Introduction
Semen Ziziphi Spinosae (SZS) and Fructus Gardeniae
(FG) are two Chinese herbal medicines frequently used in
traditional Chinese medicine (TCM). SZS is the dry ripe seed of the
deciduous shrub or small shrub Ziziphus jujuba Mill. var.
spinosa (Bunge) Hu ex H.F. Chou. FG is the dry ripe fruit of
the evergreen shrub Gardenia jasminoides Ellis (1,2),
usually used as an adjuvant medicine although it could also be used
independently (3).
Extracts obtained from SZS have been reported to
have therapeutic effects on insomnia, sleep disorders, anxiety and
neurasthenia. Our previous study showed that oil extracts obtained
from FG have the function of sedation and hypnosis (6). Thus, SZS as well as FG are suggested
to have the function of regulating the central nervous system. Our
previous study (6) also showed that
extracts obtained from SZS and FG have a synergistic effect on
sedative hypnosis. However, the effect of SZS and FG on learning
and memory has not been examined yet.
In the present study, we investigated the effects of
the extracts of SZS and FG on the learning and memory of a mouse
model using the step-through and -down passive avoidance tasks and
Morris water maze tasks. The results showed that in both of the
tasks, SZS and FG have a synergistic effect on improving the
learning and memory of mice.
Materials and methods
Materials
SZS and FG oils were prepared by the Pharmacon
Institute of FMMU using the supercritical CO2 extraction
method. The compound oil of SZS and FG contains each herb at a 2:1
ratio. Scopolamine hydrobromide, cycloheximide, sodium nitrite and
ethanol were purchased from Xuzhou Laien Pharmaceutical Co., Ltd.
(Xuzhou, China), Sigma-Aldrich, Shanghai Zhizheng Chemical
Engineering Co., Ltd. (Shanghai, China) and Xi’an Chemical Reagent
Factory (Xi’an, Shanxi, China), respectively. Step-through and
-down apparatuses were made by the Equipment Division of the
Medical College of the Xi’an Jiaotong University, China. Morris
water maze was constructed by the Chengdu TME Technology Co., Ltd.
Female Kunming (KM) mice, clean (CL) animals (n=70), weighing 18–22
g, were obtained from the Animal Center of the Medical College of
the Xian Jiaotong University.
Animal grouping and drug administration. Four
experiments were designed to assess the effect of SZS and FG oils
on improving murine learning and memory. In each experiment, the
mice were randomized into seven groups (n=10): the saline control
group, the learning and memory-impaired model group, the compound
oil high-dose group, the compound oil medium-dose group, the
compound oil low-dose group, the SZS oil only group and the FG oil
only group. At 9:00 a.m. each day, for 15 days, the mice in each
group were administered the following intragastrically: the saline
control and the learning and memory-impaired model groups were
administered normal saline; the compound oil high-, medium- and
low-dose groups were administered 5.4, 2.7 and 1.35 g/kg of
compound oil of SZS and FG, respectively; while the SZS oil only
and the FG oil only groups were given 1.8 and 0.9 g/kg of SZS and
FG oils, respectively.
Step-through and -down passive avoidance
(PA) test in memory acquisition-impaired mice model
On day 16 of each experiment, the 9:00 am
administration was carried out in the same manner for all the
animal groups as previously described. One hour after admini
stration, the animals in this experiment were injected with 1.5
mg/kg of scopolamine hydrobromide solution intraperitoneally in
order to induce memory-acquisition impairment, with the exception
of the saline control group. Ten minutes after injection, the mice
were trained for step-through and -down passive avoidance task. In
brief, for the step-through passive avoidance training, the mouse
was placed into the illuminated compartment of the mouse
step-through apparatus, facing away from the closed door connecting
the illuminated and the dark compartments. Ten seconds later, the
door connecting the 2 compartments was opened. The mouse would
enter the dark compartment through the door due to its natural
inclination. When its 4 paws touched the grid on the floor of the
dark compartment, the door was closed and a brief electric foot
shock (0.4 mA for 2 sec) was applied. The mouse was kept in the
dark compartment for 10 sec, and was then returned into its home
cage. For the step-down training, the mouse was gently placed onto
the platform of the step-down apparatus. When it stepped down and
its 4 paws touched the grid on the floor of the apparatus, an
electric foot shock (0.4 mA for 5 sec) was applied. The mouse was
then returned to its cage. The same training was repeated 1 h
later. This time, however, the latency period for the mouse to step
down the platform was measured. If the latency period was >60
sec, i.e., if the mouse stayed on the platform for a period of
>60 sec without stepping down, the mouse was returned to its
home cage given that it had already memorized the unpleasant
stimulus of stepping down onto the floor and learned to avoid it.
If the latency period was <60 sec, the training was repeated
once more 1 h later. If the latency period remained <60 sec, the
mouse was excluded from the experiment.
Assessment of memory acquisition was conducted 24 h
after the training session. For the step-through passive avoidance
task, the latency period for the mouse to enter the dark
compartment (with all four feet) and the number of times that the
mouse entered the dark compartment within 5 min (no. of errors)
were recorded. If the mouse did not enter the dark compartment in 5
min, the assessment was ended, the step-through latency was
recorded as 300 sec and the number of errors was recorded as zero.
For the step-down passive avoidance task, the latency period for
the mouse to step down the platform and the number of times that
the mouse stepped down the platform within 3 min (no. of errors)
were recorded. If the mouse did not step down in 3 min, the
assessment was ended, the step-down latency was recorded as 180 sec
and the number of errors was recorded as zero.
Step-through and -down passive avoidance
test with memory consolidation-impaired mice model
On day 16, 1 h after the 9:00 a.m. administration,
the mice included in this experiment were injected with 120 mg/kg
cycloheximide solution intraperitoneally to induce memory
consolidation impairment, with the exception of the saline control
group. Ten minutes after the injection, the mice were trained for
the step-through and -down passive avoidance task, as described
above. Assessment of memory consolidation with step-through and
-down passive avoidance task was conducted 24 h after the training
session and was performed in the same manner.
Step-through and -down passive avoidance
test with memory retrieval-impaired mice model
On day 16, 1 h after the 9:00 a.m. administration,
the mice in this experiment were trained for step-through and -down
passive avoidance task, as described above. Twenty four hours after
the training session, the mice were intragastrically administered
10 ml/kg of ethanol to induce memory retrieval impairment, with the
exception of the saline control group. Thirty minutes after the
administration, assessment of memory retrieval with step-through
and -down passive avoidance task was conducted and performed as
described previously.
Morris water maze test of spatial
learning and memory
From day 16 through 19, 1 h after the 9:00 a.m.
procedural administration, the mice in this experiment were trained
for the Morris water maze task. The water tub was 1.2 meters in
diameter and 0.50 meters in height, and the inside wall was painted
white. The tub was filled with water (∼24°C) to a depth of 0.30
meters and was rendered opaque by adding white plastic foam. The
mice were made visible by smearing picric acid (yellow color) on
their fur. The pool area was arbitrarily divided into 4 quadrants:
NE, SE, NW and SW. A transparent circular platform (10 cm diameter)
was arbitrarily placed in the center of the NW quadrant, 1 cm below
the water level. The position of the hidden platform was kept
constant throughout the training session. A digital camera
connected to a computer recording system was mounted over the pool.
Several colored shapes were placed around the pool and hung or
pasted around the walls of the testing room as visual cues. Their
positions remained unchanged during the entire experiment. Each
mouse was subjected to 4 training trials each day (with a 5-min
break between trials) for 4 consecutive days. On each trial the
mouse was gently released into the water at a different location
facing the wall of the pool. The time it spent to find the hidden
platform (escape latency) was recorded. When the animal had found
the platform, it was allowed to stay on the platform for 15 sec and
was then removed from the maze. If the mouse failed to locate the
platform in 60 sec, it was gently guided onto the platform and
allowed to remain there for 15 sec. On day 19, following the last
training trial, the mice were injected with 120 mg/kg sodium
nitrite solution subcutaneously with a view to induce memory
impairment, with the exception of the saline control group, which
was injected with the same volume of normal saline.
On day 20, 1 h after the 9:00 a.m. procedural
administration, the mice were assessed for the Morris water maze
tasks (the position of the platform unchanged) and the escape
latency was recorded as described previously. Subsequently, the
transfer test (probe trial) was carried out in which the platform
was removed from the pool, and the number of times each mouse
crossed the previous platform location within 120 sec was
recorded.
Results
Extracts of SZS and FG synergistically
improve learning and memory of mice in step-through and -down
passive avoidance task
Scopolamine, cycloheximide and ethanol were used to
induce impairment of memory acquisition, consolidation and
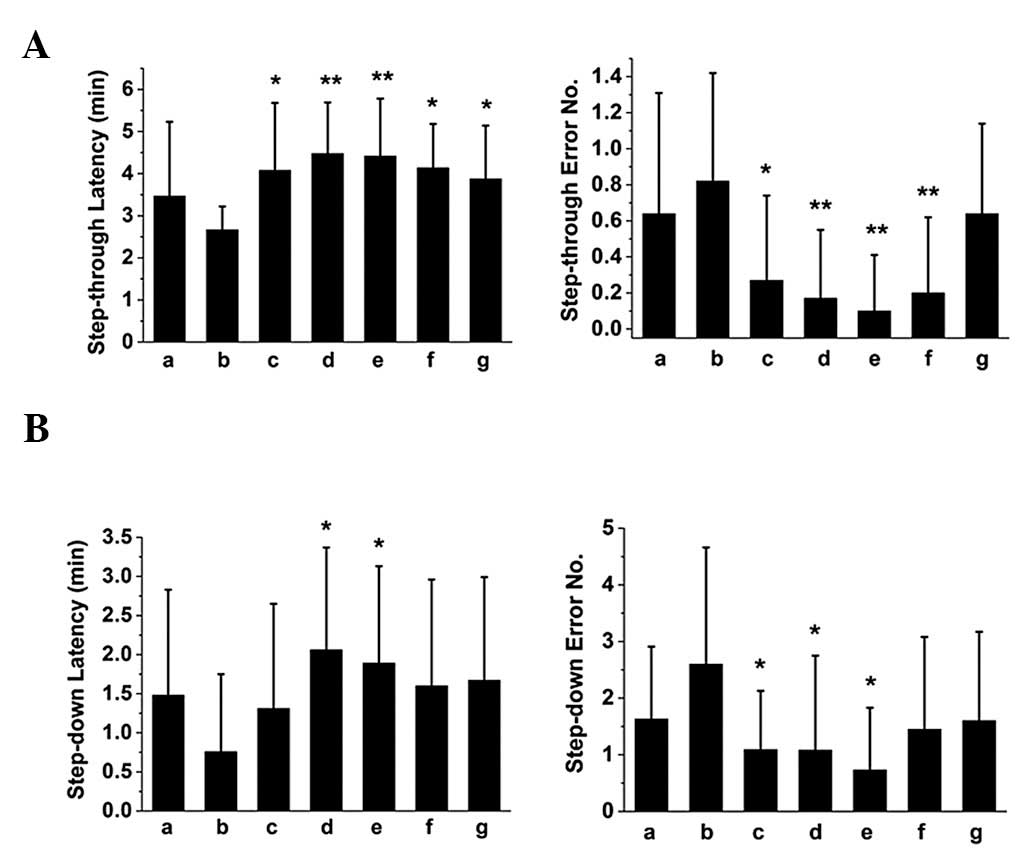
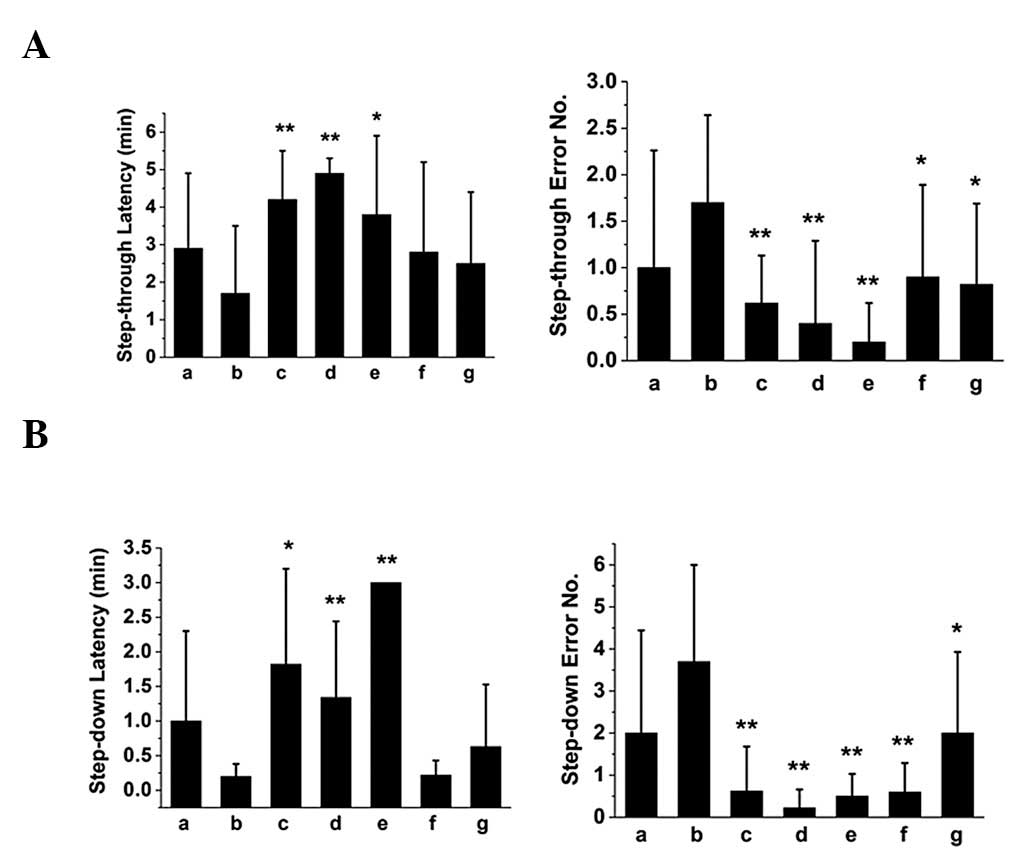
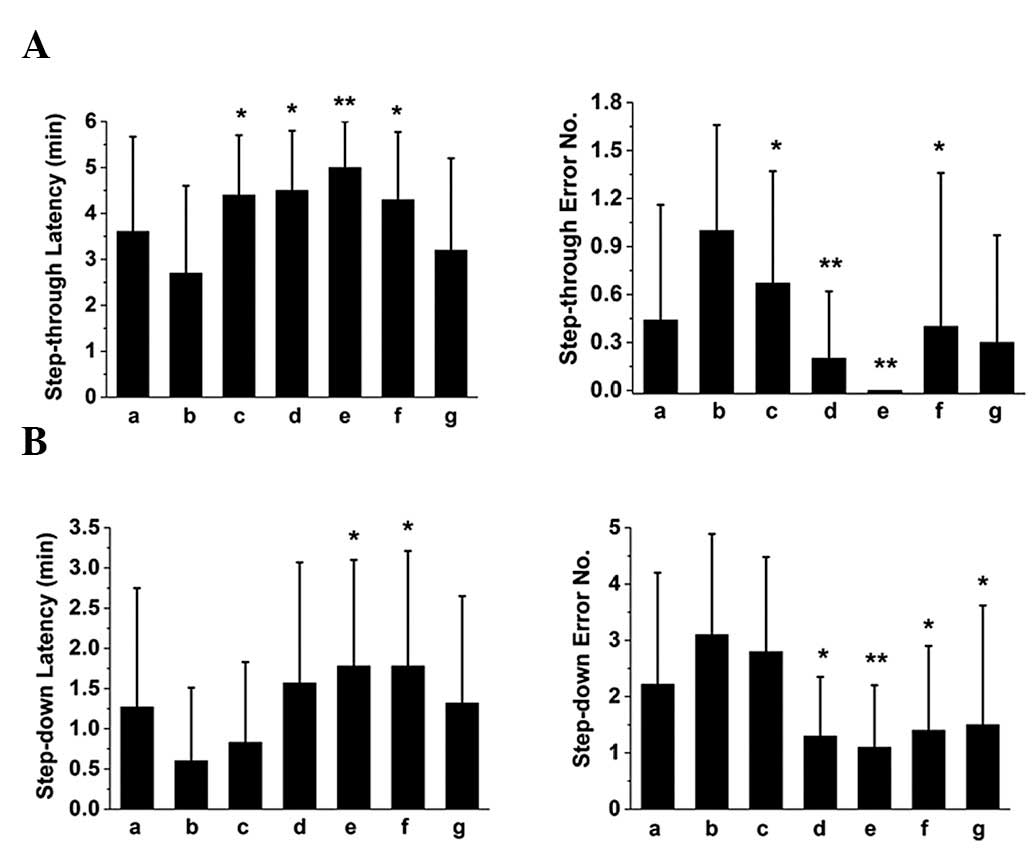
retrieval in mice, respectively. The results of step-through and
-down passive avoidance tasks showed that SZS and FG oils improved
the performance of mice with memory impairment in memory
acquisition (Fig. 1), consolidation
(Fig. 2) and retrieval (Fig. 3), since they were able to prolong
the latency and reduce the error number. Notably, the compound oil
of SZS and FG was the most effective, suggesting a synergistic
effect for improving learning and memory in mice.
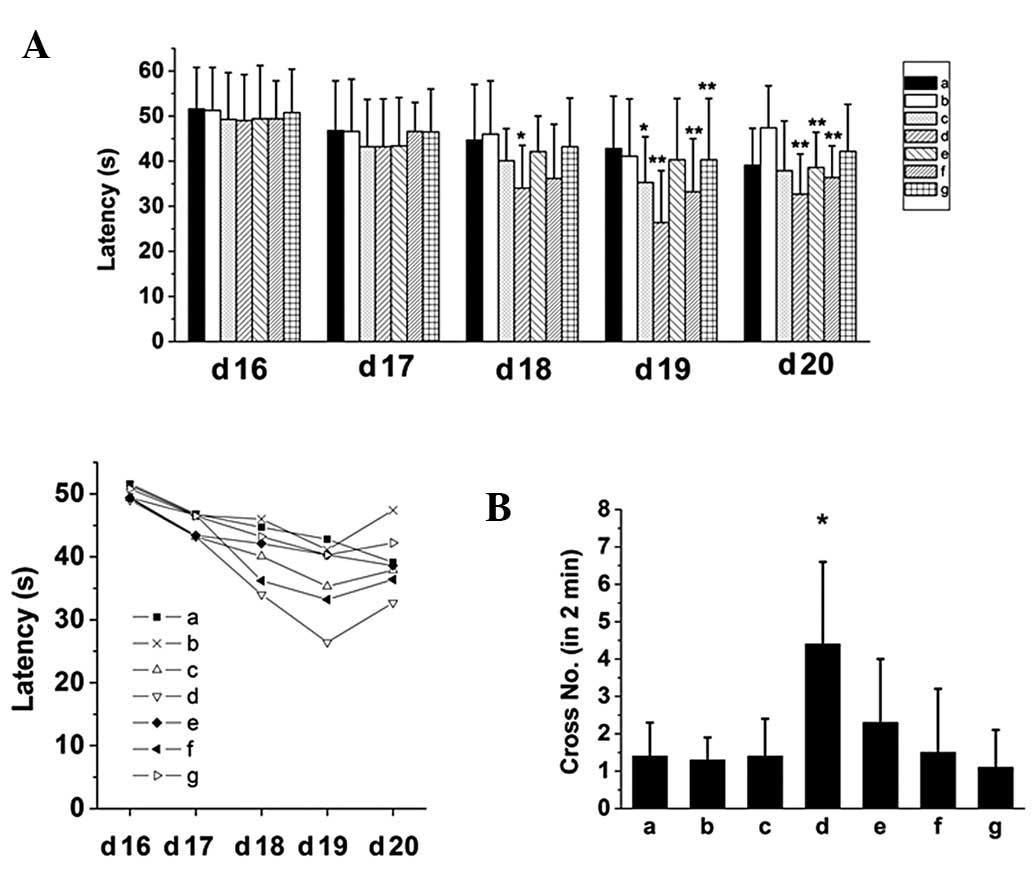
Effect of SZS and FG oils on the learning
and memory of mice in the Morris water maze test
The results of the Morris water maze test
demonstrated that the compound oil of SZS and FG improved the
learning and memory in mice treated with sodium nitrite, a drug
that induces memory retrieval-impairment, and the effects were the
best at a medium, or 2.7 g/kg dose (Fig. 4). Although SZS as well as FG oils
improved the performance when used separately, the improvement was
not markedly effective.
Discussion
SZS and FG were originally described in the Shennong
Herbal (Shen Nong Ben Cao Jin), the oldest and the most classic
Chinese materia medica book and have been used frequently by TCM
doctors until present. SZS is classified as a tranquilizing herb
that can tonify yin and nourish the heart. The indications
of its clinical application include yin-blood deficiency and
loss of nourishment of the heart and mind when manifested as
emotional upset, insomnia and amnesia (4). FG is classified as a heat-clearing
herb. It can purge fire and is especially good at clearing the heat
of the heart and eliminating moody emotions. The indications of its
clinical application include excess heat syndromes, such as
depression, restlessness, irritability and delirium arising from
heat suppressed in the heart (5).
Although classified into different categories in TCM literature,
based on their actions and properties, SZS and FG are usually used
in combination to achieve better effects.
According to modern medical and pharmacological
theories, the effective part of SZS and FG lies in the extracts
(6). Our experiments demonstrated
that the extracts of SZS and FG improved learning and memory in
mice, however, SZS was more efficient compared to FG. Notably, SZS
and FG have a synergistic effect, and the effect was the most
prominent when the compound oil dose was medium (2.7 g/kg body
weight).
The effective ingredients or bioactive components of
SZS and FG in improving learning and memory have not been
specifically investigated. However, due to the composition of SZS
and FG oils prepared with supercritical CO2, extraction
is relatively simple and is rich in oleic and linoleic acids; as
indicated by our previous studies (6–8), these
two free fatty acids may therefore be candidate ingredients. Oleic
acid can be acylated in vivo into oleamide and the latter
has been reported to improve learning and memory in mice (9–11).
Linoleic acid can be transformed in vivo into linolenic and
further into arachidonic acid. Linolenic acid is able to increase
membrane fluidity and plays an important role in neuron
proliferation, nerve conduction and synapse formation (12–14).
Arachidonic acid is one of the most abundant polyunsaturated fatty
acids in the body and is essential for maintaining the physical
property and function of the membrane (15–19).
Arachidonic acid can also regulate hippocampal long-term
potentiation (9). The metabolic
product of arachidonic acid vasopressin may also improve learning
and memory (20–22). Our study showed that SZS and FG oils
have a synergistic effect on improving learning and memory.
However, the mechanism of this synergy requires further
investigation.
References
|
1
|
Yan ZH: Chinese Materia Medica. 2nd
edition. People’s Medical Publishing House; Beijing: pp. 148–150.
2010
|
|
2
|
Hou SL: Detailed annotation of eight
hundred traditional Chinese medicines. Science and Technology Press
of Henan; Zhengzhou: pp. 680–682. 2009
|
|
3
|
Li J: Formulas of traditional Chinese
medicine. Higher Education press; Beijing: pp. 233–234. 2006
|
|
4
|
Zhang TM: Chinese Materia Medica. Higher
Education press; Beijing: pp. 697–698. 2006
|
|
5
|
Zhang TM: Chinese Materia Medica. Beijing:
Higher Education press; pp. 380–382. 2006
|
|
6
|
Li B, Xia C and Yuan B: Sedative and
hypnotic effects of oil of spine date seeds from different
extraction technologies in mice. J Xian Jiaotong Univ Med Sci.
29:227–229. 2008.(In Chinese).
|
|
7
|
Zhang Z, Li B, Chen Y, et al: Assay of the
compositions of the compound oil of semen Ziziphi spinosae and
fructus Gardeniae. J Shanxi Med Univ. 38:792–793. 2009.
|
|
8
|
Li B, Chen Y, Zhang Z, et al: Effect of
compound jujube seed oil and gardenia oil on improving the ability
of learning and memory in mice. J Xian Jiaotong Univ Med Sci.
31:673–676. 7072010.
|
|
9
|
Akanmu MA, Adeosun SO and Ilesanmi OR:
Neuropharmacological effects of oleamide in male and female mice.
Behav Brain Res. 182:88–94. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Micale V, Cristino L, Tamburella A, et al:
Enhanced cognitive performance of dopamine D3 receptor ‘knock-out’
mice in the step-through passive-avoidance test: assessing the role
of the endocannabinoid/endovanilloid systems. Pharmacol Res.
61:531–536. 2010.
|
|
11
|
Ahmadi S, Malekmohammadi N and Zarrindast
MR: Repeated histamine pretreatment decreased amnesia induced by
post-training administration of the drug in a step-down inhibitory
avoidance test in mice. Arch Iran Med. 13:209–216. 2010.PubMed/NCBI
|
|
12
|
Yang X, Sheng W, Sun GY, et al: Effects of
fatty acid unsaturation numbers on membrane fluidity and
α-secretase-dependent amyloid precursor protein processing.
Neurochem Int. 58:321–329. 2011.PubMed/NCBI
|
|
13
|
Saha SS, Chakraborty A, Ghosh S, et al:
Comparative study of hypocholesterolemic and hypolipidemic effects
of conjugated linolenic acid isomers against induced biochemical
perturbations and aberration in erythrocyte membrane fluidity. Eur
J Nutr. 51:483–495. 2012. View Article : Google Scholar
|
|
14
|
Lauretani F, Bandinelli S, Bartali B, et
al: Omega-6 and omega-3 fatty acids predict accelerated decline of
peripheral nerve function in older persons. Eur J Neurol.
14:801–808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Villegas-Comonfort S, Serna-Marquez N,
Galindo-Hernandez O, et al: Arachidonic acid induces an increase of
β-1,4-galactosyltransferase I expression in MDA-MB-231 breast
cancer cells. J Cell Biochem. 113:3330–3341. 2012.
|
|
16
|
Rossen NS, Hansen AJ, Selhuber-Unkel C, et
al: Arachidonic acid randomizes endothelial cell motion and
regulates adhesion and migration. PLoS One. 6:e251962011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Das UN: Long-chain polyunsaturated fatty
acids in growth and development of brain and memory. Nutrition.
19:62–65. 2003. View Article : Google Scholar
|
|
18
|
Fu ZY, Wang YP and Chen Y: Observation of
insulin exocytosis by a pancreatic β cell line with total internal
reflection fluorescence microscopy. Chin Med Sci J. 26:60–63.
2011.
|
|
19
|
Matsuyama M and Yoshimura R: Arachidonic
acid pathway: A molecular target in human testicular cancer
(Review). Mol Med Rep. 2:527–531. 2009.PubMed/NCBI
|
|
20
|
DeCostanzo AJ, Voloshyna I, Rosen ZB, et
al: 12-Lipoxygenase regulates hippocampal long-term potentiation by
modulating L-type Ca2+ channels. J Neurosci.
30:1822–1831. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alescio-Lautier B and Soumireu-Mourat B:
Role of vasopressin in learning and memory in the hippocampus. Prog
Brain Res. 119:501–521. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Engelmann M, Wotjak CT, Ebner K and
Landgraf R: Behavioral impact of intraseptally released vasopressin
and oxytocin in rats. Exp Physiol. 85:S125–S130. 2000. View Article : Google Scholar : PubMed/NCBI
|