Introduction
Cervical cancer (CC) is the second most frequent
malignant tumor disease among the various types of female cancer
and threatens female life expectancy and health. There have been
495,000 new cases recently worldwide, with the age bracket of
patients shifting into younger age groups. Additionally, patients
(15–35%) with high-grade cervical intraepithelial neoplasia (CIN)
or CC are often not detected at first screening.
A causal link between human papillomavirus (HPV)
infection and CC has been established. A large number of HPV
genotypes has been identified (1),
and the HPV strains are divided into high- (HR) and low-risk (LR)
categories on the basis of their association with cervical lesions.
HR HPV, especially 13 HR HPV genomes (i.e., genotypes 16, 18, 31,
33, 35, 39, 45, 51, 52, 56, 58, 59 and 68), are more frequently
found in pre-malignant or malignant tumors. Infection by HR HPV has
been demonstrated in ∼100% of CC. Clear scientific evidence
indicates that screening based on validated tests for the DNA of
oncogenic HPV as primary examination as well as the application of
an appropriate protocol is more effective compared to screening
based on cytology in preventing invasive types of cancer of the
uterine cervix (2). Cytological
analysis combined with HR HPV examination raises the detection rate
of CC, and is able to reduce ≥grade 3 cervical intraepithelial
neoplasia at 5 years (3).
Furthermore, HPV testing is an important ancillary diagnostic tool
for distinguishing which women are at risk to progress to the
squamous intraepithelial lesion (SIL) stage, decreasing the number
of colposcopy referrals and follow-up tests (4). As a result, the detection of HR HPV in
cervical samples constitutes one of the most important steps in
improving the efficacy of cervical carcinoma screening programs and
in triaging patients with ambiguous or borderline cervical smears.
An increasing number of studies has confirmed the effectiveness of
HR HPV analysis in CC screening programs (5–8). The
Hybrid Capture® II High-Risk HPV DNA test®
(HC II) was approved by the U.S. Food and Drug Administration in CC
screening in women aged ≥30 years (9), and has been widely used worldwide.
Recently, a standardized PCR-based technique, the HPV real-time
fluorescent PCR assay (Kaipu Biochemistry Co., Ltd., Wenzhou,
China), was commercialized for the detection of the 13 HR HPV
genotypes. This novel assay uses amplification of target DNA (L1
gene) by PCR and nucleic acid hybridization for the detection of HR
HPV genotypes in cervical cells collected into a transport medium.
This assay could be used in clinical diagnostic laboratories,
however, limited data are currently available on its performance
and reliability. Specifically, no data exist on its comparison with
the HC II test.
The aim of this study was to examine the performance
of two commercially available tests, HC II test and real-time
fluorescent PCR assay, and to detect the presence of HR HPV in
cervical samples of women who attended the Department of Gynecology
and Obstetrics of China-Japan Friendship Hospital (Beijing, China)
for CC screening. This study provides clinicians with an
additional, lower-cost choice in CC screening compared to the HC II
test.
Materials and methods
Study population
From July 15th, 2009 to January 10th, 2011, 1,252
women underwent CC screening at the Department of Gynecology and
Obstetrics of China-Japan Friendship Hospital (Beijing, China).
Written informed consent was obtained from all the patients. The
study was approved by the ethics committee of China-Japan
Friendship Hospital. The patient mean age was 40.8±9.5 years
(range, 19–71); 90% of patients were aged ≥30 years and 86% of them
were 30–65 years. Specimens for the liquid-based cytology test
(LCT), HC II test and real-time fluorescent PCR assay were
collected from the women during their visit. Colposcopy was
performed when ≥1 of the results of these three tests was abnormal,
and a histological sample was taken when a lesion was identified.
Women exhibiting acute genital inflammation were excluded from this
study.
Sample collection
The women were asked to avoid vaginal douching,
vaginal drug administration 3 days prior to their visit to the
hospital as well as sexual activity 24 h prior to their visit.
During the collection of cervical cells, we firstly cleaned and
polished the cervix with cotton pledgets. Secondly, cervical
samples were obtained with cervix brushes rotated for three times
on any days of the menstrual cycle, except those during menstrual
bleeding. Thirdly, we removed samples from the brushes and placed
them into sample tubes containing preserving fluid and marked the
name of the patient as well as the date. Specimens were preserved
for <24 h at 2–8°C or for 30 days at −20°C. Repeated specimen
thawing was avoided.
LCT
Smears were stained using the AutoCyte Prep staining
machine and observed by professional gynecology-pathology doctors
with cytological diagnosis aptitude. Cytologic diagnosis was
performed according to the Cervical Cytology Criteria of the 2001
Bethesda System of the International Cancer Society. According to
this system, abnormalities of cervical squamous epithelium include
atypical squamous cells (ASC), low- (LSIL) and high-grade squamous
intraepithelial lesions (HSIL), as well as squamous cell carcinomas
(SCC).
HPV DNA testing using the HC II test
HR HPV DNA testing was performed using the automated
HC II test (Qiagen, Gaithersburg, MD, USA). HC II test is a
sandwich capture molecular hybridization assay that utilizes
chemiluminescent detection to provide a semi-quantitative result.
The test was calibrated to detect ∼5,000 genome/ml equivalents of
target HPV, represented by a Relative Light Unit (RLU) measurement
greater than or equal to the cut-off value calculated in each run
by a series of standards. A measurement less than the cut-off value
was scored as negative. The samples were analyzed for the presence
of HR HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and
68. Three positive and 3 negative controls (provided by the
manufacturer) were included in each run.
HPV DNA testing using real-time
fluorescent PCR assay
This assay included: i) DNA extraction, involving
the release of HPV and cellular DNA by lysing cervical specimens
under denaturing conditions at elevated temperatures in the
presence of protease. The assay did not require DNA purification.
ii) PCR amplification and hybridization reaction, involving the use
of biotinylated primers in order to define a sequence of ∼150 bp in
length within the polymorphic L1 region of the HPV genome. The
primers, pooled in the same PCR Master mix, were designed to
amplify viral DNA from the same 13 HR HPV types included in the HC
II test. Capture probes representing regions internal to the
amplified sequences were used to identify HPV of human DNA. PCR
amplification and hybridization reaction were performed using the
Roche LightCycler®. Thirty cytological samples were
detected simultaneously, and each time negative and positive
controls were established. Following the reaction, Roche
LightCycler® computer was used to calculate the cycle
threshold (CT) of each sample. CT was the cycle number of each
reaction flourescence signal that reached the setting threshold.
iii) Result interpretation involved the baseline and threshold
exceeding culmination of the amplication curve of the negative
control, while the CT value did not indicate detection (blank).
Evaluation of response effectiveness depended on the blank negative
control and a CT of ≤36 in the positive control. When the CT value
of the samples was ≤40, samples were evaluated as positive, while
samples were considered negative with a CT >40. According to the
manufacturer’s instructions, this test was able to detect the
genotypes of the 13 HR HPV types at 5,000 copies/ml.
Colposcopy and biopsy
Colposcopic examination of the cervix was performed
in women with either HR HPV-positive results or cytological
abnormalities, or both. Negative cytological results and the two HR
HPV DNA tests used predicted a low risk of cervical neoplasia. A
histological sample was obtained when a lesion was identified, and
4 histological samples were obtained at 3°, 6°, 9° and 12° of the
normal transformation zone. Endocervical canal curettage was
performed when colposcopic examination was unsatisfactory. The
patients with high-grade lesions underwent loop electrosurgical
excision procedure (LEEP), cold knife conization (CKC) or
hysterectomy for further diagnosis and treatment. The most severe
diagnosis was determined as the final histopathological diagnosis.
The diagnostic criteria of high-grade lesions were defined as equal
to or more severe than CINII (CINII+).
Statistical analysis
Sensitivity, specificity and accuracy of the
real-time fluorescent PCR assay, the HC II test and the LCT were
determined against the presumed HPV status based on the combination
of the two HPV tests used and histological pathological results.
The 95% confidence intervals (CI) were calculated using Fisher’s
exact test. Agreement between the two HPV tests used was assessed
by Cohen’s κ, with values of 0.00–0.40 indicating poor, 0.40–0.75
fair and 0.75–1.00 excellent agreement. The χ2 test was
performed for group comparisons. All the tests were two-sided, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Outcomes of 1,252 specimens examined
using HC II test and real-time fluorescent PCR assay
The comparison between HC II test and real-time
fluorescent PCR assay of 1,252 specimens is shown in Table I. Among the 1,252 women studied, the
two tests gave similar results for 1,155 samples, with an overall
level of agreement of 92.25% (Cohen’s κ=0.814) indicating excellent
agreement. However, the examination of 97 samples using the two
tests gave discordant results. HPV genotyping of these samples are
to be performed in future studies. Assuming that samples with
positive results examined using the two tests were infected by HR
HPV, and that samples with negative results were HR HPV-negative or
infected by LR HPV, ‘conditional’ sensitivity and specificity can
be evaluated for the two tests used.
 | Table IComparison between the outcomes of
1,252 samples examined using HC II test and real-time fluorescent
PCR assay. |
Table I
Comparison between the outcomes of
1,252 samples examined using HC II test and real-time fluorescent
PCR assay.
| HC II test
outcome | Real-time fluorescent
PCR assay outcome, n
| Total, n |
|---|
| HPV-positive | HPV-negative |
|---|
| HPV-positive | 321 | 59 | 380 |
| HPV-negative | 38 | 834 | 872 |
| Total | 359 | 893 | 1,252 |
Correlation between histopathological
outcomes and HPV test
There were 477 patients who underwent colposcopic
examination and biopsy. Histopathological results were considered
to be the ‘gold standard’. Sherman et al (10) found that the cumulative incidence
rate was only 0.79%, when CIN or CC and negative HPV cases were not
detected using cervical cytological examination, while CIN and
cancer cases were not identified in these histopathology samples.
On these grounds, women with normal LCT results and negative
results from the two HR HPV DNA tests used were classified into the
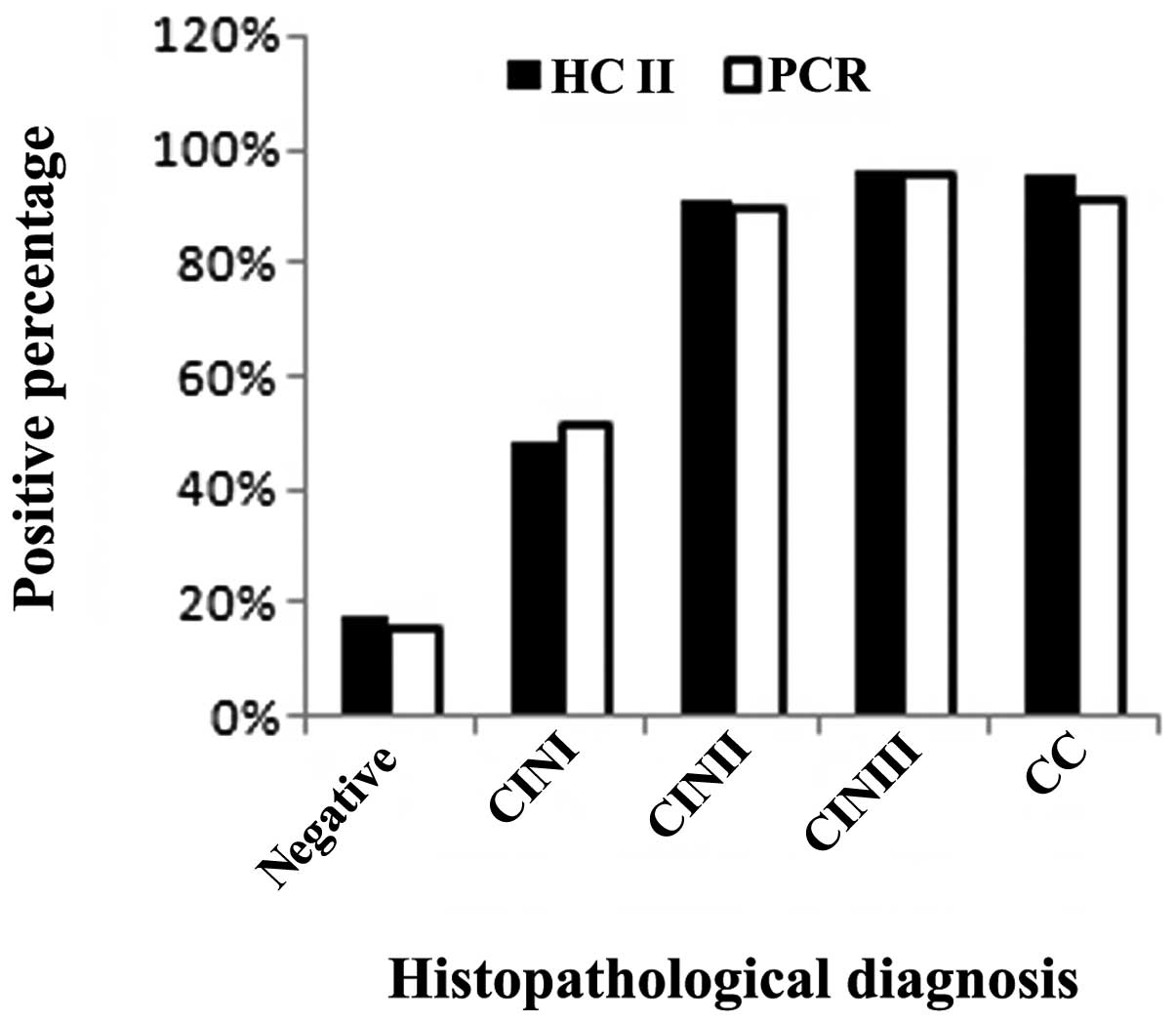
‘negative’ group (women without CIN or CC). As shown in Fig. 1, 1,015 specimens were
histopathologically negative (normal or inflammation). Among these
samples, 182 (17.93%) were positive using the HC II test, while 162
(15.96%) were positive using the real-time fluorescent PCR assay.
Additionally, 56 samples were diagnosed as CINI using
histopathological examination of which 27 (48.21%) were positive
using the HC II test and 29 (51.79%) were positive using the
real-time fluorescent PCR assay. Fifty-five samples were diagnosed
as CINII using histopathological examination of which 50 (90.90%)
were positive using the HC II test, while 49 (89.09%) were positive
using the real-time fluorescent PCR assay. A further 104 samples
were diagnosed as CINIII using histopathological examination of
which 100 (96.15%) were positive using the HC II test, while 99
(95.19%) were positive using the real-time fluorescent PCR assay.
Furthermore, 22 samples were diagnosed as CC using
histopathological examination of which 21 (95.45%) were positive
using the HC II test, while 20 (90.90%) were positive using the
real-time fluorescent PCR assay. We compared the negative and
positive histopathological groups, where the HR HPV infection rate
was 19.51 and 94.48%, respectively, when samples were examined
using the HC II test. A statistically significant difference
between two groups was observed (χ2 = 411.57;
P<0.05).
Due to the fact that CINII and CINIII patients did
not undergo the CINI morphology stage, the difficulty to
distinguish HPV infection from the changes of CINI at
cytopathological appearance under a colposcope, and the significant
proportion of samples that would undergo spontaneous regression, we
classified histopathological results of CINII, CINIII and CC into
the positive group in this study, and normal or inflammation and
CINI samples into the negative group. Sensitivity and specificity
were also calculated at this point. The correlation between the two
HPV tests used and histopathological outcomes is shown in Table II.
 | Table IICorrelation between HPV tests and
histopathological outcomes. |
Table II
Correlation between HPV tests and
histopathological outcomes.
| Histopathological
outcome | HC II test outcome,
n | Real-time fluorescent
PCR assay outcome, n | Total, n |
|---|
|
|
|---|
| HPV-positive | HPV-negative | HPV-positive | HPV-negative |
|---|
| HPV-positive | 171 | 10 | 168 | 13 | 181 |
| HPV-negative | 209 | 862 | 191 | 880 | 1,071 |
| Total | 380 | 872 | 359 | 893 | 1,252 |
In this study, there were 181 patients with CINII+,
94.48% of whom were positive using the HC II test and 92.82% of
whom were positive using the real-time fluorescent PCR assay. Out
of the 1,071 samples of the negative histopathology group, there
were 862 (80.49%) negative samples using the HC II test and 880
(82.17%) negative samples using the real-time fluorescent PCR
assay. Out of the 872 negative samples using the HC II test, there
were 862 patients without CINII+, indicating a negative predictive
value of 98.85%. Out of the 893 negative samples using the
real-time fluorescent PCR assay, there were 880 patients without
CINII+, indicating a negative predictive value of 98.54%.
Discussion
A causal link between HR HPV infection and CC has
been established. According to Sharma et al (11), conducting a population-based HPV
survey targeting women >35 years of age would be sufficient to
estimate the CC risk in a certain country. This study has shown the
difference of the HR HPV infection rate between the normal or
inflammation group and the CINII+ group (χ2 = 411.57;
P<0.05).
Numerous methods can be utilized to detect HR HPV
infection. The HC II test has been considered the best method to
screen for CC. Real-time fluorescent PCR is a novel assay developed
to use TaqGold™ DNA polymerase, which minimizes the amount of
non-specific amplification and increases the sensitivity of the
assay. Furthermore, this PCR protocol is based on a 5′-exonuclease
assay and real-time detection of fluorescence accumulation.
Fluorescence release during each amplification cycle is directly
proportional to the amount of amplicon generated. Therefore, it is
considered to be an accurate method for estimating viral load
(?).
The aim of this study was to compare: i) the
performance of two commercially available assays (HC II test and
real-time fluorescent PCR assay) which cover the same HR HPV
genotypes for the detection of the 13 HR HPV types as well as ii)
the sensitivity, specificity and accuracy of the two tests for
CINII+ screening in a group of 1,252 women. The overall correlation
of the two tests was 92.25%, with a Cohen’s κ value of 0.814,
indicating good agreement. However, 97 samples yielded different
results (7.75% of all samples): 59 were positive using the HC II
test and negative using the real-time fluorescent PCR assay. Of
these 59 women, 10 were diagnosed as CINII+, while 38 were positive
using the real-time fluorescent PCR assay and negative using the HC
II test. Of these 38 women, 8 patients were diagnosed as CINII+.
Although neither of the two tests was able to detect all of the
CINII+ cases during CC screening, LCT and HR HPV tests may be
combined in order to increase accuracy.
Previous studies (12,13)
have demonstrated that the Digene HC II assay may have
cross-reactivity with LR HPV types, known to cause cytological
abnormalities that do not progress to cancer. Subsequent studies
are to investigate whether the real-time PCR HPV test is likely to
recognize the LR HPV type as HR HPV. Regardless of the discordant
samples, the two HPV tests used showed a good correlation and
yielded comparable results. In clinical practice, when choosing the
most appropriate HPV test, gynecologists should consider whether
the test has good negative predictive value and a satisfactory
sensitivity of detecting clinical lesions, and whether or not the
test is reproducible and has unitive standard.
This study suggests that the real-time fluorescent
PCR assay possesses the above characteristics. The sensitivity of
this assay detecting CINII+ is ∼92.82%, and the negative predictive
value is ∼98.54%. An additional characteristic of the real-time
fluorescent PCR assay constitutes its excellent quality combined
with its reasonable price, expending only a half of the HC II test.
As a result, it could reduce the expense of CC screening in
developing countries. However, due to the fact that it has the
ability to amplify shorter fragments, it is considered to have a
higher analytical sensitivity and a lower clinical specificity, as
well as to be adaptable for less well-preserved specimens.
In conclusion, the present study suggests that
real-time fluorescent PCR assay and HC II test are easily
implemented in a clinical laboratory and that they provide
comparable results. Although this study found a number of modest
differences between the two tests, a fact which will be further
studied in future studies, it provides gynecologists with an
additional, low-cost choice in CC screening.
References
|
1
|
Munoz N, Bosch FX, de Sanjose S, Herrero
R, Castellsaque X, Shah KV, Snijders PJ and Meijer CJ;
International Agency for Research on Cancer Multicenter Cervical
Cancer Study Group: Epidemiologic classification of human
papilomavirus types associated with cervical cancer. N Engl J Med.
348:518–527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ronco G, Biggeri A, Confortini M, et al:
Health technology assessment report: HPV DNA based primary
screening for cervical cancer precursors. Epidemiol Prev.
36:e1–e72. 2012.(In Italian).
|
|
3
|
Budenholzer B: ACP Journal Club. Adding
HPV testing to cytology screening reduced ≥grade 3 cervical
intraepithelial neoplasia at 5 years. Ann Intern Med. 157:JC2–JC7.
2012.PubMed/NCBI
|
|
4
|
Leinonen M, Nieminen P, Kotaniemi-Talonen
L, Malila N, Tarkkanen J, Laurila P and Anttila A: Age-specific
evaluation of primary human papillomavirus screening vs.
conventional cytology in a randomized setting. J Natl Cancer Inst.
101:1612–1623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davey DD and Zarbo RJ: Human
papillomarivus testing - are you ready for a new era in cervical
cancer screening? Arch Pathol Lab Med. 127:927–929. 2003.
|
|
6
|
Damasus-Awatai G and Freeman-Wang T: Human
papillomavirus and cervical screening. Curr Opin Obstet Gynecol.
15:473–477. 2003. View Article : Google Scholar
|
|
7
|
Petry KU, Menton S, Menton M, et al:
Inclusion of HPV testing in routine cervical cancer screening for
woman above 29 years in Germany: results for 8,466 patients. Br J
Cancer. 88:1570–1577. 2003.PubMed/NCBI
|
|
8
|
Wright TC Jr, Schiffman M, Solomon D, et
al: Interim guidance for the use of human papillomavirus DNA
testing as an adjunct to cervical cytology for screening. Obstet
Gynecol. 103:304–309. 2004. View Article : Google Scholar
|
|
9
|
Saslow D, Runowicz CD, Solomon D, et al:
American Cancer Society guideline for the early detection of
cervical neoplasia and cancer. CA Cancer J Clin. 52:342–362. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sherman ME, Lorincz AT, Scott DR, et al:
Baseline cytology, human papillomavirus testing, and risk for
cervical neoplasia: a 10-year cohort analysis. J Natl Cancer Inst.
95:46–52. 2003.PubMed/NCBI
|
|
11
|
Sharma M, Bruni L, Diaz M, Castellsaqué X,
de Sanjosé SD, Bosch FX and Kim JJ: Using HPV prevalence to predict
cervical cancer incidence. Int J Cancer. Sep 11–2012.(Epub ahead of
print). View Article : Google Scholar
|
|
12
|
Castle PE, Schiffman M, Burk RD, et al:
Restricted cross-reactivity of hybrid capture 2 with nononcogenic
human papillomavirus types. Cancer Epidemiol Biomarkers Prev.
11:1394–1399. 2002.PubMed/NCBI
|
|
13
|
Poljak M, Marin IJ, Seme K and Vince A:
Hybrid Capture II HPV test detects at least 15 human papillomavirus
genotypes not included in its current high-risk probe cocktail. J
Clin Virol. 25:S89–S97. 2002. View Article : Google Scholar : PubMed/NCBI
|