Introduction
Bone morphogenetic protein 7 (BMP7) is a member of
the transforming growth factor-β (TGF-β) superfamily and was
initially identified as a protein that may induce bone and
cartilage growth in the bone matrix. Previous studies suggested
that BMP7 acts on the skeletal system and is also expressed in the
neural tissues of adult animals together with its receptor
(1), playing an important role in
the process of neural histogenesis (2). It was previously confirmed that the
injection of BMP7 into the cisterns prior to focal cerebral
ischemia in adult rats significantly improved motor function and
reduced the volume of the cerebral infarct. The injection of BMP7
into the cisterns at 24 h following ischemia did not reduce the
volume of the cerebral infarct, although it significantly promoted
the motor function recovery of the affected rat limbs (3,4). Chou
et al(5) reported that BMP7
promoted the proliferation of neural progenitor cells in the
infarction region-contralateral subventricular zone of middle
cerebral artery occlusion (MCAO) rats and observed that the
proliferated neural progenitor cells migrated to the cortex of the
region surrounding the infarction through the corpus callosum.
Nestin is considered to be one of the markers of neural precursor
cells (6) and nestin-positive cells
may be considered as neural progenitor cells. The glial fibrillary
acidic protein (GFAP) is a specific cytoskeletal protein mainly
present in astrocytes and it is considered as a marker of astrocyte
maturation (7). The astrocytes are
activated at the time of cerebral ischemia-reperfusion injury,
reflected by hypertrophic and edematous astrocyte bodies, increased
and extended apoptosis and enhanced GFAP expression demonstrated by
immunohistochemical staining. The significance of GFAP may be
closely associated with the repair of the central lesion. The aim
of this experiment was to investigate the effect of BMP7, injected
via the caudal vein at 2 h following focal cerebral ischemia, on
the expression of nestin and GFAP after cerebral
ischemia-reperfusion injury in adult rats and assess the
neuroprotective effect of BMP7 following cerebral
ischemia-reperfusion injury, as well as its underlying
mechanism.
Materials and methods
Animal model
A total of 40 adult healthy male Sprague-Dawley rats
of clean grade, weighing 230–250 g, were provided by the Qingdao
Experimental Animal Center (2009SCXK0010). The animals were kept in
the laboratory at room temperature (23±2°C) under natural
illumination during an acclimatization period of 1 week prior to
the experiment. Ten rats randomly received a sham operation and the
remaining 30 rats were subjected to a 2-h ischemia and 24-h
reperfusion by ligation of left external and internal carotid
arteries (8). The Bederson’s scale
(9) was adopted to evaluate the
neurological deficits after the postoperative animals regained
consciousness and 20 animals (10 unsuccessful animals were
excluded) with a Bederson’s score of ≥2 were equally randomized
into the BMP7 treatment and control groups.
This study was reviewed and approved by our
Institutional Animal Care and Use Committee.
Drug administration
BMP7 (BS0504P; Beijing Biosynthesis Biotechnology
Co. Ltd., Beijing, China), as the investigational drug, was
dissolved in sterile water for injection. The rats in the treatment
group were intervened with 250 μl BMP7 (0.1 mg/kg) via tail vein
injection using a microsyringe immediately after the 2 h-ischemia
reperfusion, whereas the rats in the control and sham operation
groups were injected with an equal volume of sterile water for
injection.
Neurological deficit scoring
The Bederson’s scale (9) was used for neurological deficit
scoring after the animals had regained consciousness: grade 0, no
signs of neurological deficits; grade 1, rats with infarction
flexed the forelimb contralateral to the injured hemisphere when
their tails were raised, while extending the normal forelimb to the
ground; grade 2, apart from the signs of grade 1, there was a
significantly decreased resistance to lateral push without
circling; grade 3, rats exhibited the same behavior as in grade 2,
with circling.
Triphenyl tetrazolium chloride (TTC)
staining
Five animals were selected from each group and were
anesthetized by intraperitoneal injection of 0.3 ml 10% chloral
hydrate at 24 h after reperfusion. The rats were decapitated and
the brains were removed. Five continuous coronal brain sections
were obtained at 2-mm intervals, starting 2 mm from the polus
frontalis. The sections were immersed in 1% TTC solution and
placed in a 37°C incubator for 30 min. The staining effect was as
follows: normal brain tissue appeared red, whereas infarcted brain
tissue appeared more pale. After capturing images of the stained
brain tissue sections, the Image-Pro Plus image analysis system,
version 6.0 (Fryer Co, Chicago, IL, USA) was used to calculate the
infarction area and the total brain section area in each brain
sample. The following formula was used to calculate the infarction
area per brain section and the total brain section area and,
subsequently, the percentage of cerebral infarct volume of each
rat: percentage of cerebral infarct volume = (infarction area of
brain section/total area of brain section) ×100%.
Preparation of paraffin sections
Five animals were selected from each group,
anesthetized with an intraperitoneal injection of 0.3 ml 10%
chloral hydrate and positioned in dorsal recumbency. Physiological
saline and 4% formaldehyde solution were administered to perform
cardiac perfusion fixation. The rats were decapitated and their
brains were removed. Ethanol was used for dehydration at the
conventional gradient, xylene for vitrification and paraffin for
embedding. Continuous coronal 2-μm sections were obtained from the
posterior of the optic chiasma, mounted on microscopic slides,
treated with polylysine and stored for later use.
Apoptosis detection
The sections were routinely deparaffinized and
hydrated. After washing with distilled water, apoptosis assessment
was conducted according to the instructions in the terminal
deoxynucleotidyl transferase (TdT)-mediated biotinylated
deoxyuridine triphosphate nick end-labelling (TUNEL) apoptosis test
kit (Wuhan Boster Biotech. Co. Ltd., Wuhan, China). Cells with
nuclei containing brown particles observed under a light microscope
were considered as positive. In some sections, TdT was replaced by
0.01 mol/l−1 phosphate-buffered saline (PBS), which
yielded no positive reaction. Four sections per rat were obtained
and positive cells were counted in four random visual fields in the
cortex, corpus striatum and hippocampus under high-power
magnification.
Immunohistochemical staining
The sections mentioned above were routinely
deparaffinized and hydrated. After washing with distilled water,
nestin and GFAP immunohistochemical staining was performed in
compliance with the instructions (Wuhan Boster Biotech. Co. Ltd.).
Cells with cytoplasm or nuclei containing brown particles under a
light microscope were considered as positive. In some sections, the
primary antibody was replaced with 0.01 mol/l PBS, which yielded no
positive reaction. Four sections per rat were obtained and positive
cells were counted in four random visual fields in the cortex,
corpus striatum and hippocampus under a light microscope
(magnification, ×400) to calculate the number of positive cells per
visual field.
Statistical method
SPSS statistical software, version 11.0 (SPSS Inc.,
Chicago, IL, USA) was adopted to conduct data analysis. Data are
expressed as means ± standard deviation. One-factor analysis of
variance was adopted with a significance level (α) of 0.05.
Results
Neurological deficit scores
No postoperative neuromotor dysfunction was
identified in the sham operation group. The majority of the animals
in the model group regained consciousness 2 h after the operation.
The rats developed right limb dysfunction after waking up and
presented with right forelimb flexion when their tails were raised
and suspended in mid-air. The Bederson’s score in the BMP7
treatment group (1.7±0.48) was significantly lower compared to that
in the control group (2.7±0.48), with a statistically significant
difference (t=4.66, P<0.01).
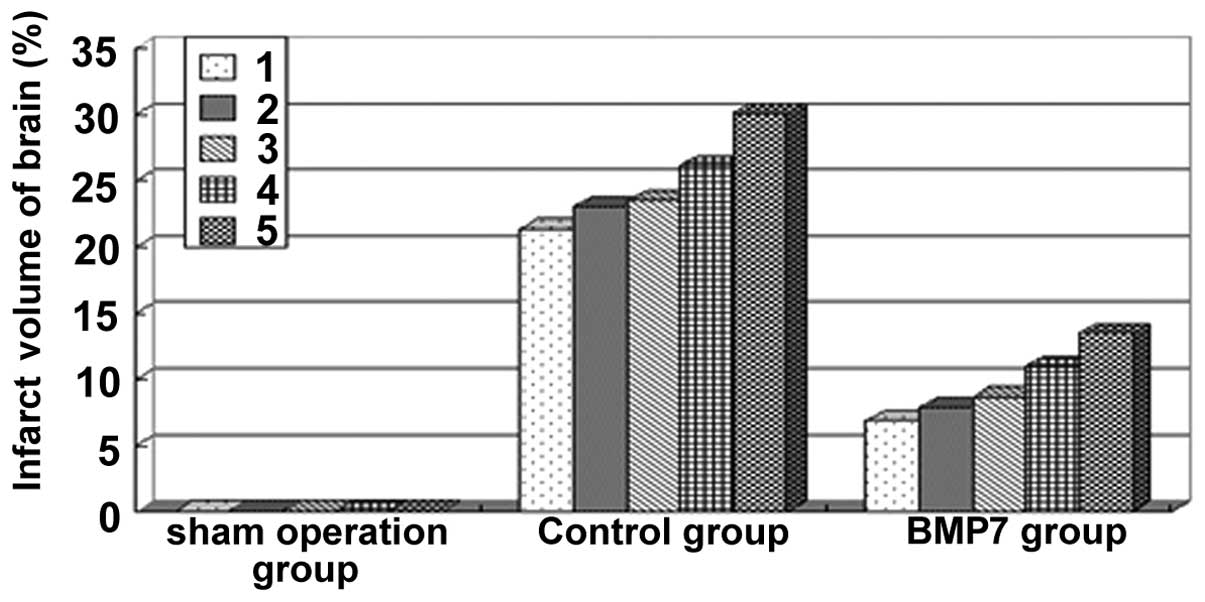
Cerebral infarct volume percentage in
rats from each group
The TTC staining results suggested that the brains
of the rats from the sham operation group were all stained red and
the cerebral infarct volume percentage in the model group was
significant. The cerebral infarct volume percentage in the BMP7
group (9.57±2.67%) was significantly decreased compared to the
control group (24.81±3.41%), with a statistically significant
difference (t=7.87, P<0.01) (Fig.
1).
Number of TUNEL-positive cells
The cells were evenly distributed in the brain
sections from rats in the sham operation group. Scattered
TUNEL-positive cells were identified in the cerebral cortex. The
number of TUNEL-positive cells in the control group was higher
compared to that in the sham operation group and these cells were
mainly distributed in the ischemic cortex, corpus striatum and
hippocampus. The numbers of TUNEL-positive cells in the ischemic
cortex, corpus striatum and hippocampus in the BMP7 treatment group
were significantly lower compared to those in the control group and
the differences were statistically significant (t=17.31, t=12.45
and t=17.38, respectively; P<0.01). Apart from the sham
operation group, the majority of TUNEL-positive cells were located
in the corpus striatum, as determined by intra-group comparison,
followed by the hippocampus and cortex, with a statistically
significant difference (t=2.77–6.71, P<0.05). The results are
shown in Table I.
 | Table ITUNEL-positive cells in brain tissue
(means ± standard deviation per visual field). |
Table I
TUNEL-positive cells in brain tissue
(means ± standard deviation per visual field).
| Groups | n | Cortex | Corpus striatum | Hippocampus |
|---|
| Sham | 5 | 1.8±0.84 | 3.2±0.71 | 3.0±0.84 |
| Control | 5 | 13.4±1.14a | 17.8±1.48a | 15.4±1.14a |
| BMP7 | 5 | 3.6±0.55b | 7.4±1.14b | 5.0±0.70b |
Nestin expression in rat brains
Nestin-positive cells were almost absent in the sham
operation group, whereas increased numbers of nestin-positive cells
were identified in the control group and were mainly distributed in
the ischemic cortex, corpus striatum and hippocampus. The numbers
of nestin-positive cells in the ischemic cortex, corpus striatum
and hippocampus in the BMP7 group were significantly higher
compared to those in the control group, with statistically
significant differences (t=16.03, t=8.28 and t=12.68, respectively;
P<0.01). Except for the sham operation group, the majority of
nestin-positive cells were located in the ischemic cortex, as
determined by intra-group comparison, followed by the corpus
striatum and hippocampus, with statistically significant
differences (t=2.22–7.77, P<0.05). The results are shown in
Fig. 2 and Table II.
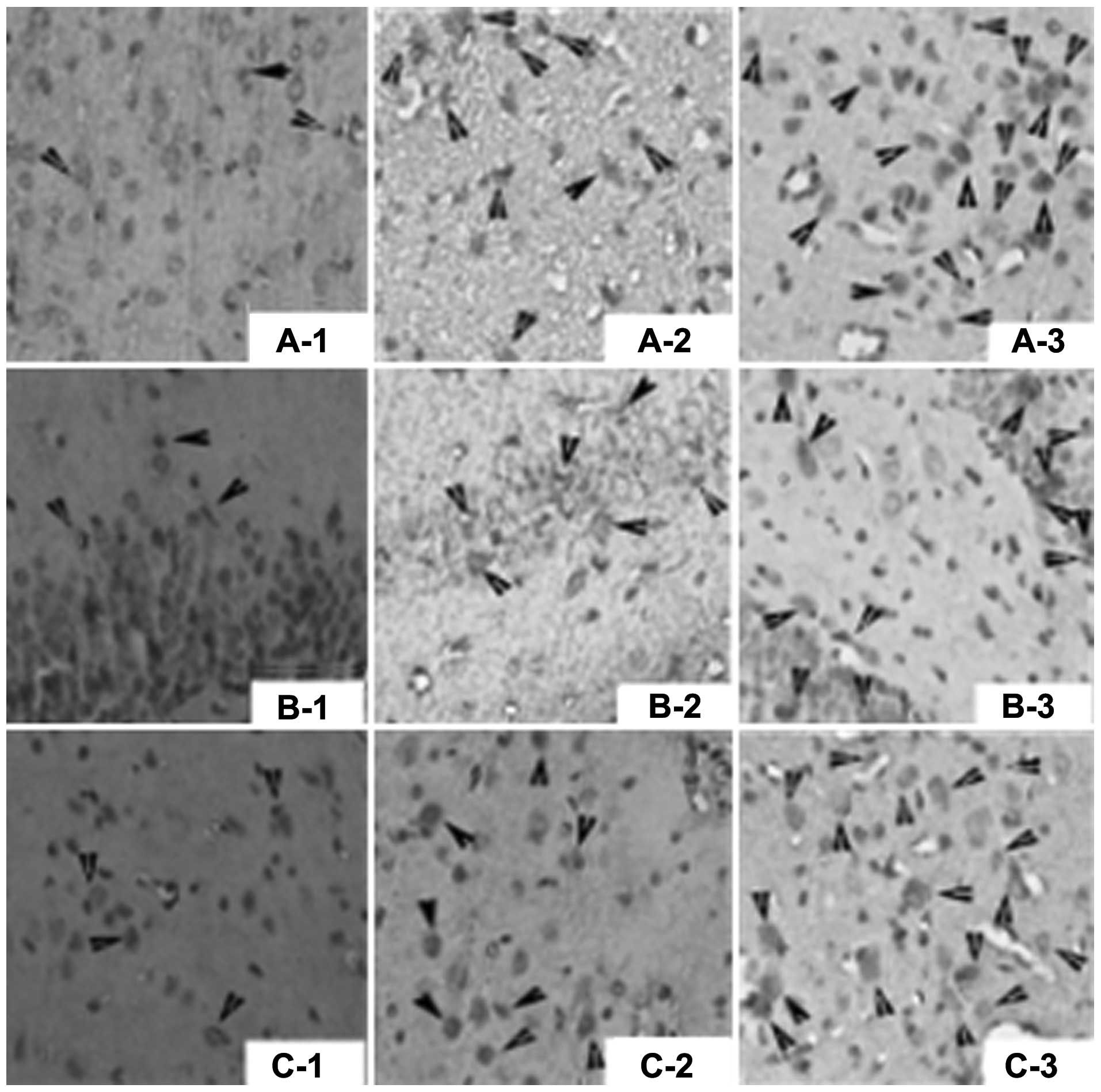 | Figure 2Nestin-positive cells (arrows) shown
by immunohistochemical assay (magnification, ×400). (A)
Nestin-positive cells in the cortex in: A-1, sham operation group;
A-2, control group; and A-3, bone morphogenetic protein 7 (BMP7)
group. (B) Nestin-positive cells in the hippocampus in: B-1, sham
operation group; B-2, control group; and B-3, BMP7 group. (C)
Nestin-positive cells in the striatum in: C-1, sham operation
group; C-2, control group; and C-3, BMP7 group. |
 | Table IINestin-positive cells in brain tissue
(means ± standard deviation per visual field). |
Table II
Nestin-positive cells in brain tissue
(means ± standard deviation per visual field).
| Groups | n | Cortex | Corpus striatum | Hippocampus |
|---|
| Sham | 5 | 2.8±0.84 | 2.8±0.84 | 2.4±0.55 |
| Control | 5 | 8.6±0.55a | 7.2±1.30a | 5.8±0.84a |
| BMP7 | 5 | 15.8±0.84b | 13.6±1.14b | 12.0±0.70b |
GFAP expression in in rat brains
GFAP-positive cells were rare in the sham operation
group (concentration of GFAP antibody, 1:150), whereas increased
numbers of GFAP-positive cells were identified in the control group
and were mainly distributed in the ischemic cortex, corpus striatum
and hippocampus. The numbers of GFAP-positive cells in the ischemic
cortex, corpus striatum and hippocampus in the BMP7 group were
significantly higher compared to those in the control group, with
statistically significant differences (t=9.47, t=20.42 and t=7.73,
respectively; P<0.01). Apart from the sham operation group, the
majority of GFAP-positive cells were located in the hippocampus, as
determined by intra-group comparison, followed by the ischemic
cortex and corpus striatum, with statistically significant
differences (t=2.67–14.64, P<0.05). The results are presented in
Fig. 3 and Table III.
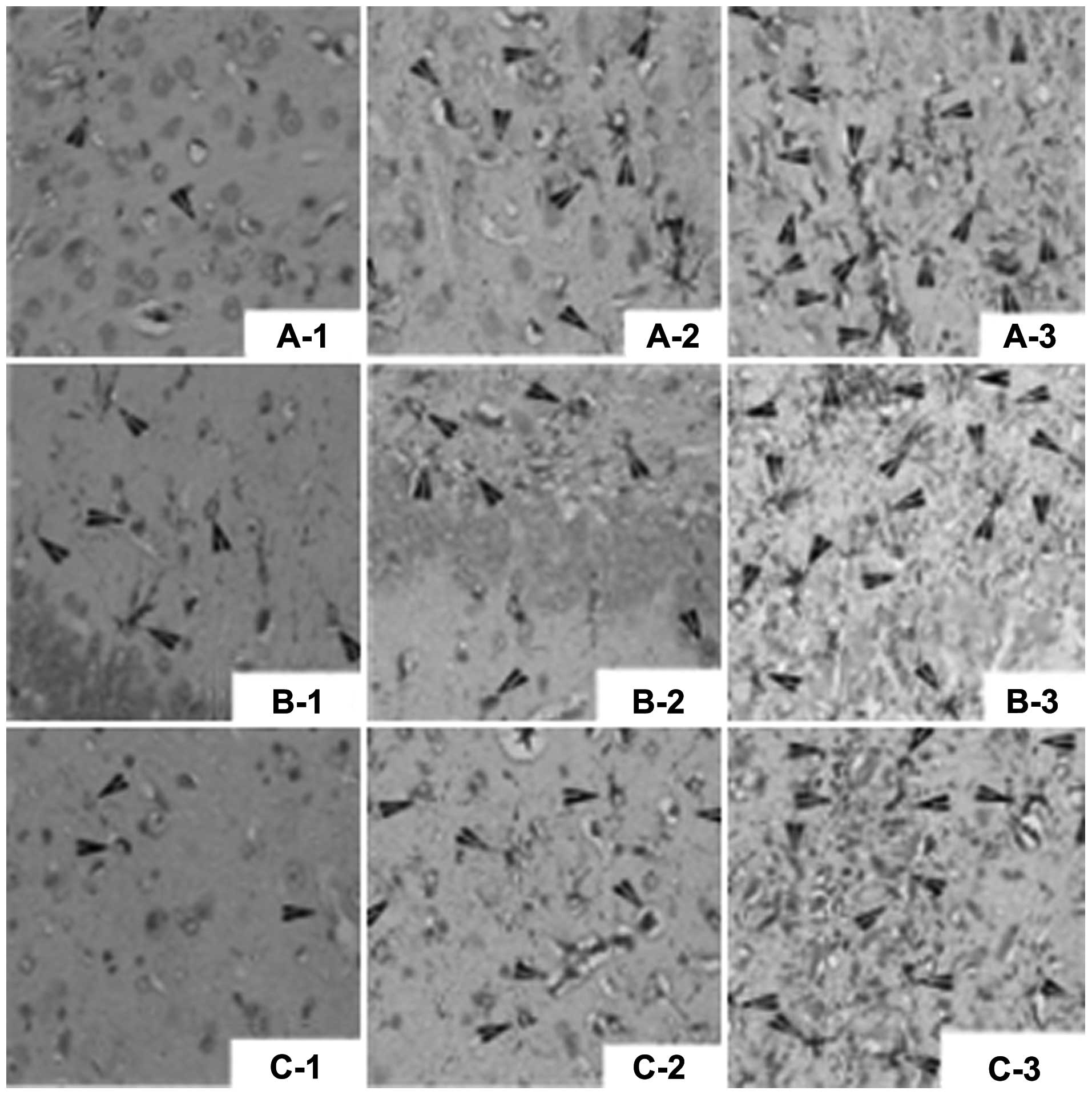 | Figure 3Glial fibrillary acidic protein
(GFAP)-positive cells (arrows) shown by immunohistochemical assay
(magnification, ×400). (A) GFAP-positive cells in the cortex in:
A-1, sham operation group; A-2, control group; and A-3, bone
morphogenetic protein 7 (BMP7) group. (B) GFAP-positive cells in
the hippocampus in: B-1, sham operation group; B-2, control group;
and B-3, BMP7 group. (C) GFAP-positive cells in the striatum in:
C-1, sham operation group; C-2, control group; and C-3, BMP7
group. |
 | Table IIIGFAP-positive cells in brain tissue
(means ± standard deviation per visual field). |
Table III
GFAP-positive cells in brain tissue
(means ± standard deviation per visual field).
| Groups | n | Cortex | Corpus striatum | Hippocampus |
|---|
| Sham | 5 | 4.0±1.0 | 2.8±0.84 | 7.0±0.71 |
| Control | 5 | 9.4±1.14a | 6.0±0.71a | 13.2±0.84a |
| BMP7 | 5 | 15.4±0.84b | 14.2±0.55b | 17.0±0.71b |
Discussion
Neurogenesis remains active in the brain of adult
animals and its main function is to replace the apoptotic neurons
in the specific encephalic region. For example, the granular cells
of the dentate gyrus regularly undergo apoptosis, whereas neural
progenitor cells proliferate with the same rate to maintain a
stable number of granulosa cells (10). It was demonstrated that when an
infarction develops in the unilateral cortex and corpus striatum in
rats with middle cerebral artery embolism, the neural progenitor
cells in the subventricular zone and dentate gyrus increasingly
proliferate, migrate to the surroundings of the infarcted region
and differentiate into neurons and astrocytes (11). Therefore, the injured brain cells in
patients with apoplexy are expected to exhibit neural regenerative
potential. It was previously confirmed that BMP7 may promote DNA
synthesis in brain cells cultured in vitro and
simultaneously promote the dendrite growth of sympathetic neurons
cultured in vitro(12). In
rats without BMP type I receptors, the expression of netrin, a
nerve growth factor that induces axon growth, is inhibited
(13). Therefore, the activation of
BMP signal transduction plays an important role in cell
proliferation and neural regeneration.
Nestin is a type IV intermediate filament protein,
which is distributed in the cytoplasm, is mainly expressed in the
stem cells of the neuroepithelium and participates in the
construction of the cytoskeleton. When the nerve cell migration is
almost completed, the nestin expression begins to decrease and
stops with cell maturation. Nestin-positive cells cultured in
vitro may differentiate into the precursor cells of neurons and
gliocytes; therefore, nestin is considered to be an important
protein marker of neural stem cells. Nestin-positive cells are
mainly distributed in blood vessels, submeningeal spaces, choroid
plexus and among ependymal epithelial cells, as well as in the
subependymal region (14).
Following ischemic injury, the expression of nestin is
significantly increased. In addition to the regions mentioned
above, nestin expression is also detected in the central ischemic
region and its surroundings. The results of our experiment
demonstrated that, compared to the same region in the control
group, BMP7 injection via the caudal vein after a 2-h MCAO
ischemia-reperfusion was able to increase the expression of nestin
in the injured cortex, corpus striatum and hippocampal dentate
gyrus in rats 24 h after suffering a stroke. Therefore, BMP7 may
exert a neuroprotective effect by promoting neural
regeneration.
Astrocytes are widely distributed in the central
nervous system. Apart from their supporting and nutritional
functions, astrocytes also play an important role in nerve tissue
repair and regeneration. The application of immunohistochemical
staining for GFAP may reflect the morphological changes exhibited
by astrocytes (15,16). GFAP is rarely expressed under
physiological conditions and is mainly involved in the structure of
neurons and the regulation of substance metabolism, as well as
cytoskeleton recombination and the functioning of the blood-brain
barrier; it is also involved in intercellular signal transduction
pathways (17). Nawashiro et
al(18) established a cerebral
ischemia model by using wild-type mice lacking GFAP and
demonstrated that the cortex infarction volume was significantly
increased compared to the control group, indicating that astrocytes
and GFAP may exert a neuroprotective effect on brain tissue
suffering ischemia-reperfusion injury. GFAP is expressed by a large
number of astrocytes at the time of cerebral ischemia, which may be
associated with the phagocytosis of harmful extracellular
neurohumors, the maintenance of intracephalic environment stability
and neuron survival and plasticity repair (19). The present experiment confirmed that
GFAP expression was increased after cerebral ischemia and was
mainly distributed in the ischemic cortex, corpus striatum and
hippocampus. BMP7 may upregulate the expression of GFAP after
cerebral ischemia and provide protection for ischemic and anoxic
neurons.
In conclusion, BMP7 may upregulate the expression of
nestin and GFAP and promote neural regeneration, protecting animal
cells against ischemic injury.
Acknowledgements
This study was supported by grant-in-aids for the
Natural Science Foundation of Shandong province (grant no.
ZR2009CM121).
Abbreviations:
|
GFAP
|
glial fibrillary acidic protein
|
|
TGF-β
|
transforming growth factor-β
|
|
TTC
|
tetrazolium chloride
|
|
BMP7
|
bone morphogenetic protein 7
|
|
MCAO/R
|
middle cerebral artery
occlusion/reperfusion
|
|
TUNEL
|
terminal deoxynucleotidyl
transferasemediated biotinylated deoxyuridine triphosphate nick
end-labelling
|
References
|
1
|
Chen HL, Lein PJ, Wang JY, et al:
Expression of bone morphogenetic proteins in the brain during
normal aging and in 6-hydroxydopamine-lesioned animals. Brain Res.
994:81–90. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qin L, Wine-Lee L, Ahn KJ, et al: Genetic
analyses demonstrate that bone morphogenetic protein signaling is
required for embryonic cerebellar development. J Neurosci.
26:1896–1905. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawamata T, Ren J, Chan TC, et al:
Intracisternal osteogenic protein-l enhances functional recovery
following focal stroke. Neuroreport. 9:1441–1445. 1998. View Article : Google Scholar
|
|
4
|
Schallert T, Fleming SM, Leasure JL, et
al: CNS plasticicy and assessment of forelimb sensorimotor outcome
in unilateral rat models of stroke, cortical ablation, parkinsonism
and spinal cord injury. Neuropharmacology. 39:777–787. 2000.
View Article : Google Scholar
|
|
5
|
Chou J, Harvey BK, Chang CF, et al:
Neuroregenerative effects of BMP7 after stroke in rats. J Neurol
Sci. 240:21–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Almazan G, Vela JM, Molina-Holgado E, et
al: Re-evaluation of nestin as a marker of oligodendrocyte lineage
cells. Microsc Res Tech. 52:753–756. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gomes FC, Paulin D and Moura Neto V: Glial
fibrillary acidic protein (GFAP): modulation by growth factors and
its implication in astrocyte differentiation. Braz J Med Biol Res.
32:619–631. 1999. View Article : Google Scholar
|
|
8
|
Longa EZ, Weinstein PR, Carlson S, et al:
Reversible middle cerebral artery occlusion without craniectomy in
rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bederson JB, Pitts LH, Tsuji M, et al: Rat
middle cerebral artery occlusion: evaluation of the model and
development of a neurologic examination. Stroke. 17:472–476. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wiltrout C, Lang B, Yan Y, et al:
Repairing brain after stroke: a review on post-ischemic
neurogenesis. Neurochem Int. 50:1028–1041. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang PB, Liu Y, Li J, et al:
Ependymal/subventricular zone cells migrate to the peri-infarct
region and differentiate into neurons and astrocytes after focal
cerebral ischemia in adult rats. Academic Journal of the First
Medical College of PLA. 25:1201–1206. 2005.PubMed/NCBI
|
|
12
|
Lein P, Johnson M, Guo X, et al:
Osteogenic protein-1 induces dendritic growth in rat sympathetic
neurons. Neuron. 15:597–605. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, Wilson S and Reh T: BMP receptor lb
is required for axon guidance and cell survival in the developing
retina. Dev Biol. 256:34–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu PC, Lu SD, Huang YL, et al: The
expression of nestin in ischemia-injured brain of adult rat. Acta
Physiologica Sinica. 54:294–299. 2002.(In Chinese).
|
|
15
|
Chen HX, Zhang LM, Zhang YZ, et al: Effect
of agmatine on the neurons and astrocytes in hippocampus of
chronically stressed rats. Chin Pharmacol Bull. 25:21–25. 2009.
|
|
16
|
Zhang JJ: Advances in astrocytes. Acta
Pharmacologica Sinica. 22:788–791. 2006.(In Chinese).
|
|
17
|
Timmer M, Cesnulevicius K, Winkler C, et
al: Fibroblast growth factor (FGF)-2 and FGF receptor 3 are
required for the development of the substantia nigra, and FGF-2
plays a crucial role for the rescue of dopaminergic neurons after
6-hydroxydopamine lesion. J Neurosci. 27:459–471. 2007. View Article : Google Scholar
|
|
18
|
Nawashiro H, Brenner M, Fukui S, et al:
High susceptibility to ischemia in GFAP-null mice. J Cereb Blood
Flow Metab. 20:1040–1044. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu D, Smith CL, Barone FC, et al:
Astrocytic demise precedes delayed neuronal death in focal ischemic
rat brain. Brain Res Mol Brain Res. 68:29–41. 1999. View Article : Google Scholar : PubMed/NCBI
|