatin M, EGF, RA, dexamethasone and ITS. HNF4α was expressed
in iPS cells cultured with oncostatin M, dexamethasone, ITS and
their combinations.
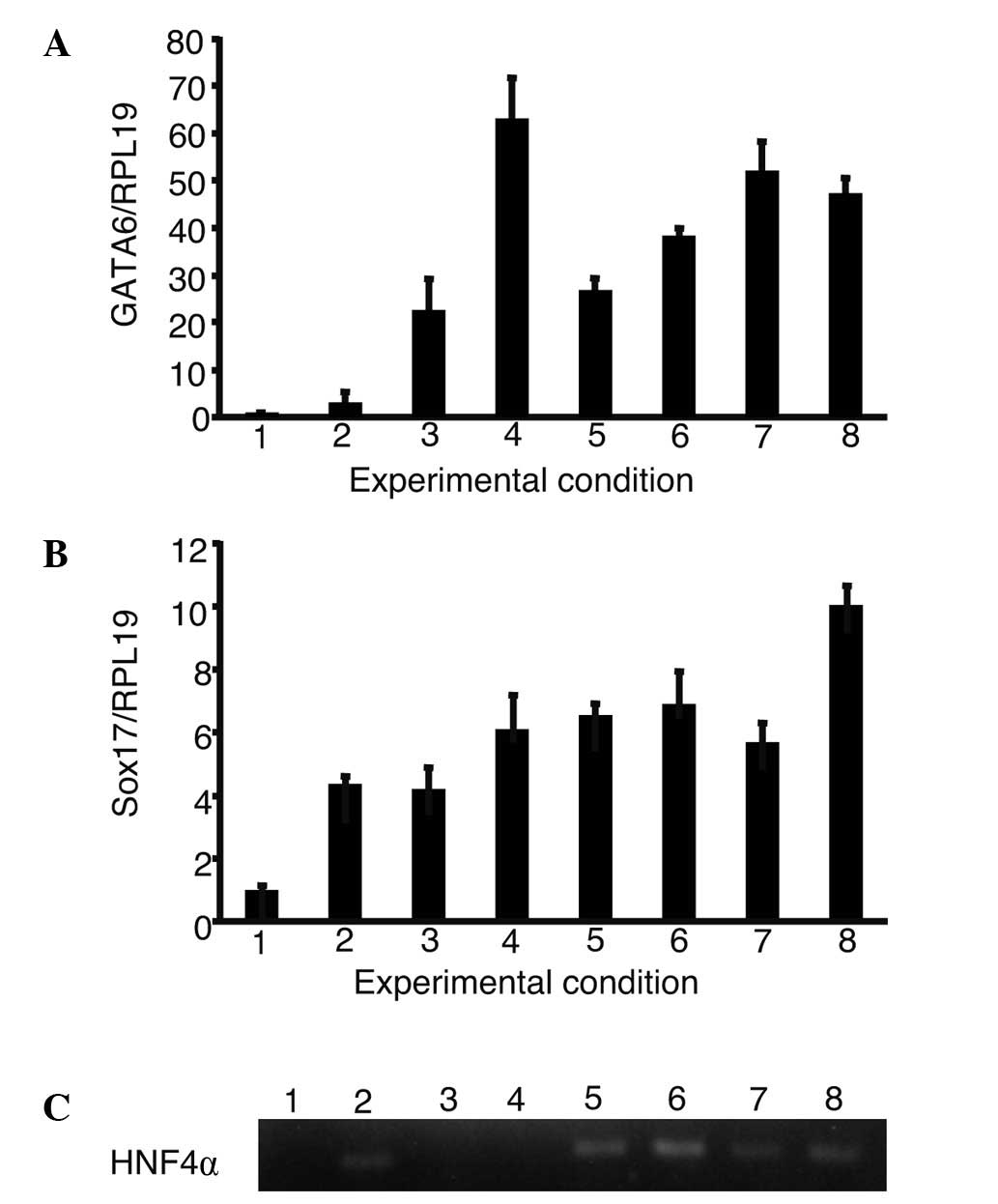 | Figure 3.The expression of GATA6, Sox17 and
HNF4α in the presence of growth factors. iPS cells were cultured
using a single or a combination of growth factors. The expression
of GATA6, Sox17 and HNF4α were analyzed. (A and B) The relative
expression levels were normalized against iPS cells in ReproFF
media. (C) The expression of HNF4α was analyzed using gel
electrophoresis as it was too low to be normalized in iPS cells. (A
and B) Columns and (C) lanes 1–8 show the following: 1, ReproFF; 2,
oncostatin M; 3, epidermal growth factor; 4, retinoic acid; 5,
dexamethasone; 6, ITS; 7, oncostatin M + epidermal growth factor +
retinoic acid and 8, oncostatin M + epidermal growth factor +
retinoic acid + dexamethasone + ITS. Error bar, standard error. |
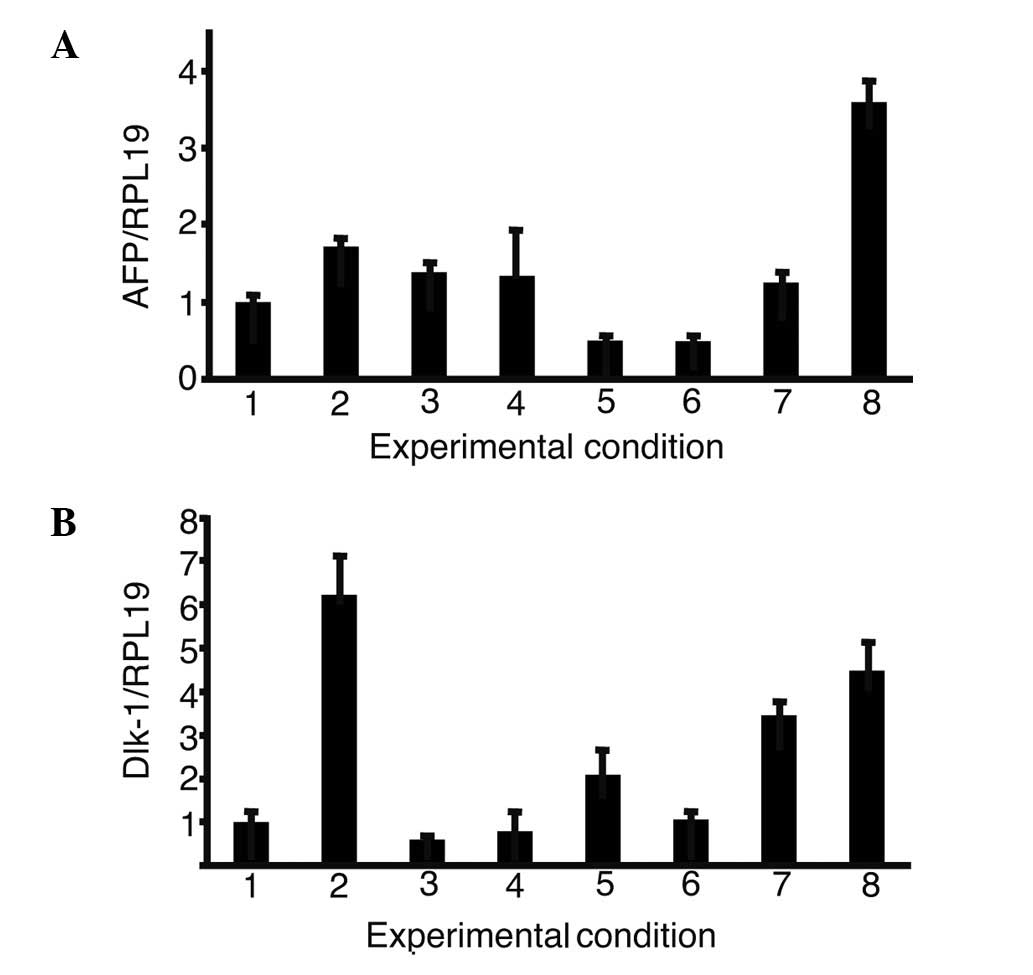
Expression of α-fetoprotein (AFP) and
δ-like (Dlk)-1 in the presence of growth factors
The expression of AFP and Dlk-1, markers of hepatic
progenitor cells, was then analyzed in iPS cells with single or a
combination of growth factors (Fig.
4). The combination of oncostatin M, EGF, RA, dexamethasone and
ITS (OERDITS) resulted in the greatest increase in the expression
of AFP. Dlk-1 expression was stimulated with either oncostatin M or
OERDITS. Thus, OERDITS was regarded as the most suitable
combination of growth factors to promote the differentiation of iPS
cells into hepatocytes.
Expression of AFP, Dlk-1, γ-GTP and Nanog
in the presence of growth factors
FoxA2, GATA4, HEX and C/EBPα were introduced with
iPS cells and cultured with OERDITS (Fig. 5). The expression of AFP and Dlk-1
was comparable to that observed in fetal liver cells with a
combination of FoxA2, GATA4, HEX and C/EBPα (FGHA). The expression
of γ-GTP with FGHA was the strongest, although it was lower than
that shown by fetal liver cells. All the conditions significantly
suppressed the expression of Nanog.
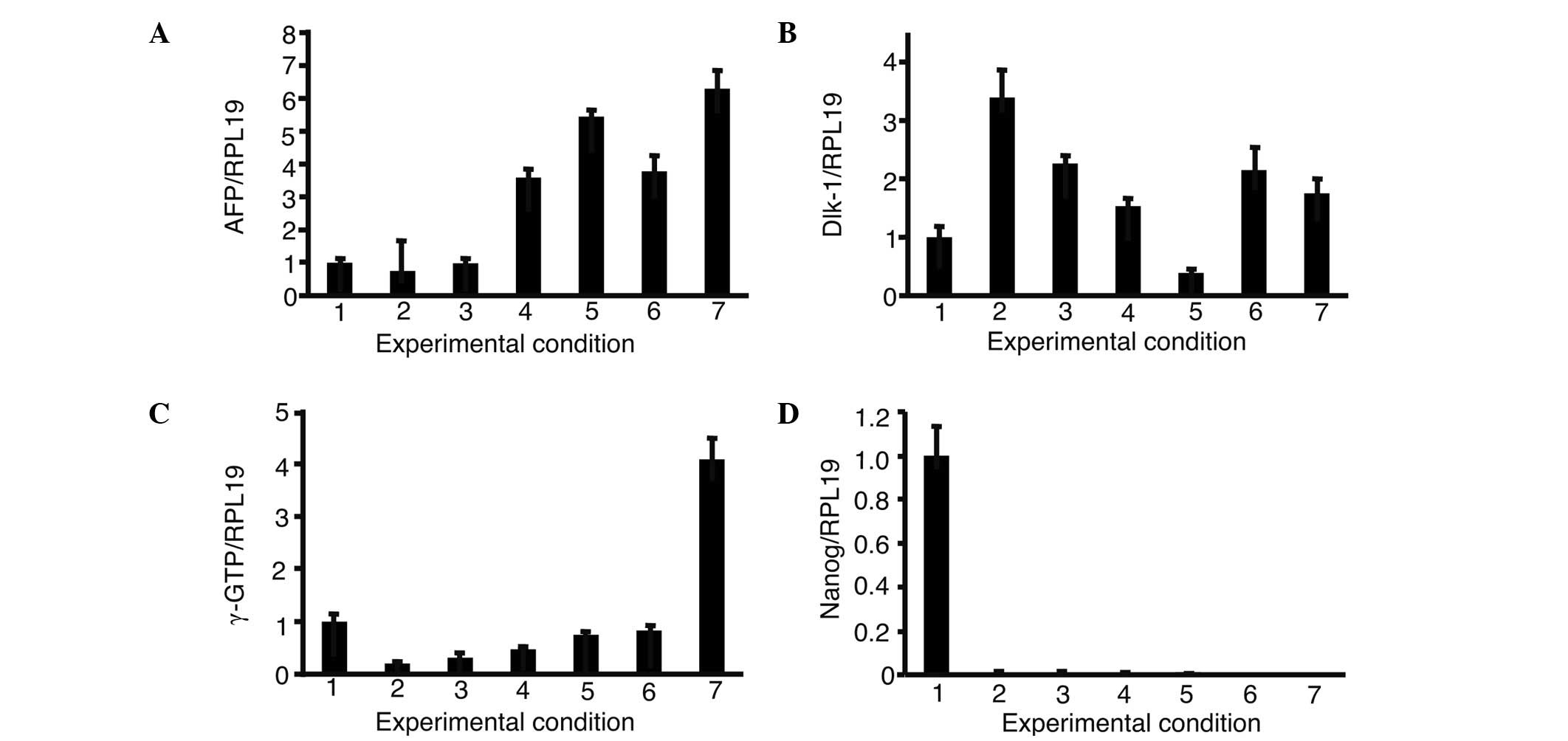 | Figure 5.Expression of (A) AFP, (B) Dlk-1, (C)
γ-GTP and (D) Nanog in the presence of growth factors. iPS cells
were introduced with a combination of 3 or 4 growth factors from
FoxA2, GATA4, HEX and C/EBPα and cultured in oncostatin M, EGF, RA,
dexamethasone and ITS. The expression of AFP, Dlk-1, γ-GTP and
Nanog was analyzed. The relative expression levels were normalized
against iPS cells in ReproFF media. Columns: 1, ReproFF; 2, GHA; 3,
FHA; 4, FGA; 5, FGH; 6, FGHA and 7, fetal liver. FGHA: F, FoxA2; G,
GATA4; H, HEX; A, C/EBPα. Error bar, standard error. |
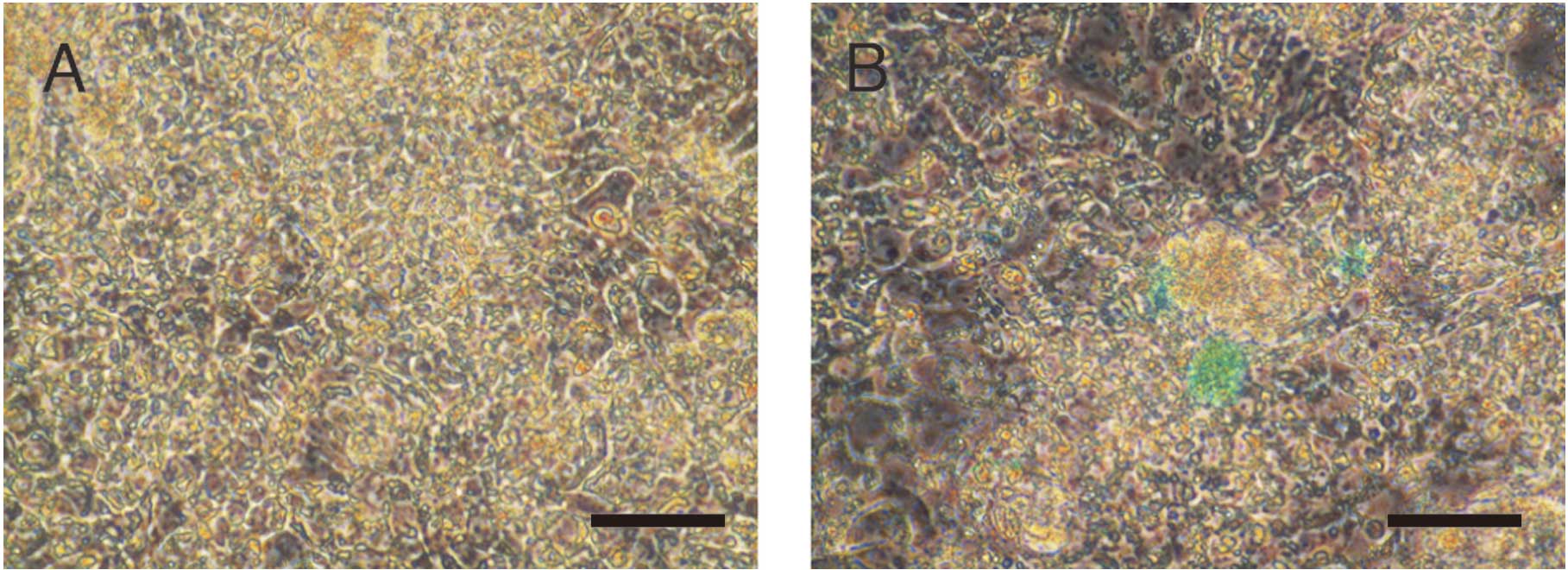
Indocyanine green (ICG) uptake in iPS
cells cultured at varying conditions
ICG was added in the iPS cell culture medium 8 days
after the introduction of FGHA and cultured in OERDITS. ICG was
taken-up by iPS cells (Fig. 6).
Discussion
Stepwise differentiation protocols are currently
employed to promote the differentiation of iPS cells into
hepatocytes (15,17–19).
Current protocols involve the sequential application of growth
factors. DeLaForest et al(15) used LY294002, an inhibitor of
phosphatidyl-inositol 3 kinase. Si-Tayeb et al(17) changed the oxygen concentration to 4
and 20%. Song et al(20)
added supplements (N2 and B27). Takayama et al(19) transduced Sox17, HEX and HNF4α.
However, these protocols require more than 20 days for iPS cells to
differentiate into hepatocytes. Shorter induction periods are
necessary for the transplantation of cells to patients diagnosed
with hepatic failure. Thus, we developed a new protocol that
promoted the differentiation of iPS cells into hepatocytes within 8
days. This study has also investigated growth factors that
increased the expression of transcription factors. The OERDITS
medium was found to stimulate the expression of GATA6, Sox17 and
HNF4α. Sox17 is essential for the differentiation of iPS cells into
endodermal cells, whereas HNF4α promotes the maturation of
hepatocytes (21). In addition, the
introduction of GATA6, Sox17 and HNF4α was not necessary with the
OERDITS medium. However, the OERDITS medium did not increase the
expression of FoxA2, GATA4 and HEX. Therefore, the introduction of
FoxA2, GATA4 and HEX was necessary for the induction of cell
differentiation.
AFP expression was lowest in iPS cells introduced
with FoxA2, GATA4 and HEX (Fig. 4),
suggesting that C/EBPα was necessary for iPS cells to differentiate
into hepatocytes. Thus, the combination of GATA4, FoxA2, HEX and
C/EBPα was essential for the generation of hepatocytes from iPS
cells.
The role of activin A in the endodermal
differentiation of iPS cells depends on its concentration. The
concentration of 100 ng/ml of activin A was shown to promote
differentiation of human embryonic stem (ES) cells into endoderm
cells (6). Activin A maintains
pluripotency, regulating the Nanog expression (7). No endodermal markers were expressed
with activin A at 20 ng/ml. Xiao et al(22) succeeded in the long-term feeder-free
culture of human ES cells (H1) for >150 days and 20 passages
using 5 ng/ml of activin A. However, the stepwise protocols
employed high concentrations of activin A. Activin A was not
analyzed in the present study as we had succeeded in the passage
culture of 201B7 cells using a medium with activin A at 10 ng/ml
(in preparation for submission).
Albumin was not expressed in iPS cells cultured with
FGHA and OERDITS (data not shown). Future investigations should
therefore be performed on the application of the extracellular
matrix (23). Moreover, exposure to
FoxA2, GATA4, HEX and C/EBPα and culturing with OERDITS
supplementation potentially serves as a single-step inducer for the
differentiation of iPS cells into hepatic progenitor-like
cells.
Acknowledgements
This study was supported by the
Grant-in-Aid for Encouragement of Scientists (no. 22931047) and the
Research Grant-in-Aid for Scientific Research (C) (no. 23591002)
from the Japan Society for the Promotion of Science (JSPS).
References
|
1.
|
Inoue K, Watanabe T, Maruoka N, Kuroki Y,
Takahashi H and Yoshiba M: Japanese-style intensive medical care
improves prognosis for acute liver failure and the perioperative
management of liver transplantation. Transplant Proc. 42:4109–4112.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Alam S, Azam G, Mustafa G, et al: Natural
course of fulminant hepatic failure: the scenario in Bangladesh and
the differences from the west. Saudi J Gastroenterol. 15:229–233.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Chistiakov DA and Chistiakov PA:
Strategies to produce hepatocytes and hepatocyte-like cells from
pluripotent stem cells. Hepatol Res. 42:111–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Schuldiner M, Yanuka O, Itskovitz-Eldor J,
Melton DA and Benvenisty N: Effects of eight growth factors on the
differentiation of cells derived from human embryonic stem cells.
Proc Natl Acad Sci USA. 97:11307–11312. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Tomizawa M, Toyama Y, Ito C, et al:
Hepatoblast-like cells enriched from mouse embryonic stem cells in
medium without glucose, pyruvate, arginine, and tyrosine. Cell
Tissue Res. 333:17–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Yao S, Chen S, Clark J, et al: Long-term
self-renewal and directed differentiation of human embryonic stem
cells in chemically defined conditions. Proc Natl Acad Sci USA.
103:6907–6912. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Shin M, Alev C, Wu Y, Nagai H and Sheng G:
Activin/TGF-beta signaling regulates Nanog expression in the
epiblast during gastrulation. Mech Dev. 128:268–278. 2011.
View Article : Google Scholar
|
|
8.
|
Morrisey EE, Tang Z, Sigrist K, et al:
GATA6 regulates HNF4 and is required for differentiation of
visceral endoderm in the mouse embryo. Genes Dev. 12:3579–3590.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Artus J, Piliszek A and Hadjantonakis AK:
The primitive endoderm lineage of the mouse blastocyst: sequential
transcription factor activation and regulation of differentiation
by Sox17. Dev Biol. 350:393–404. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Cirillo LA, Lin FR, Cuesta I, Friedman D,
Jarnik M and Zaret KS: Opening of compacted chromatin by early
developmental transcription factors HNF3 (FoxA) and GATA-4. Mol
Cell. 9:279–289. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Keng VW, Yagi H, Ikawa M, et al: Homeobox
gene HEX is essential for onset of mouse embryonic liver
development and differentiation of the monocyte lineage. Biochem
Biophys Res Commun. 276:1155–1161. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Tomizawa M, Garfield S, Factor V and
Xanthopoulos KG: Hepatocytes deficient in CCAAT/enhancer binding
protein α (C/EBPα) exhibit both hepatocyte and biliary epithelial
cell character. Biochem Biophys Res Commun. 249:1–5. 1998.
|
|
13.
|
Yamasaki H, Sada A, Iwata T, et al:
Suppression of C/EBPα expression in periportal hepatoblasts may
stimulate biliary cell differentiation through increased
Hnf6 and Hnf1b expression. Development.
133:4233–4243. 2006.
|
|
14.
|
Kheolamai P and Dickson AJ: Liver-enriched
transcription factors are critical for the expression of hepatocyte
marker genes in mES-derived hepatocyte-lineage cells. BMC Mol Biol.
10:352009. View Article : Google Scholar
|
|
15.
|
DeLaForest A, Nagaoka M, Si-Tayeb K, et
al: HNF4A is essential for specification of hepatic progenitors
from human pluripotent stem cells. Development. 138:4143–4153.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Yamada T, Yoshikawa M, Kanda S, et al: In
vitro differentiation of embryonic stem cells into hepatocyte-like
cells identified by cellular uptake of indocyanine green. Stem
Cells. 20:146–154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Si-Tayeb K, Noto FK, Nagaoka M, et al:
Highly efficient generation of human hepatocyte-like cells from
induced pluripotent stem cells. Hepatology. 51:297–305. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Song Z, Cai J, Liu Y, et al: Efficient
generation of hepatocyte-like cells from human induced pluripotent
stem cells. Cell Res. 19:1233–1242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Takayama K, Inamura M, Kawabata K, et al:
Efficient generation of functional hepatocytes from human embryonic
stem cells and induced pluripotent stem cells by HNF4α
transduction. Mol Ther. 20:127–137. 2012.
|
|
20.
|
Song H, Hollstein M and Xu Y: p53
gain-of-function cancer mutants induce genetic instability by
inactivating ATM. Nat Cell Biol. 9:573–580. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Takayama K, Inamura M, Kawabata K, et al:
Efficient and directive generation of two distinct endoderm
lineages from human ESCs and iPSCs by differentiation
stage-specific SOX17 transduction. PLoS One. 6:e217802011.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Xiao L, Yuan X and Sharkis SJ: Activin A
maintains self-renewal and regulates fibroblast growth factor, Wnt,
and bone morphogenic protein pathways in human embryonic stem
cells. Stem Cells. 24:1476–1486. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Shiraki N, Yamazoe T, Qin Z, et al:
Efficient differentiation of embryonic stem cells into hepatic
cells in vitro using a feeder-free basement membrane substratum.
PLoS One. 6:e242282011. View Article : Google Scholar : PubMed/NCBI
|