Introduction
Epigenetics is the study of inherited genetic
changes that occur without changes to the naked DNA sequence,
including DNA methylation, histone modification, chromatin
remodeling and non-coding RNAs (1).
DNA methylation is the most widely researched epigenetic alteration
in human tumors. Human tumor cells exhibit abnormal DNA methylation
patterns including the hypermethylation of CpG islands in
tumor-suppressor genes (TSGs) and a global loss of DNA methylation
in the genome (2). These changes,
correlated with the inactivation of TSGs and the activation of
oncogenes, may promote tumor progression (3). Abnormal expression of DNA
methyltransferase (DNMT) and demethylase (MBD2) are regarded as
important causes for the aberrant DNA methylation that occurs in
tumors (4). High levels of DNMT and
MBD2 expression may contribute to tumor progression through the
hypermethylation-mediated inactivation of TSGs in CpG islands
(5). In this study, the
gastrointestinal stromal tumor (GIST) was used to analyze the
expression of DNMT (DNMT1, DNMT2, DNMT3A, DNMT3B and DNMT3L) and
MBD2. This study is considered to be useful for future epigenetic
investigations on GIST.
Materials and methods
Ethics statement
The experimental procedures were approved by the
Ethics Committee of The First Affiliated Hospital of the Chongqing
Medical University (Chongqing, China).
Clinical specimens
Fifteen pairs of adult GIST and matched non-tumor
tissues (located >2 cm from the tumor area) were obtained from
the gastric resection specimens in The First Affiliated Hospital of
the Chongqing Medical University. The GIST samples were confirmed
by pathological analysis (including KIT: +, CD34: +, DOG-1: +, SMA:
+, S-100: −). The samples were prepared for immunohistochemistry
and western blot analysis to determine the expression of DNMT and
MBD2.
Immunohistochemistry
The streptavidin-peroxidase (SP) method was
performed. Primary antibodies specific to DNMT1, DNMT2, DNMT3A,
DNMT3B, DNMT3L and MBD2 were purchased from Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA. The SP and DAB kits were
obtained from the Beijing Zhongshan Company (Beijing, China).
Evaluation of staining
(immunohistochemistry)
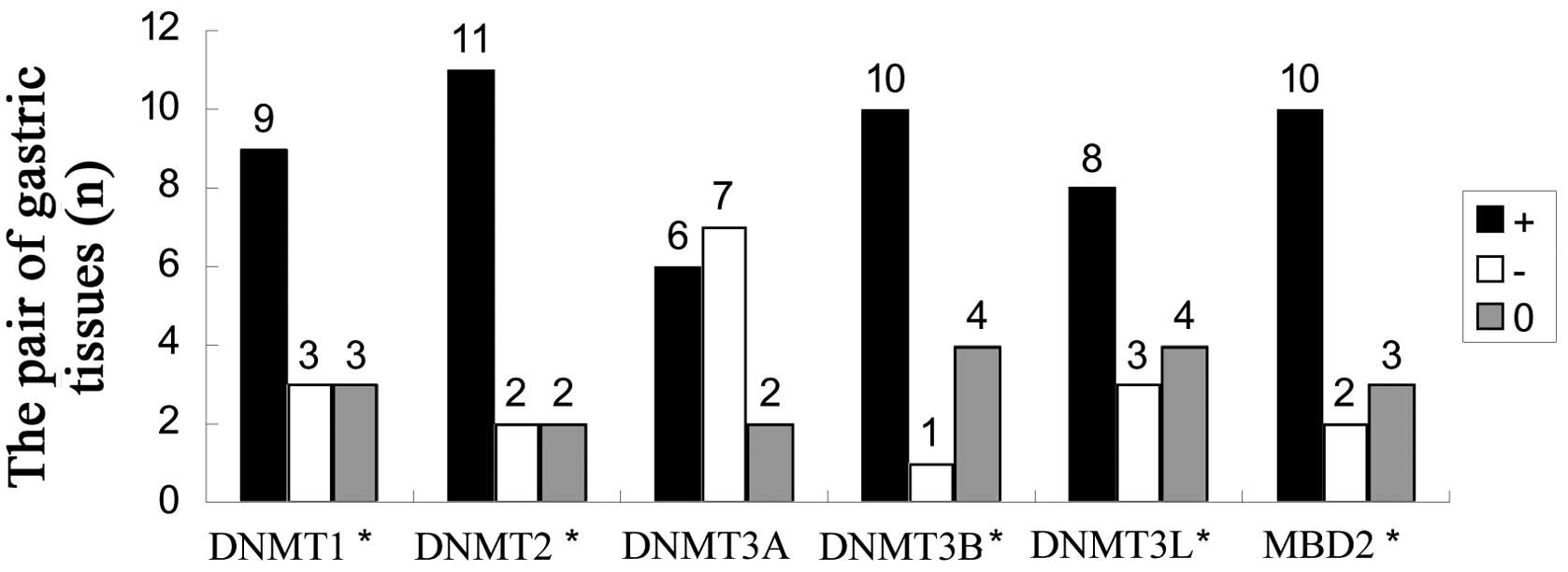
Expression of DNMT and MBD2 were assessed by scoring
the staining intensity and proportion. The staining intensity was
determined as negative, 0; light, 1; moderate, 2 or strong, 3. The
staining proportion was determined as 1 (≤25%), 2 (≤50%), 3 (≤75%)
or 4 (>75%). The two values were multiplied for each slide to
produce a terminal score. In case the score was higher in the tumor
compared to the matched control, the pair of tissues was marked
‘+’. The opposite condition was marked ‘−’. In case the scores were
the same, the pair was marked ‘0’. Terminal scores of 0–2 were
defined as negative expression, while 3–12 were defined as positive
expression.
Western blot analysis
The western blot analysis was carried out as
decribed in a previous study (6).
Materials used in the analysis were obtained from the Beyotime
Company (Jiangsu, China).
Statistical analysis
Data were presented as mean ± SD. Standard
statistical analysis was performed using the SPSS 17.0 package
software. The Wilcoxon signed-rank, χ2, Fisher’s exact
and Student’s t-tests were used in this study. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of DNMT and MBD2 in GIST
tissues
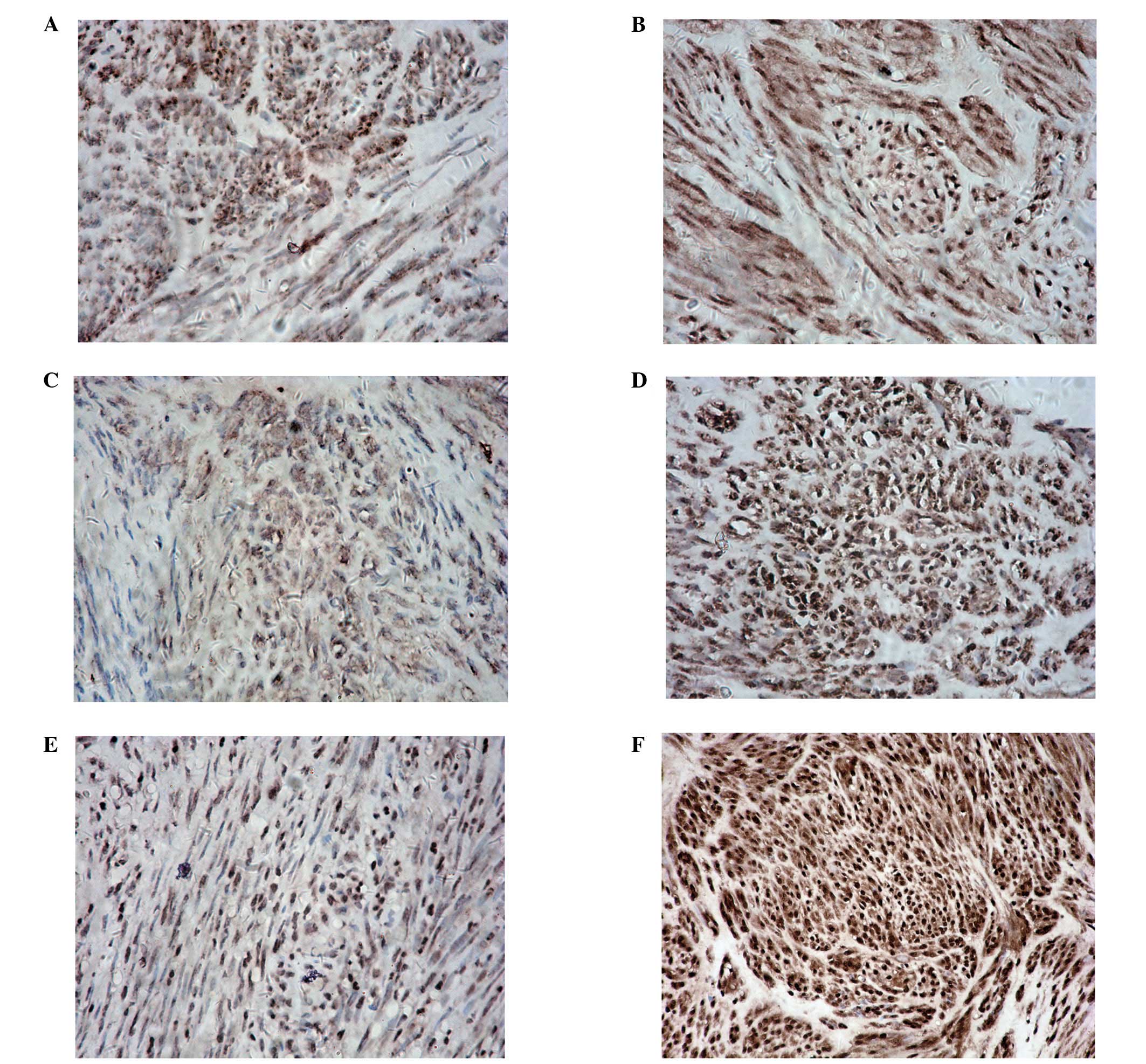
GIST was detected to express DNMT and MBD2 proteins
(Fig. 1). With the exception of
DNMT3A, expression of DNMT and MBD2 were significantly higher in
GIST tissues compared to matched non-tumor tissues (Figs. 2 and 3).
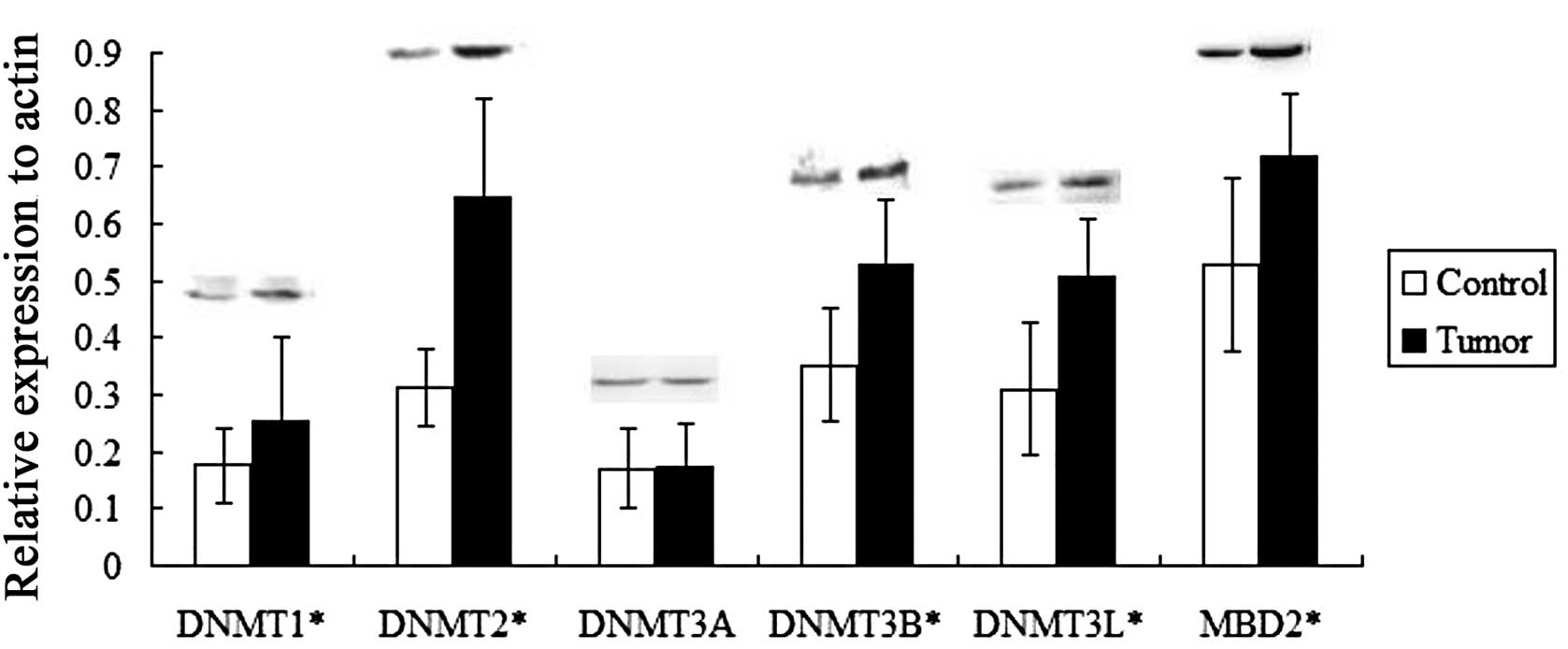 | Figure 3Differential expression of DNA
methyltransferases (DNMTs) and methyl-CpG-binding domain protein 2
(MBD2) in gastrointestinal stromal tumor (GIST) tissues and matched
non-tumor tissues by western blot analysis. 0.176±0.065,
0.312±0.068, 0.171±0.068, 0.352±0.099, 0.31±0.115 and 0.529±0.151
were corresponding to the relative expression of DNMT1, 2, 3A, 3B,
3L and MBD2, respectively, to actin in non-tumor tissues.
0.253±0.147, 0.645±0.174, 0.175±0.075, 0.529±0.112, 0.506±0.103 and
0.719±0.109 were corresponding to the relative expression of DNMT1,
2, 3A, 3B, 3L and MBD2, respectively, to Actin in GIST. With the
exception of DNMT3A, expression of DNMT and MBD2 was significantly
higher in GIST tissues compared to matched non-tumor tissues
(*P<0.05, t-test, mean ± SD, n=15). Actin was used as
the internal control. |
Association between expression of DNMTs
and MBD2 and clinical parameters of GIST
There was no statistically significant association
between expression of DNMTs, MBD2 and gender or age in GISTs as
detected by the χ2 and the Fisher’s exact tests
(P>0.05). However, significant associations were observed
between DNMT1 expression and the mitotic index; and DNMT3B
expression and tumor size, Helicobacter pylori infection;
DNMT3L expression and Helicobacter pylori infection in GISTs
(P<0.05) (Table I).
 | Table IPositive DNMT and MBD2 expression in
differential clinical characteristics of GIST. |
Table I
Positive DNMT and MBD2 expression in
differential clinical characteristics of GIST.
| Clinical
characteristics | No. | Expression (%) |
|---|
|
|---|
| DNMT1 | DNMT2 | DNMT3A | DNMT3B | DNMT3L | MBD2 |
|---|
| Gender | | | | | | | |
| Male | 6 | 1 (16.7) | 4 (66.7) | 2 (33.3) | 5 (83.3) | 5 (83.3) | 6 (100) |
| Female | 9 | 4 (44.4) | 9 (100) | 5 (55.6) | 8 (88.9) | 7 (77.8) | 8 (88.9) |
| P-value | | 0.58 | 0.14 | 0.61 | 1 | 1 | 1 |
| Age (years) | | | | | | | |
| >50 | 11 | 4 (36.4) | 9 (81.8) | 6 (54.5) | 10 (90.9) | 9 (81.8) | 10 (90.9) |
| ≤50 | 4 | 1 (25) | 4 (100) | 1 (25) | 3 (75) | 3 (75) | 4 (100) |
| P-value | | 1 | 1 | 0.57 | 0.48 | 1 | 1 |
| Tumor size (cm) | | | | | | | |
| >5 | 12 | 5 (41.7) | 10 (83.3) | 5 (41.7) | 12 (100) | 11 (91.7) | 12 (100) |
| ≤5 | 3 | 0 (0) | 3 (100) | 2 (66.7) | 1 (33.3) | 1 (33.3) | 2 (66.7) |
| P-value | | 0.51 | 1 | 0.57 | 0.03 | 0.08 | 0.2 |
| Mitotic index
(HPF) | | | | | | | |
| ≤5/50 | 11 | 1 (9.1) | 10 (90.9) | 4 (36.4) | 9 (81.8) | 8 (72.7) | 10 (90.9) |
| >5/50 | 4 | 4 (100) | 3 (75) | 3 (75) | 4 (100) | 4 (100) | 4 (100) |
| P-value | | 0.01 | 0.48 | 0.28 | 1 | 0.52 | 1 |
| H. pylori
infection | | | | | | | |
| Positive | 13 | 5 (38.5) | 11 (84.6) | 7 (53.8) | 13 (100) | 12 (92.3) | 13 (100) |
| Negative | 2 | 0 (0.0) | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 1 (50) |
| P-value | | 0.52 | 1 | 0.47 | 0.01 | 0.03 | 0.13 |
Discussion
GIST is the most common mesenchymal tumor in the
gastrointestinal tract that usually arises in the stomach (60%)
(7). Seventy percent of GISTs
present with non-specific clinical symptoms and the risk
assessments for GIST are determined by tumor size, anatomic site
and mitotic activity (8). Early
events in GIST development are activating mutations in KIT
or PDGFRA, which occur in most GISTs and encode for mutated
tyrosine receptor kinases that are the causes for uncontrolled
kinase activity and result in alterations to the cell cycle and
apoptosis (9,10). Immunoreactive detection of KIT
(CD117), DOG1, CD34, SMA, CALDES, DES, and S-100 is a useful method
for the differential diagnosis of GIST from other mesenchymal
tumors (11,12). Tyrosine kinase inhibitor imatinib
mesylate and surgery are known to be traditional treatments for
GISTs. However, certain GISTs (∼10–15% in adults, 85–90% in
children) are characterized by the lack of KIT or
PDGFRA mutations (13,14),
suggesting a complex pathogenesis of GIST.
DNMT and MBD2 are important functional proteins in
epigenetics. DNMT1 methylates hemimethylated CpG palindromes in DNA
and is referred to as a ‘maintenance’ DNA methylation enzyme that
faithfully copies the DNA methylation pattern from the parent to
the daughter strand of DNA after replication. DNMT2 contains the
catalytic signature motifs of conventional (cytosine-5) DNMTs. It
has comparably low DNMT activity, but has shown transfer RNA (tRNA)
methyltransferase activity (15).
DNMT3A and DNMT3B have been termed de novo
methyltransferases and are thought to be involved in the
establishment of methylation patterns. DNMT3L lacks the
methyltransferase catalytic domain but is involved in the
establishment of DNA methylation by recruiting or activating other
de novo DNMTs (16–18). MBD2 is capable of binding
specifically to methylated DNA and can repress transcription from
methylated gene promoters (19).
Notably, MBD2 has also been reported to function as a demethylase
to activate transcription (20), as
DNA methylation causes gene silencing.
Epigenetic alterations, such as promoter
hypermethylation leading to chromatin remodeling and the silencing
of cancer-related genes are highly involved in tumor development
(21). A common assumption is that
the abnormal expression of DNMTs and MBD2 may contribute to tumor
progression through hypermethylation-mediated TSG inactivation in
CpG islands. A number of studies have proven that tumors exhibit a
high expression of DNMT and MBD2 (19,22).
However, their involvement in GIST has yet to be elucidated.
In this study, we found almost all the DNMTs, with
the exception of DNMT3A, while MBD2 proteins were significantly
higher in GIST tissues compared to matched control tissues. This
result is consistent with previous studies on the DNMT expression
in tumors, suggesting that, besides KIT and the
PDGFRA mutation, epigenetic alterations may also be
potentially involved in the pathogenesis of GIST. Nevertheless, the
associations between DNMT and clinical parameters in GIST indicate
the underlying diagnostic value of DNMT expression for GIST. Thus,
more GIST samples are required to verify the above-mentioned
results. Additionally, to confirm the DNA methylation status of
tumor-related genes in GISTs and the potential suppressive effect
of the DNMT inhibitor, such as 5-aza-2′-deoxycytidine on GIST
further investigations are required in the future.
In conclusion, GIST may exhibit high expression of
DNMT and MBD2 protein, thus demonstrating potential associations
between DNMT expression and clinical parameters in GIST.
References
|
1
|
Esteller M: Cancer epigenetics for the
21st century: what’s next? Genes Cancer. 2:604–606. 2011.
|
|
2
|
Daniel FI, Cherubini K, Yurgel LS, de
Figueiredo MA and Salum FG: The role of epigenetic transcription
repression and DNA methyltransferases in cancer. Cancer.
117:677–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bender CM, Pao MM and Jones PA: Inhibition
of DNA methylation by 5-aza-2′-deoxycytidine suppresses the growth
of human tumor cell lines. Cancer Res. 58:95–101. 1998.
|
|
4
|
Fang JY, Cheng ZH, Chen YX, Lu R, Yang L,
Zhu HY and Lu LG: Expression of Dnmt1, demethylase, MeCP2 and
methylation of tumor-related genes in human gastric cancer. World J
Gastroenterol. 10:3394–3398. 2004.PubMed/NCBI
|
|
5
|
Vertino PM, Yen RW, Gao J and Baylin SB:
De novo methylation of CpG island sequences in human fibroblasts
overexpression DNA (cytosine-5-)-methyltransferase. Mol Cell Biol.
16:4555–4565. 1996.PubMed/NCBI
|
|
6
|
Jung Y, Park J and Kim TY, Park JH, Jong
HS, Im SA, Robertson KD, Bang YJ and Kim TY: Potential advantages
of DNA methyltransferase 1 (DNMT1)-targeted inhibition for cancer
therapy. J Mol Med (Berl). 85:1137–1148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Foo WC, Liegl-Atzwanger B and Lazar AJ:
Pathology of gastrointestinal stromal tumors. Clin Med Insights
Pathol. 5:23–33. 2012.
|
|
8
|
Fletcher CD, Berman JJ, Corless C,
Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti
H, Rubin BP, Shmookler B, Sobin LH and Weiss SW: Diagnosis of
gastrointestinal stromal tumors: a consensus approach. Int J Surg
Pathol. 10:81–89. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirota S, Isozaki K, Moriyama Y, Hashimoto
K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M,
Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y and Kitamura
Y: Gain-of-function mutations of c-kit in human gastrointestinal
stromal tumors. Science. 279:577–580. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heinrich MC, Corless CL, Demetri GD,
Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den
Abbeele AD, Druker BJ, Kiese B, Eisenberg B, Roberts PJ, Singer S,
Fletcher CD, Silberman S, Dimitrijevic S and Fletcher JA: Kinase
mutations and imatinib response in patients with metastatic
gastrointestinal stromal tumor. J Clin Oncol. 21:4342–4349. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miettinen M, Sobin LH and Lasota J:
Gastrointestinal stromal tumors of the stomach: a
clinicopathologic, immunohistochemical, and molecular genetic study
of 1765 cases with long-term follow-up. Am J Surg Pathol. 29:52–68.
2005. View Article : Google Scholar
|
|
12
|
Novelli M, Rossi S, Rodriguez-Justo M,
Taniere P, Seddon B, Toffolatti L, Sartor C, Hogendoorn PC, Sciot
R, Van Glabbeke M, Verweij J, Blay JY, Hohenberger P, Flanagan A
and Dei Tos AP: DOG1 and CD117 are the antibodies of choice in the
diagnosis of gastrointestinal stromal tumours. Histopathology.
57:259–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Janeway KA, Liegl B, Harlow A, Le C,
Perez-Atayde A, Kozakewich H, Corless CL, Heinrich MC and Fletcher
JA: Pediatric KIT wild-type and platelet-derived growth factor
receptor alpha-wild-type gastrointestinal stromal tumors share KIT
activation but not mechanisms of genetic progression with adult
gastrointestinal stromal tumors. Cancer Res. 67:9084–9088. 2007.
View Article : Google Scholar
|
|
14
|
Demetri GD, von Mehren M, Antonescu CR,
DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF,
Schuetze S, Sundar HM, Trent JC and Wayne JD: NCCN Task Force
report: update on the management of patients with gastrointestinal
stromal tumors. J Natl Compr Canc Netw. 8(Suppl 2): S1–S44.
2010.PubMed/NCBI
|
|
15
|
Schaefer M, Hagemann S, Hanna K and Lyko
F: Azacytidine inhibits RNA methylation at DNMT2 target sites in
human cancer cell lines. Cancer Res. 69:8127–8132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ooi SK, Qiu C, Bernstein E, Li K, Jia D,
Yang Z, Erdjument-Bromage H, Tempst P, Lin SP, Allis CD, Cheng X
and Bestor TH: DNMT3L connects unmethylated lysine 4 of histone H3
to de novo methylation of DNA. Nature. 448:714–717. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bourc’his D, Xu GL, Lin CS, Bollman B and
Bestor TH: Dnmt3L and the establishment of maternal genomic
imprints. Science. 294:2536–2539. 2001.PubMed/NCBI
|
|
18
|
Jia D, Jurkowska RZ, Zhang X, Jeltsch A
and Cheng X: Structure of Dnmt3a bound to Dnmt3L suggests a model
for de novo DNA methylation. Nature. 449:248–251. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu D, Hunter SB, Vertino PM and Van Meir
EG: Overexpression of MBD2 in glioblastoma maintains epigenetic
silencing and inhibits the antiangiogenic function of the tumor
suppressor gene BAI1. Cancer Res. 71:5859–5870. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fuso A, Nicolia V, Cavallaro RA and Scarpa
S: DNA methylase and demethylase activities are modulated by
one-carbon metabolism in Alzheimer’s disease models. J Nutr
Biochem. 22:242–251. 2011.PubMed/NCBI
|
|
21
|
Rountree MR, Bachman KE, Herman JG and
Baylin SB: DNA methylation, chromatin inheritance, and cancer.
Oncogene. 20:3156–3165. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding WJ, Fang JY, Chen XY and Peng YS: The
expression and clinical significance of DNA methyltransferase
proteins in human gastric cancer. Dig Dis Sci. 53:2083–2089. 2008.
View Article : Google Scholar : PubMed/NCBI
|