Introduction
The development of cervical cancer (CC) has been
causally linked to oncogenes E6 and E7 of high-risk human
papillomaviruses (HPV). The HPV E6 and E7 oncogenes integrate into
the genome of infected cervical cells and inactivate the
tumor-suppressor proteins p53 and retinoblastoma, which results in
epithelial immortalization and ultimate malignant transformation
through a multistage process (1).
Due to active and indefinite growth of cancer cells, reactive
oxygen species (ROS) production from the mitochondria is
accordingly increased (2).
Excessive ROS is likely to induce oxidative stress and subsequent
cell apoptosis through mitochondria or/and direct injury to
protein, DNA and lipid (3–5). In fact, the chemo- or radiotherapy for
cancers is through ROS increase and apoptotic induction of cancer
cells (6,7).
Organisms have developed multiple antioxidant
systems to protect against oxidative damage, among which
peroxiredoxin 3 (Prx3) plays an important role in regulating
cellular ROS level and apoptosis (8). As a mitochondrial scavenger of ROS,
Prx3 has been demonstrated to play an antioxidant role under
cellular oxidative conditions (9,10) and
the involvement of Prx3 in CC carcinogenesis was previously
reported (11). Nevertheless, the
significance of Prx3 in CC development and/or progression has not
been clear. The present study was conducted to investigate the
characteristics of Prx3 expression, and thereby contributing to the
understanding of the mechanism of tumor growth and invasion.
Materials and methods
Patients and samples
After the approval of the Institutional Review
Boards of Tsinghua University Second Hospital (Beijing, China), we
collected invasive squamous cervical cancer (ISCC) samples from
patients between August, 2008 and July, 2010. The samples were
collected after obtaining informed consent from the patients. The
samples were processed into formalin-fixed and paraffin-embedded
tissue blocks. At the same time, adjacent normal epithelial tissues
were used as the controls.
Examination of HPV infection
HPV-infection status of the samples was examined
using HPV gene array test kit (Hybribio Limited, Hong Kong, China).
Briefly, DNA was extracted from cervical tissues and amplified by
polymerase chain reaction (PCR). The PCR products were then
hybridized on HPV genoarray membrane that contained probes
corresponding to 21 HPV types. Biotin was used as the positive
control and distilled water as the negative control. The membrane
was visualized through NBT/BCIP to determine the status of HPV
infection.
To detect HPV16 mRNA expression in CCs, we performed
quantitative real-time PCR (qRT-P C R) using
SYBR®-GreenER™ Two-Step qRT-PCR kits (Invitrogen,
Carlsbad, CA, USA). Total RNA was extracted from paraffin-embedded
tissue sections. After cDNA synthesis, the qRT-PCR program and the
calculation for HPV16 expression were performed as previously
described (10). The primer
sequences for HPV16 E6/E7 were: forward,
5′-GTTACTGCGACGTGAGGTATATG-3′; reverse,
5′-CATTTATCACATACAGCATATGGATTC-3′. β-actin (forward,
5′-ACGTTGACATCCGAAAGACC-3′; reverse, 5′-CCACCGATCCACACAGAGTA-3′)
was used as the internal control.
Immunohistochemistry
To detect Prx3 protein in cervical tissues, we
performed immunohistochemistry as previously described with a minor
modification (9). Consecutive
slides were incubated using mouse monoclonal antibodies against
human Prx3 and Ki67 respectively (1:1000 dilution for Prx3 and
1:150 dilution for Ki67; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA). Immunohistochemical staining scores were evaluated
as the percentage of positive cells and counted by two independent
persons not aware of the patient information.
Results
In the present study, we included 68 patients with
ISCC (age, 45.96±9.89 years). Thirty patients had FIGO stage I and
38 had stage II, of which 12 were cancer-positive in the lymph
nodes. The patients did not receive chemo- or radiotherapy prior to
the operation.
Expression of high-risk HPV is higher in
cancer cells compared to the adjacent normal epitheliums
As determined by the HPV genoarray test kit, all
samples were infected with high-risk HPV. Among these, fifty-six
samples were positive for HPV16, seven for HPV18 and five for
HPV33. In the fifty-six HPV16-positive samples, the expression of
HPV16 E6/E7 mRNA relative to β-actin was significantly higher in
cancer samples compared to the adjacent normal epitheliums
(8996±409 vs. 198±56, P=0.000 by analysis of variance).
Expression of Prx3 and HPV16 is
positively correlated in CC samples
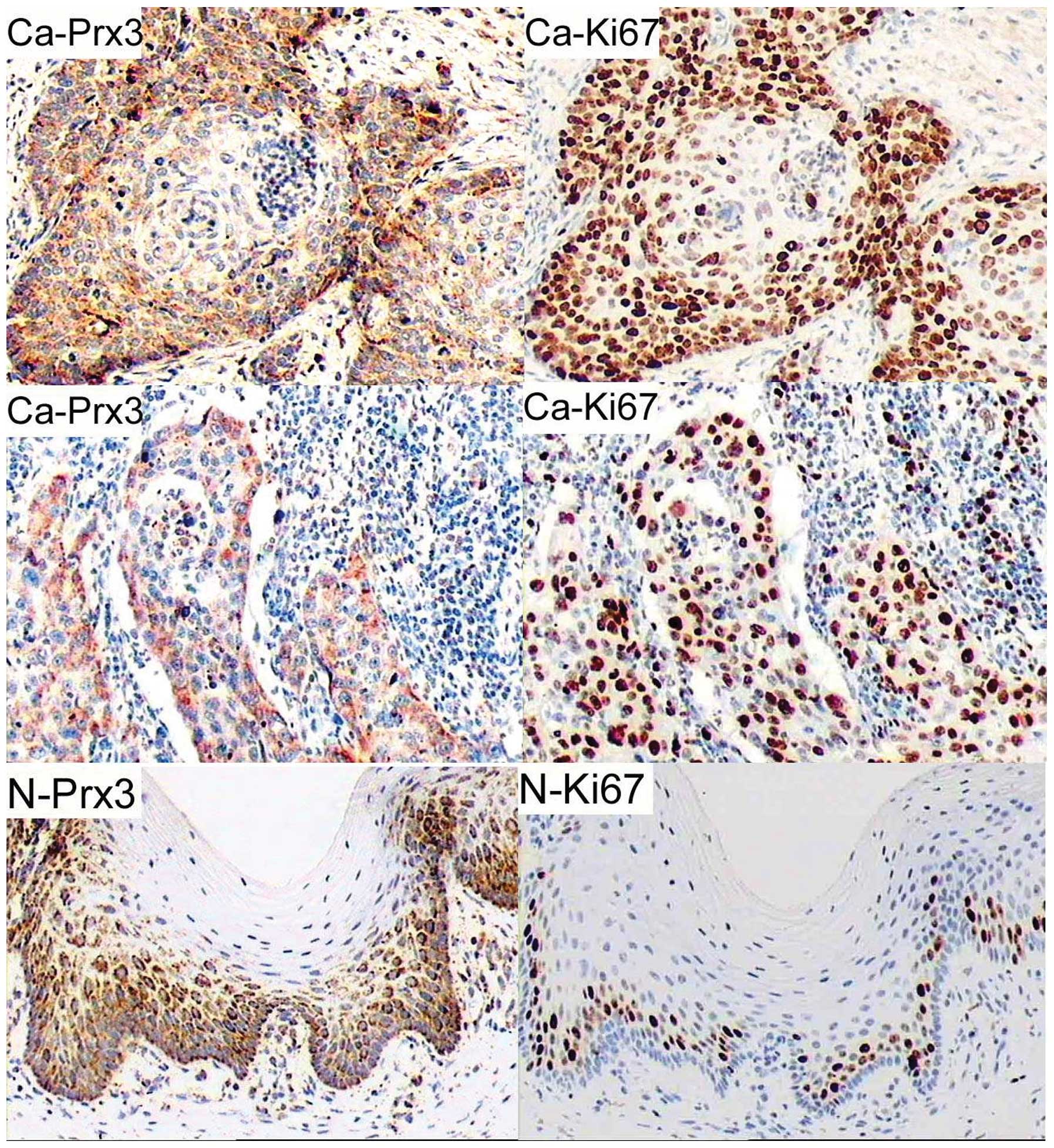
As shown in Fig. 1,
we observed strong cytoplasmic staining for Prx3 in the cancer
nests and the surrounding cells or in invasive cancerous cells,
while Prx3-positive cells in the adjacent epithelium were located
mainly in the basal layer. The number of positive cells for Prx3 in
cancerous areas was significantly higher compared to that in
non-cancerous areas (65.00±21.08 vs. 41.04±13.09%, P=0.000 by
Student’s t-test). Of the clinical and pathological
characteristics, cell differentiation (grade) was significantly
associated with Prx3 expression (Pearson’s correlation coefficient
was −0.648, P=0.000). In addition, the positive cells for Prx3 in
CCs were correlated with the expression of HPV16 E6/E7 (Pearson’s
correlation coefficient was 0.726, P=0.000).
Expression of Prx3 and Ki67 is positively
correlated in CC samples
Since Prx3 was predominantly expressed in cells with
active proliferation, immunohistochemistry was performed using
mouse monoclonal antibody against human Ki67. As shown in Fig. 1, the number of positive cells for
Ki67 was significantly higher in cancerous cervices compared to
adjacent epitheliums (70.89±21.49 vs. 17.12±10.28%, P=0.000 by
Student’s t-test). Notably, the staining pattern of Prx3 was
consistent with that of Ki67 (Fig.
1; Pearson’s correlation coefficient was 0.801, P=0.000). In
cancerous sections, Ki67-positive cells were located in the cancer
nests and the surrounding areas or in invaded cancerous tissues,
while Ki67-positive cells in the adjacent normal epitheliums were
located mainly in the basal layer.
Discussion
In the present study, we demonstrated increased
expression of Prx3 in CC and provided evidence that Prx3 was
upregulated in the cells with active proliferation. The pattern of
Prx3 expression in CC was consistent with that of Ki67, indicating
that Prx3 is a potential marker for cell proliferation.
In their study, Riethdorf et al (12) demonstrated that high-risk HPV
oncogenes activated telomerase and played a critical role in human
carcinogenesis. High-risk HPV genomes were reported to
preferentially integrate near the c-myc gene (13). The latter cooperated with HPV E6/E7
to promote the malignant transformation of cervical epitheliums
(14). As a target gene of c-myc,
Prx3 played an important role in mitochondrial homeostasis
maintenance and neoplastic transformation (15). Our findings showed that the HPV16
E6/E7 expression was increased and was positively associated with
the expression of Prx3 in CC. The interaction between HPV, c-myc
and Prx3 in CC development or/and progression requires additional
investigation.
As an established marker for cell proliferation,
Ki67 has been suggested to be a predictor for high-risk HPV
infection and an independent prognostic parameter for
recurrence-free survival of CC (16,17).
Our findings showed that the Ki67-positive cells were mainly
located in the surrounding areas of cancer nests or in invaded
cancerous tissues, implying active growth of the cells in these
areas. Since ROS production was increased in the cells with active
proliferation (2), the upregulation
of Prx3 in the same areas might be a natural response to oxidative
stress in the cancer microenvironment. In their previous study,
Nonn et al (18) reported
that Prx3 played a protective role against drug-induced oxidative
stress and subsequent apoptosis of cancer cells, thus it would be
useful to investigate the significance of Prx3 in the prognosis,
recurrence and chemo- or radiotherapy-resistance of CC.
References
|
1
|
Scheffner M, Munger K, Byrne JC and Howley
PM: The state of the p53 and retinoblastoma genes in human cervical
carcinoma cell lines. Proc Natl Acad Sci USA. 88:5523–5527. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beevi SS, Rasheed MH and Geetha A:
Evidence of oxidative and nitrosative stress in patients with
cervical squamous cell carcinoma. Clin Chim Acta. 375:119–123.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cotran RS, Kumar V and Robbins SL: Robbins
Pathologic Basis of Disease. 5th edition. W.B. Saunders Company;
Philadelphia: 1994
|
|
4
|
Ding B, Chi SG, Kim SH, et al: Role of p53
in antioxidant defense of HPV-positive cervical carcinoma cells
following H2O2 exposure. J Cell Sci.
120:2284–2294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh M, Sharma H and Singh N: Hydrogen
peroxide induces apoptosis in HeLa cells through mitochondrial
pathway. Mitochondrion. 7:367–373. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Srinivas P, Gopinath G, Banerji A, Dinakar
A and Srinivas G: Plumbagin induces reactive oxygen species, which
mediate apoptosis in human cervical cancer cells. Mol Carcinog.
40:201–211. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alexandre J, Batteux F, Nicco C, et al:
Accumulation of hydrogen peroxide is an early and crucial step for
paclitaxel-induced cancer cell death both in vitro and in vivo. Int
J Cancer. 119:41–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang TS, Cho CS, Park S, Yu S, Kang SW
and Rhee SG: Peroxiredoxin III, a mitochondrion-specific
peroxidase, regulates apoptotic signaling by mitochondria. J Biol
Chem. 279:41975–41984. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Shoji W, Takano H, et al: Increased
susceptibility of MER5 (peroxiredoxin III) knockout mice to
LPS-induced oxidative stress. Biochem Biophys Res Commun.
355:715–721. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Shoji W, Oshima H, Obinata M,
Fukumoto M and Kanno N: Crucial role of peroxiredoxin III in
placental antioxidant defense of mice. FEBS Lett. 582:2431–2434.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim K, Yu M, Han S, et al: Expression of
human peroxiredoxin isoforms in response to cervical
carcinogenesis. Oncol Rep. 21:1391–1396. 2009.PubMed/NCBI
|
|
12
|
Riethdorf S, Riethdorf L, Schulz G, et al:
Relationship between telomerase activation and HPV 16/18 oncogene
expression in squamous intraepithelial lesions and squamous cell
carcinomas of the uterine cervix. Int J Gynecol Pathol. 20:177–185.
2001. View Article : Google Scholar
|
|
13
|
Couturier J, Sastre-Garau X,
Schneider-Maunoury S, Labib A and Orth G: Integration of
papillomavirus DNA near myc genes in genital carcinomas and its
consequences for proto-oncogene expression. J Virol. 65:4534–4538.
1991.PubMed/NCBI
|
|
14
|
Subramanyam D and Krishna S: c-Myc
substitutes for Notch1-CBF1 functions in cooperative transformation
with papillomavirus oncogenes. Virology. 347:191–198. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wonsey DR, Zeller KI and Dang CV: The
c-Myc target gene PRDX3 is required for mitochondrial homeostasis
and neoplastic transformation. Proc Natl Acad Sci USA.
99:6649–6654. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mimica M, Tomić S, Kardum G, Hofman ID,
Kaliterna V and Pejković L: Ki-67 quantitative evaluation as a
marker of cervical intraepithelial neoplasia and human
papillomavirus infection. Int J Gynecol Cancer. 20:116–119. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanprasertpong J, Tungsinmunkong K,
Chichareon S, et al: Correlation of p53 and Ki-67 (MIB-1)
expressions with clinicopathological features and prognosis of
early stage cervical squamous cell carcinomas. J Obstet Gynaecol
Res. 36:572–580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nonn L, Berggren M and Powis G: Increased
expression of mitochondrial peroxiredoxin-3 (thioredoxin
peroxidase-2) protects cancer cells against hypoxia and
drug-induced hydrogen peroxide-dependent apoptosis. Mol Cancer Res.
1:682–689. 2003.
|