Introduction
Capecitabine (Xeloda®), an oral
fluoropyrimidine, is a systemic prodrug of 5-fluorouracil (5-FU),
with the advantage of oral vs. intravenous administration, which is
required for 5-FU. Capecitabine is effective in the treatment of
various malignancies, including colorectal, breast and gastric
cancer (1–4). Although capecitabine is generally
well-tolerated, hand-foot syndrome (HFS) is the most common adverse
event associated with capecitabine and was proven to be a chronic
dose-limiting toxicity, leading to significant morbidity in
patients receiving this type of treatment (5).
HFS, also referred to as palmar-plantar
erythrodysesthesia (PPE), is a distinctive toxic reaction
associated with certain chemotherapeutic agents (6). The incidence of HFS associated with
capecitabine is 50–60% and the incidence of severe HFS (≥ grade 3)
may be 10–70% (7). HFS is
dose-dependent and its occurrence is determined by peak drug
concentration and total cumulative dose (8). The mechanism underlying the
development of HFS has not been elucidated, although the majority
of researchers consider it to be an inflammatory reaction. The
mainstay of the management of HFS is temporary treatment
interruption and, if necessary, dose reduction. Topical agents,
such as potent topical steroids and emollients are occasionally
used to reduce pain and discomfort and protect against
infections.
Since HFS was found to resemble a rat disease
(acrodynia) caused by pyridoxine (vitamin B6) deficiency,
capecitabine-associated HFS was empirically treated with pyridoxine
(9,10). Several studies investigated whether
pyridoxine is able to reduce the incidence of capecitabine-induced
HFS (15,16,30–32).
However, the results of those studies were inconclusive and no
consensus was reached. The purpose of our study was to determine
whether pyridoxine therapy is able to prevent the development of
HFS in patients being treated with capecitabine. In addition, we
performed analyses of publication bias and heterogeneity between
published studies.
Materials and methods
Search strategy and study selection
The following databases were searched using the
medical subject headings and search tags ‘capecitabine’ or
‘Xeloda’, ‘pyridoxine’ or ‘vitamin B6’, ‘hand-foot syndrome’ or
‘palmar-plantar erythrodysesthesia’: PubMed, Embase, Web of
Science, the Cochrane Library, the American Society of Clinical
Oncology (ASCO) and the ASCO Gastrointestinal Cancers Symposium.
The upper date limit of March, 2013 was applied, with no lower date
limit. A manual search for general reviews on HFS associated with
capecitabine and references cited by the included studies were also
used to complete the search. The search results were downloaded to
reference databases and then screened.
In order to be eligible for inclusion in this
meta-analysis, a study was required to meet the following criteria:
i) the patients received chemotherapy that included capecitabine;
ii) the trials included a treatment group receiving pyridoxine
during chemotherapy and a control group that did not receive
pyridoxine; iii) the evaluation of HFS in the published trials
adopted the toxicity grading of the National Cancer Institute
Common Toxicity Criteria (NCI-CTC). Trials were excluded if they
did not meet the criteria described above. Additional exclusion
criteria included the following: i) animal or in vitro
studies; ii) not primary studies (e.g., review articles, letters to
the editor); or iii) duplicate publications of other studies
previously identified in our systematic evaluation. To identify
multiple publications from the same data sets, we investigated all
author names, different institutions involved and the time period
of patient recruitment of each study. When the same author reported
results from the same patient population, the most recent or the
most complete study was included. The abstracts of all candidate
studies were read by two independent readers (L.P. and Y.Z.).
Studies that could not be classified based on the title and
abstract alone were retrieved for full-text review. Disagreements
were resolved by consensus between the two readers.
Data extraction
The final studies included were independently
assessed by two readers (L.P. and Y.Z.). Information was carefully
retrieved from the studies, using a standardized data collection
form, including the following items: first author, year of
publication, country of origin, number of patients allocated,
patient characteristics and chemotherapeutic regimen. If data from
any of the above categories were not reported in the study, the
items were treated as ‘not specified’. To ensure accuracy, targeted
data were extracted by two authors working independently. If the
study had more than one grade criteria, only data regarding HFS
incidence graded on the basis of the NCI-CTC were extracted. The
authors of the primary studies were not contacted for additional or
unreported information. We did not use prespecified quality-related
inclusion or exclusion criteria and did not weigh each study by a
quality score, since the quality score has not received general
approval for use in meta-analyses, particularly observational
studies.
Statistical methods
The primary outcome for analysis was the incidence
of HFS in patients receiving capecitabine. Dichotomous variables
were analyzed with odds ratios (ORs) with mean differences at a 95%
confidence interval (CI). The calculations were performed under the
hypothesis that the variance of the two groups was identical
(11). Two-sided P-values were
computed for the differences between dichotomous variables, which
were considered significant at P<0.05. The heterogeneity of the
individual ORs was calculated with χ2 tests according to
Peto’s method (12). A
heterogeneity test with inconsistency index (I2)
statistic and Q statistic was performed. If P>0.10 or
I2≤50%, the heterogeneity of the trial was considered
acceptable and the differences between the OR and 95% CI were
computed by the fixed-effects model. If P≤0.10 or
I2>50%, the differences between the OR and 95% CI
were computed by the random-effects model.
Publication bias was identified with funnel plots
(13,14), whereby asymmetries in the funnel
plot indicated publication bias. Intercept significance was
determined by the t-test suggested by Egger (P<0.05 was
considered representative of statistically significant publication
bias). All the calculations were performed by Stata software
version 11.0 (StataCorp, College Station, TX, USA).
Results
Study selection and characteristics
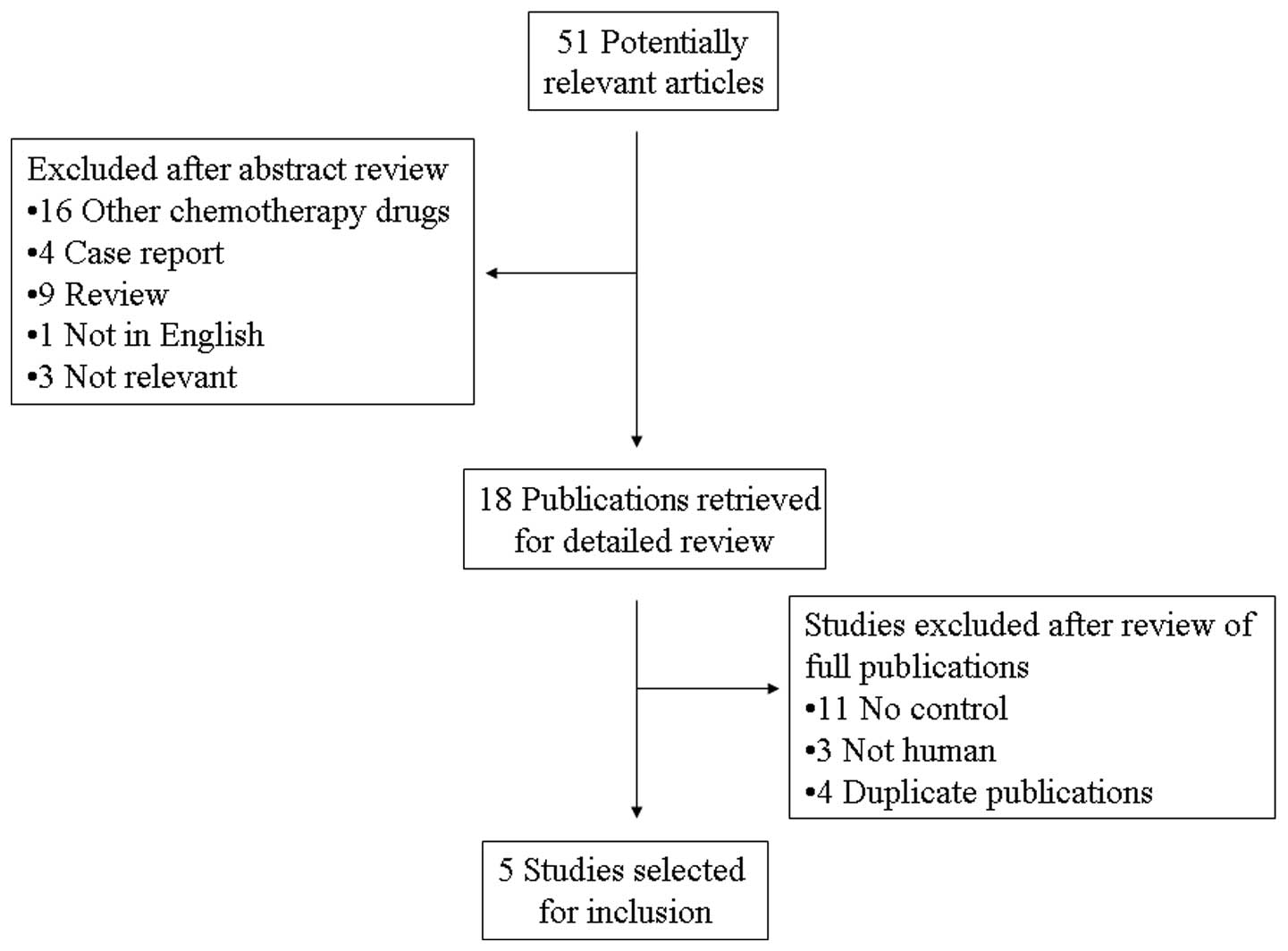
A total of 51 potentially relevant citations were
reviewed and five studies met the inclusion criteria in the search
strategy and study selection section, comprising 793 patients for
the final analysis (Fig. 1). Of the
five studies, three were prospective randomized control trials,
whereas the remaining two were retrospective studies. The major
baseline characteristics of the five eligible publications are
presented in Table I. The studies
were conducted in four countries (Japan, Korea, the United States
of America and the United Kingdom) and were published between 2003
and 2012. The dose of pyridoxine varied from 50 to 600 mg/day. All
the studies reported the incidence of HFS in patients receiving
capecitabine. As regards chemotherapy outcome, two trials (15,16)
compared the treatment outcome between the two groups according to
the response evaluation criteria in solid tumors (17). The characteristics of the included
trials are listed in Table I. The
NCI-CTC criteria are listed in Table
II.
 | Table IMain characteristics and results of
the eligible studies. |
Table I
Main characteristics and results of
the eligible studies.
| Study | Country | Site of tumor | Type of study | Chemotherapy
regimen | Enrolled
patients | Dose of pyridoxine
(mg/day) | Refs. |
|---|
|
|---|
| Pyridoxine | Control |
|---|
| Mortimer et
al | United States | Colon, breast | Retrospective | NS | 73 | 99 | 50–600 | (15) |
| Yoshimoto et
al | Japan | NS | Retrospective | X or X with CTX and
EPI | 38 | 40 | 60 | (30) |
| Kang et
al | Korea | Stomach, colon,
biliary tract, duodenum | RCT | X, XP, DXP | 180 | 180 | 200 | (31) |
| Braik et
al | United States | NS | RCT | NS | 38 | 39 | 100 | (32) |
| Corrie et
al | United Kingdom | Colorectum,
breast | RCT | NS | 53 | 53 | 150 | (16) |
 | Table IIHFS grading according to the National
Cancer Institute Common Toxicity Criteria (NCI-CTC) version
4.0. |
Table II
HFS grading according to the National
Cancer Institute Common Toxicity Criteria (NCI-CTC) version
4.0.
| Severity | Criteria |
|---|
| Grade 1 | Minimal skin changes
or dermatitis (e.g., erythema, edema or hyperkeratosis) without
pain |
| Grade 2 | Skin changes (e.g.,
peeling, blisters, bleeding, edema or hyperkeratosis) with pain;
limiting instrumental ADL |
| Grade 3 | Severe skin changes
(e.g., peeling, blisters, bleeding, edema or hyperkeratosis) with
pain; limiting self care ADL |
Meta-analysis
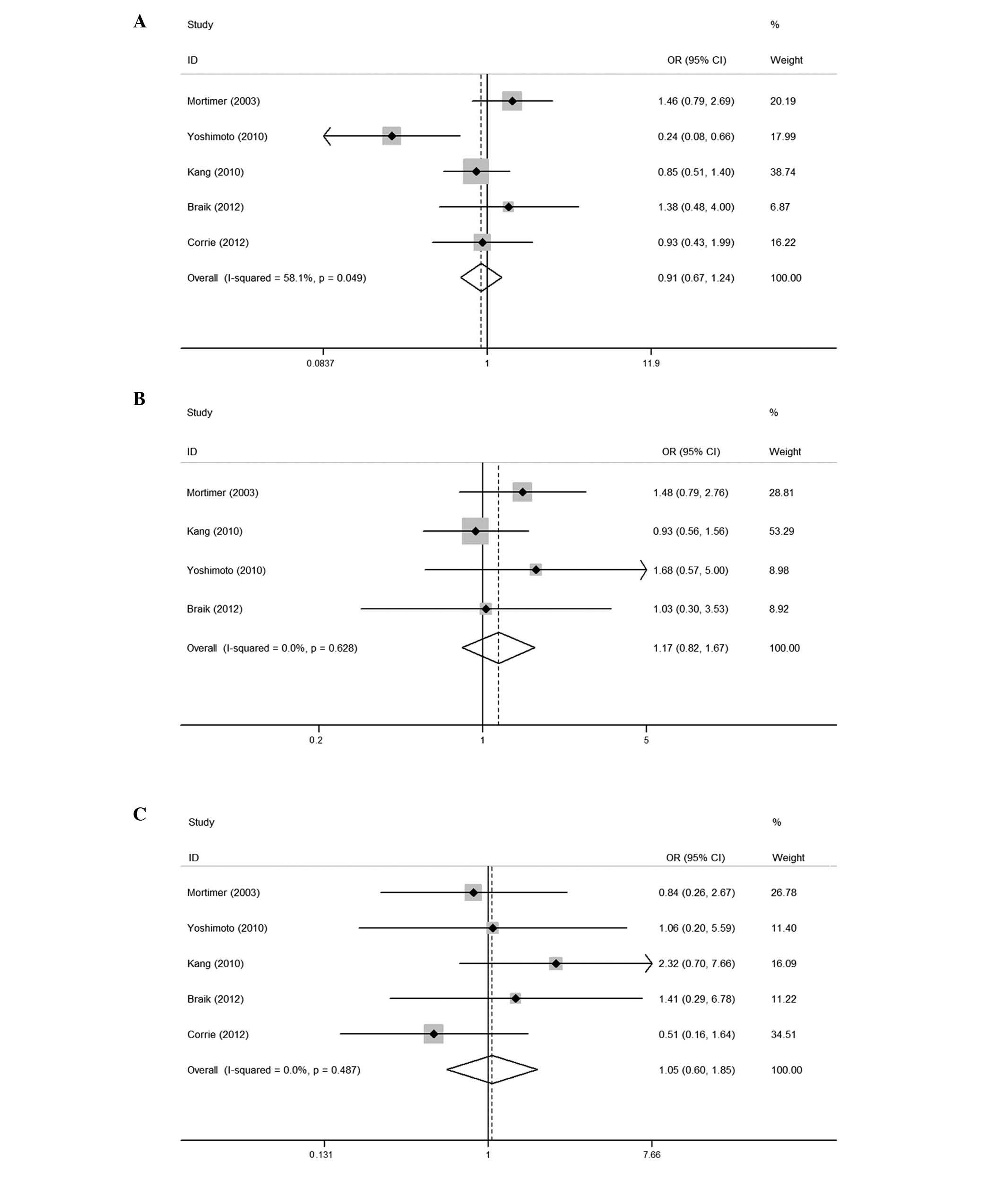
The results of the meta-analysis are shown in
Fig. 2. The five trials included in
this study evaluated capecitabine-induced HFS according to the
NCI-CTC. The pooled analysis revealed that, compared to the
placebo, pyridoxine did not reduce the number of patients with HFS
of all grades, with an OR of 0.91 (95% CI: 0.67–1.24). The combined
ORs for the studies evaluating the prophylactic use of pyridoxine
in preventing capecitabine-associated HFS of ≥ grade 2 and severe
(≥ grade 3) were 1.17 (95% CI: 0.82–1.67) and 1.05 (95% CI:
0.60–1.85), respectively, indicating that pyridoxine was not
effective in the prevention against HFS of any grade. No
significant heterogeneity was observed among the studies (all
grades: Q=9.55, I2=58.1%, P=0.049; ≥ grade 2: Q=1.74,
I2=0.0%, P=0.628; and ≥ grade 3: Q=3.44,
I2=0.0%, P=0.487).
Treatment outcomes of chemotherapy
The effects of chemotherapy on unresectable tumors
in the placebo and pyridoxine groups were compared between two
trials. No significant differences were observed in the response
rates, disease control rates or survival times between the two
groups. The results indicated that the use of pyridoxine did not
appear to increase the recurrence of tumors.
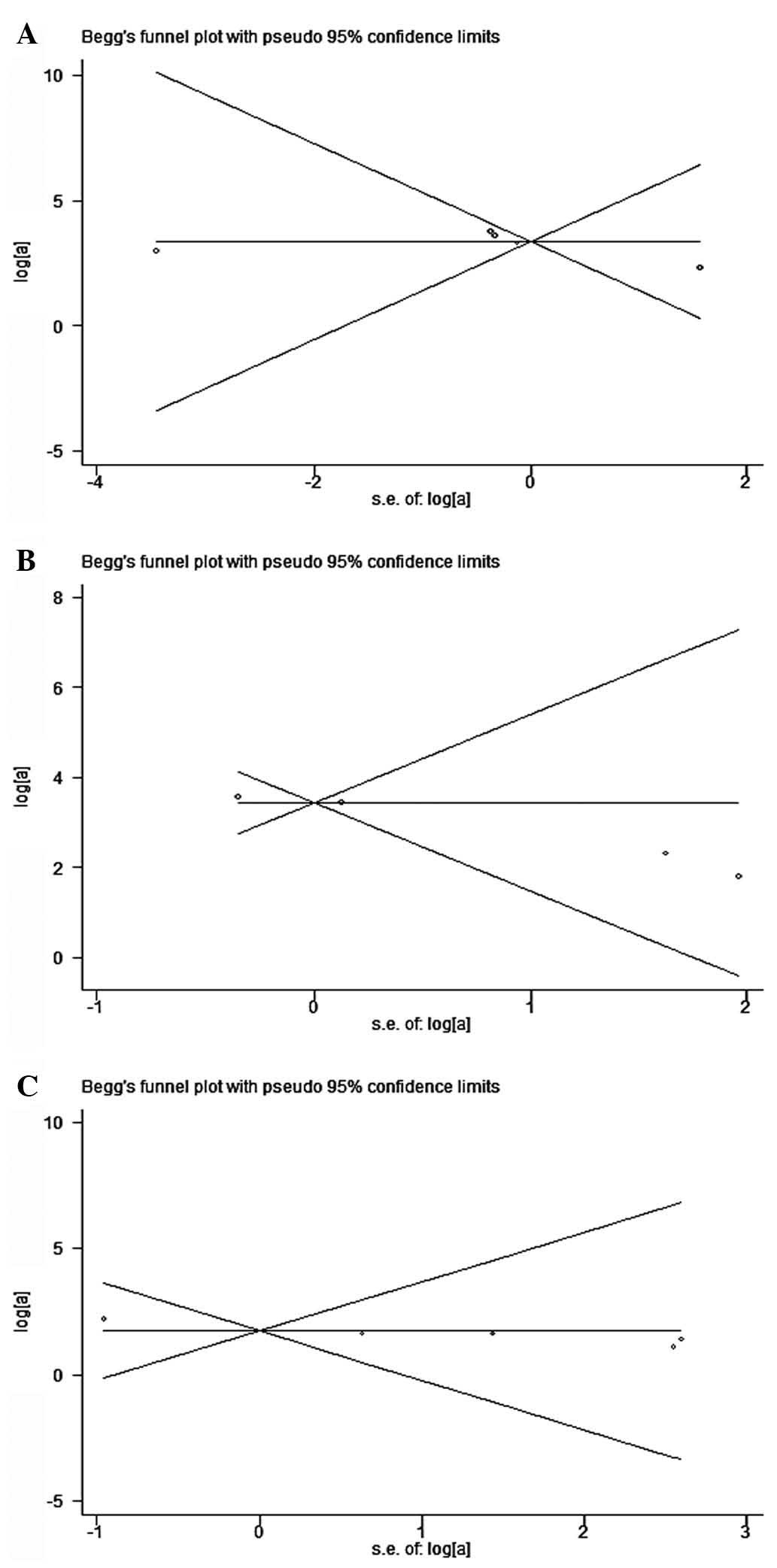
Publication bias
Begg’s funnel plot and Egger’s test were performed
to evaluate the publication bias of the eligible studies (Fig. 3). The Egger’s test score of
pyridoxine in the prevention against capecitabine-induced HFS of
all grades, ≥ grade 2 and severe (≥ grade 3) yielded P-values of
0.200, 0.11 and 0.016, respectively, indicating that there was
publication bias in severe HFS.
Discussion
Capecitabine is an oral fluoropyrimidine that is
used to treat various types of cancer. Chemotherapy regimens
including capecitabine are currently considered to be a standard of
care in the management of colorectal, breast and gastric cancers.
However, capecitabine-induced HFS is a commonly occurring adverse
event that affects the quality of life of the patients and may lead
to postponement or even interruption of capecitabine therapy.
Hand-foot syndrome, also referred to as palmar-plantar
erythrodysesthesia or palmoplantar keratoderma, is a dose-limiting
adverse effect associated with capecitabine. Several
chemotherapeutic drugs have been identified as a cause of HFS, such
as doxorubicin, liposomal doxorubicin, docetaxel and
fluoropyrimidine (18–21). The clinical manifestations present
as dysesthesia, followed by painful, symmetric, well-defined
erythema and edema. The median time of onset is 79 days, although
the range may be 11–360 days (22).
HFS is an emerging issue in capecitabine cancer treatment, leading
to additional morbidity, suboptimal dosing and poor compliance with
treatment.
The mechanism underlying the development of HFS has
not been elucidated. One suggested hypothesis was that the
keratinocytes in the skin may upregulate the enzyme thymidine
phosphorylase, which increases the accumulation of capecitabine
metabolites. Another hypothesis was that capecitabine may be
excreted by sweat glands, which are profuse in the palms and the
soles (7). It was also hypothesized
that HFS is induced by capecitabine metabolites through a
prostaglandin-like action (23).
The treatment for capecitabine-induced HFS is dose
reduction or permanent discontinuation of capecitabine. Topical
agents, such as steroids, emollients and 99% dimethyl sulfoxide are
occasionally used for relief (24).
Since HFS was found to resemble a rat disease (acrodynia) caused by
pyridoxine deficiency, capecitabine-induced HFS was empirically
treated with pyridoxine. Animal studies, case reports and other
small studies previously suggested that the administration of
pyridoxine may help prevent and treat HFS induced by chemotherapy
drugs (9,10,25–27).
It appears to be effective in preventing against HFS in at least
some individuals who experience this side effect. As pyridoxine is
a safe nutritional supplement, its prophylactic use seems
appealing. Pyridoxine is often used to treat HFS associated with
capecitabine; however, the evidence regarding its beneficial effect
is not adequate. There is no consensus to prove that pyridoxine
prevents or treats HFS. Therefore, the present meta-analysis was
conducted to determine the effect of prophylactic pyridoxine
administration in reducing the incidence of HFS in patients
receiving capecitabine.
The present meta-analysis, which was based on data
provided by five studies including a total of 793 patients to yield
statistics, compared the administration of pyridoxine to placebo
regarding the prevention of capecitabine-induced HFS. Pyridoxine
did not provide any marked benefit against capecitabine-induced
HFS. An important issue in these studies was that the dose of
pyridoxine varied from 50 to 600 mg/day. Long-term use of high
doses of pyridoxine may lead to the development of additional side
effects or health problems or interfere with the absorption and use
of other important nutrients. Since a preclinical model or
pharmacodynamic data on the dose-response relationship of
pyridoxine are not available, the optimal dose of pyridoxine cannot
be determined.
Another controversial issue was the possible effect
of pyridoxine on tumor response and patient survival. The
investigation on the correlation between HFS and clinical outcome
has been limited. It was suggested that patients who were
administered high doses of pyridoxine exhibited worse tumor
response (26). In our
meta-analysis, two of the primary studies evaluated tumor response
in the pyridoxine vs. placebo groups, which revealed no difference
in the tumor response rate. This may be explained by the different
doses of pyridoxine used in the studies, which requires further
investigation.
Our meta-analysis has several limitations. First,
two of the included studies were not randomized controlled trials
but retrospective studies. Retrospective studies limit confidence
in the further clinical utility of pyridoxine. Second, although
there was no significant heterogeneity among the primary studies,
it should be noted that, due to the small number of primary studies
analyzed in each group, the power of detection of potentially
important differences was limited. Third, the meta-analysis relied
on publication, rather than individual patient data (IPD).
Therefore, the results must be interpreted with caution, since the
IPD-based analysis provides the least bias and is more reliable
compared to the literature-based meta-analysis (28).
Biases should also be handled with caution. First,
publication bias is a major concern in all forms of meta-analysis,
as published studies are often positive (29). As for the results of insignificant
publication bias, it should be noted that when the sample size of
the studies or the number of eligible studies is limited, the power
of detecting publication bias by the linear regression model is
reduced. Considering the limited number of studies included, the
findings from our meta-analysis require confirmation by further
studies. Furthermore, the language bias could not be completely
avoided, as the inclusion criteria was restricted to studies
published in English. A selection process with rigid inclusion
criteria was adopted in ascertaining studies, thereby reducing
selection bias.
In conclusion, to the best of our knowledge, our
meta-analysis is the first study to systematically evaluate the
efficacy of prophylactic pyridoxine in preventing
capecitabine-induced HFS. Our results led to the conclusion that
pyridoxine was not able to prevent capecitabine-induced HFS.
Therefore, the use of pyridoxine may not be currently recommended
for prophylaxis against HFS. To strengthen our findings,
well-designed prospective studies may help determine the efficacy
of pyridoxine in the prevention of capecitabine-induced HFS.
Acknowledgements
We are indebted to the authors of the primary
studies. This study was supported by the Medical Science Research
Foundation of the Health Bureau of Zhejiang Province (no.
2012KYA072) and the Administration of Traditional Chinese Medicine
of Zhejiang Province (no. 2012ZB084).
References
|
1
|
Van Cutsem E, Twelves C, Cassidy J, et al:
Oral capecitabine compared with intravenous fluorouracil plus
leucovorin in patients with metastatic colorectal cancer: results
of a large phase III study. J Clin Oncol. 19:4097–4106. 2001.
|
|
2
|
Blum JL, Dieras V, Lo Russo PM, et al:
Multicenter, phase II study of capecitabine in taxane-pretreated
metastatic breast carcinoma patients. Cancer. 92:1759–1768. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Twelves C, Wong A, Nowacki MP, et al:
Capecitabine as adjuvant treatment for stage III colon cancer. N
Engl J Med. 352:2696–2704. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bang YJ: Capecitabine in gastric cancer.
Expert Rev Anticancer Ther. 11:1791–1806. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scheithauer W and Blum J: Coming to grips
with hand-foot syndrome. Insights from clinical trials evaluating
capecitabine. Oncology (Williston Park). 18:1161–1168; discussion
1173–1176. 1181–1184. 2004.PubMed/NCBI
|
|
6
|
Nagore E, Insa A and Sanmartin O:
Antineoplastic therapy-induced palmar plantar erythrodysesthesia
(‘hand-foot’) syndrome. Incidence, recognition and management. Am J
Clin Dermatol. 1:225–234. 2000.
|
|
7
|
Gressett SM, Stanford BL and Hardwicke F:
Management of hand-foot syndrome induced by capecitabine. J Oncol
Pharm Pract. 12:131–141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiara S, Nobile MT, Barzacchi C, et al:
Hand-foot syndrome induced by high-dose, short-term, continuous
5-fluorouracil infusion. Eur J Cancer. 33:967–969. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vukelja SJ, Lombardo FA, James WD and
Weiss RB: Pyridoxine for the palmar-plantar erythrodysesthesia
syndrome. Ann Intern Med. 111:688–689. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fabian CJ, Molina R, Slavik M, Dahlberg S,
Giri S and Stephens R: Pyridoxine therapy for palmar-plantar
erythrodysesthesia associated with continuous 5-fluorouracil
infusion. Invest New Drugs. 8:57–63. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yusuf S, Peto R, Lewis J, Collins R and
Sleight P: Beta blockade during and after myocardial infarction: an
overview of the randomized trials. Prog Cardiovasc Dis. 27:335–371.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Macaskill P, Walter SD and Irwig L: A
comparison of methods to detect publication bias in meta-analysis.
Stat Med. 20:641–654. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mortimer JE, Lauman MK, Tan B, Dempsey CL,
Shillington AC and Hutchins KS: Pyridoxine treatment and prevention
of hand-and-foot syndrome in patients receiving capecitabine. J
Oncol Pharm Pract. 9:161–166. 2003. View Article : Google Scholar
|
|
16
|
Corrie PG, Bulusu R, Wilson CB, et al: A
randomised study evaluating the use of pyridoxine to avoid
capecitabine dose modifications. Br J Cancer. 107:585–587. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
18
|
Lokich JJ and Moore C:
Chemotherapy-associated palmar-plantar erythrodysesthesia syndrome.
Ann Intern Med. 101:798–799. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Banfield GK, Crate ID and Griffiths CL:
Long-term sequelae of palmar-plantar erythrodysaesthesia syndrome
secondary to 5-fluorouracil therapy. J R Soc Med. 88:356P–357P.
1995.PubMed/NCBI
|
|
20
|
Zimmerman GC, Keeling JH, Burris HA, et
al: Acute cutaneous reactions to docetaxel, a new chemotherapeutic
agent. Arch Dermatol. 131:202–206. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gordon KB, Tajuddin A, Guitart J, Kuzel
TM, Eramo LR and VonRoenn J: Hand-foot syndrome associated with
liposome-encapsulated doxorubicin therapy. Cancer. 75:2169–2173.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abushullaih S, Saad ED, Munsell M and Hoff
PM: Incidence and severity of hand-foot syndrome in colorectal
cancer patients treated with capecitabine: a single-institution
experience. Cancer Invest. 20:3–10. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin E, Morris JS and Ayers GD: Effect of
celecoxib on capecitabine-induced hand-foot syndrome and antitumor
activity. Oncology (Williston Park). 16:31–37. 2002.PubMed/NCBI
|
|
24
|
Lopez AM, Wallace L, Dorr RT, Koff M,
Hersh EM and Alberts DS: Topical DMSO treatment for pegylated
liposomal doxorubicin-induced palmar-plantar erythrodysesthesia.
Cancer Chemother Pharmacol. 44:303–306. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Andres R, Mayordomo JI, Lara R, et al:
Gemcitabine/capecitabine in patients with metastatic breast cancer
pretreated with anthracyclines and taxanes. Clin Breast Cancer.
6:158–162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chalermchai T, Tantiphlachiva K,
Suwanrusme H, Voravud N and Sriuranpong V: Randomized trial of two
different doses of pyridoxine in the prevention of
capecitabine-associated palmar-plantar erythrodysesthesia. Asia Pac
J Clin Oncol. 6:155–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rossi D and Catalano G: Pyridoxine as
prophylactic therapy for palmar-plantar erythrodysesthesia
associated with administration of pegylated liposomal doxorubicin
(caelyx): a single-center experience. Oncology. 73:277–278. 2007.
View Article : Google Scholar
|
|
28
|
Stewart LA and Parmar MK: Meta-analysis of
the literature or of individual patient data: is there a
difference? Lancet. 341:418–422. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoshimoto N, Yamashita T, Fujita T, et al:
Impact of prophylactic pyridoxine on occurrence of hand-foot
syndrome in patients receiving capecitabine for advanced or
metastatic breast cancer. Breast Cancer. 17:298–302. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kang YK, Lee SS, Yoon DH, et al:
Pyridoxine is not effective to prevent hand-foot syndrome
associated with capecitabine therapy: results of a randomized,
double-blind, placebo-controlled study. J Clin Oncol. 28:3824–3829.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Braik T, Yim B, Evans AT, et al: A
randomized trial to determine if vitamin B6 can prevent hand and
foot syndrome in cancer patients treated with capecitabine
chemotherapy. J Clin Oncol. 30(Suppl): abstr 9085. 2012.
|