Introduction
Imidocarb
[1,3-bis[3-(4,5-dihydro-1h-imidazol-2-yl)phenyl]urea] is a
carbanilide derivative and chemotherapeutic, chemoprophylactic
agent with antiprotozoal activity. Imidocarb is usually
administered as dipropionate salt (1–3). In
veterinary medicine, it is used in cattle, horses, sheep, and
domestic animals including cats and dogs, for the treatment of
anaplasmosis and babesiosis (4–8).
Findings of recent studies show that significant residues of
imidocarb were detected in bovine and ovine tissues and milk
following the administration of 14C-imidocarb
dipropionate (2,3,9). For
this reason, maximum residue limits (MRL) of imidocarb have been
set by CODEX, Europe, Middle East and Africa and other countries,
including Japan and Korea. The MRLs imposed by the Korea Food and
Drug Administration (KFDA) are 0.3 mg/kg for bovine muscle, 1.5
mg/kg for bovine liver, 0.05 mg/kg for bovine fat, 2 mg/kg for
bovine kidney and 0.05 mg/kg for bovine milk (10).
Recently, Ishii et al(5) and Inoue et al(6) reported a liquid chromatographic method
with detection by tandem mass spectrometry (LC-MS/MS) for
monitoring imidocarb in bovine tissue and milk. However, simple
analytical methods, such as high-performance liquid chromatography
(HPLC), for monitoring imidocarb residues in various animal tissues
are not well developed. In general, if it provides satisfactory
sensitivities for determination levels of residues less than their
MRLs, HPLC is preferred over LC-MS/MS due to cost benefits and ease
of handling. Therefore, we have developed an HPLC method to
quantify imidocarb residues in animal tissues using a solid-phase
extraction (SPE) clean-up process. Wang et al(8) suggested an HPLC method for imidocarb
residue determination in swine tissue, however, this method did not
involve a solid-phase clean-up process. Tarbin and Shearer
(11) also reported a method for
determining imidocarb using HPLC with SPE, however, that method was
applied only to a bovine kidney sample. Thus, the aim of the
present study was to develop a sensitive and economic method for
imidocarb detection in beef and milk samples using HPLC with
DAD.
Materials and methods
Chemical and reagents
Imidocarb, trifluoroacetic acid, ammonium hydroxide
(NH3 content 20.8–30.0%) and acetic acid were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile, methanol and
hexane HPLC-grade solvents were provided by JT Baker (Phillipsburg,
NJ, USA). Sodium sulfate was purchased from Junsei Chemical (Tokyo,
Japan). Any other chemicals and solvents were of analytical grade.
Oasis weak cation exchange (WCX) (60 mg/3 ml) was obtained from
Waters Corporation (Milford, MA, USA). Buffer solutions prepared
for HPLC were filtered through a 0.45-μm GHP membrane filter (Pall,
Ann Arbor, MI, USA). Animal extract samples were filtered through a
0.45-μm GHP syringe filter (Pall). Beef and commercial whole milk
were purchased from large markets. Preliminary analysis indicated
that the samples were analyte-free.
Standard solutions
Stock solutions of 1,000 μg/ml imidocarb were
prepared in water and stored at −4°C. The working solutions for
HPLC injections were prepared daily from stock solution mixtures of
0.1% trifluoroacetic acid in water and acetonitrile (85:15,
v/v).
Sample preparation
Beef (5 g) and milk (5 ml + 5 g of
Na2SO4) samples were transferred to 50-ml
conical tubes. Acetonitrile:methanol:trifluoroacetic acid (10 ml,
495:500:5, v/v/v) was added and the resulting solution was
vortex-mixed for 10 min and centrifuged at 3,500 × g for 20 min.
The supernatant was gently transferred to 15-ml conical tubes and
re-extracted with 5 ml of acetonitrile:methanol:trifluoroacetic
acid (495:500:5, v/v/v), vortex-mixed for 10 min and centrifuged at
3,500 × g for 20 min. The first and second extracts were combined
and the resulting solution was evaporated to dryness under nitrogen
at 50°C. The resulting evaporation residue was dissolved in 2 ml of
water and 0.5 ml of hexane, after which the solution was
vortex-mixed for 1 min. The samples were then added to a Waters
Oasis™ WCX cartridge (60 mg) after the cartridge was conditioned
with 3 ml of methanol and equilibrated with 3 ml of water. The
loaded cartridge was washed with 3 ml of methanol and 2% ammonium
hydroxide. The analyte was eluted with 3 ml of
acetonitrile:methanol:trifluoroacetic acid (50:45:5, v/v/v)
followed by evaporation under a nitrogen stream at 50°C. The
concentrated residues were then dissolved in 5 ml (beef sample) and
1 ml (milk sample) of mobile phase [0.1% trifluoroacetic acid in
water and acetonitrile (85:15, v/v)] and filtered through a 0.45-μm
GHP syringe filter.
Chromatographic quantification
Imidocarb residue levels were quantified via HPLC
using an Agilent series 1100 instrument (Palo Alto, CA, USA). The
HPLC columns were equipped with a quart pump (G1311A), degasser
(G1322A), autosampler (G1313A), column oven (G1316A) and
diode-array detection (DAD) detector (G1315B). The samples (20 μl)
were separated on a C18 column (Waters Xbridge, 4.6×250
mm, particle size: 5 μm; Waters) maintained at 20°C. The mobile
phase consisted of 0.1% trifluoroacetic acid in water and
acetonitrile (85:15, v/v). The flow rate was fixed at 1.0 ml/min
and the DAD detector was set at 260 nm.
Method validation
The method developed was validated to ensure the
criteria specified by the CODEX guidelines for specificity,
linearity, limits of detection (LOD) and quantification (LOQ),
accuracy and precision (12). Blank
samples (beef muscle and commercial milk) were assessed for matrix
interferences. Linearity was evaluated for each of the investigated
samples (beef muscle and commercial milk) using samples spiked with
six concentration levels [0.5, 1, 2, 3, 4 and 5 times the permitted
limit (MRL)]. Each sample was analyzed four times. Calibration
curves were calculated via least-squares linear regression analysis
of the peak area ratio of each analyte.
The LOD calculations were based on the standard
deviation of the y-intercepts (σ) and the slope (S) determined by
regression analyses, using the equation LOD=3.3 σ/S. The LOQ was
calculated using the equation LOQ=10 σ/S (13).
Recoveries were obtained for fortified samples at
concentrations of 0.5, 1 and 2 times their MRLs. Five samples were
prepared for each concentration level. The responses obtained when
imidocarb was added to blank samples prior to extraction were
compared with those in which imidocarb was added after extraction.
In the inter-laboratory investigations, recoveries and precisions
were assessed using fortified samples at MRL concentrations (1
time). Five samples were prepared for each concentration level.
Method precision was expressed as the relative standard deviation
(RSD). The accepted criteria for the analytical method are shown in
Table I.
 | Table IThe accepted criteria for the
analytical method. |
Table I
The accepted criteria for the
analytical method.
| Recovery | Relative standard
deviation (%) |
|---|
|
|
|---|
| Concentration
(μg/kg) | Acceptable recovery,
range (%) | Concentration
(μg/kg) | Within laboratory
(%) | Between laboratories
(%) |
|---|
| ≤1 | 50–120 | ≤ 1 | 35 | 53 |
| >1, ≤10 | 60–120 | >1, ≤10 | 30 | 45 |
| >10, ≤100 | 70–110 | >10, ≤100 | 20 | 32 |
| ≤100, ≤1,000 | 70–110 | ≤100, ≤1,000 | 15 | 23 |
| ≤1,000 | 70–110 | ≤1,000 | 10 | 16 |
Results and Discussion
An HPLC analysis method was developed to detect
imidocarb residues in beef and milk. The imidocarb chemical
structure is shown in Fig. 1. The
appropriate mobile-phase conditions were established by varying the
ratio of 0.1% trifluoroacetic acid solution and acetonitrile. The
effect of the organic solvent on imidocarb retention was
investigated by varying the acetonitrile ratios in the mobile
phase; 11, 12, 15 and 20% acetonitrile ration resulted in imidocarb
retention times of ~17, 12.5, 7.4 and 3.5 min, respectively. The
linear isocratic mobile phase [consisting of 0.1% trifluoroacetic
acid in water and acetonitrile (85:15, v/v)] showed optimal
separation given the intensities of the analyte peaks, where the
imidocarb retention time was 7.4 min.
To extract imidocarb from the samples, a mixture of
acetonitrile and methanol was used. The recovery of imidocarb was
<30% by acetonitrile extraction. Optimal recovery was obtained
utilizing a mixture of acetonitrile:methanol:trifluoroacetic acid
(495:500:5, v/v/v). The average recovery was >80% and,
therefore, highly satisfactory. A SPE clean-up using a WCX
cartridge was performed following extractions of the
acetonitrile:methanol:trifluoroacetic acid (495:500:5, v/v/v). No
difference was observed between SPE and non-SPE in terms of
recovery. However, the interference peak was not observed in either
the beef or milk sample after clean-up with SPE using a WCX
cartridge. Thus, highly satisfactory chromatograms and recoveries
were obtained using these procedures.
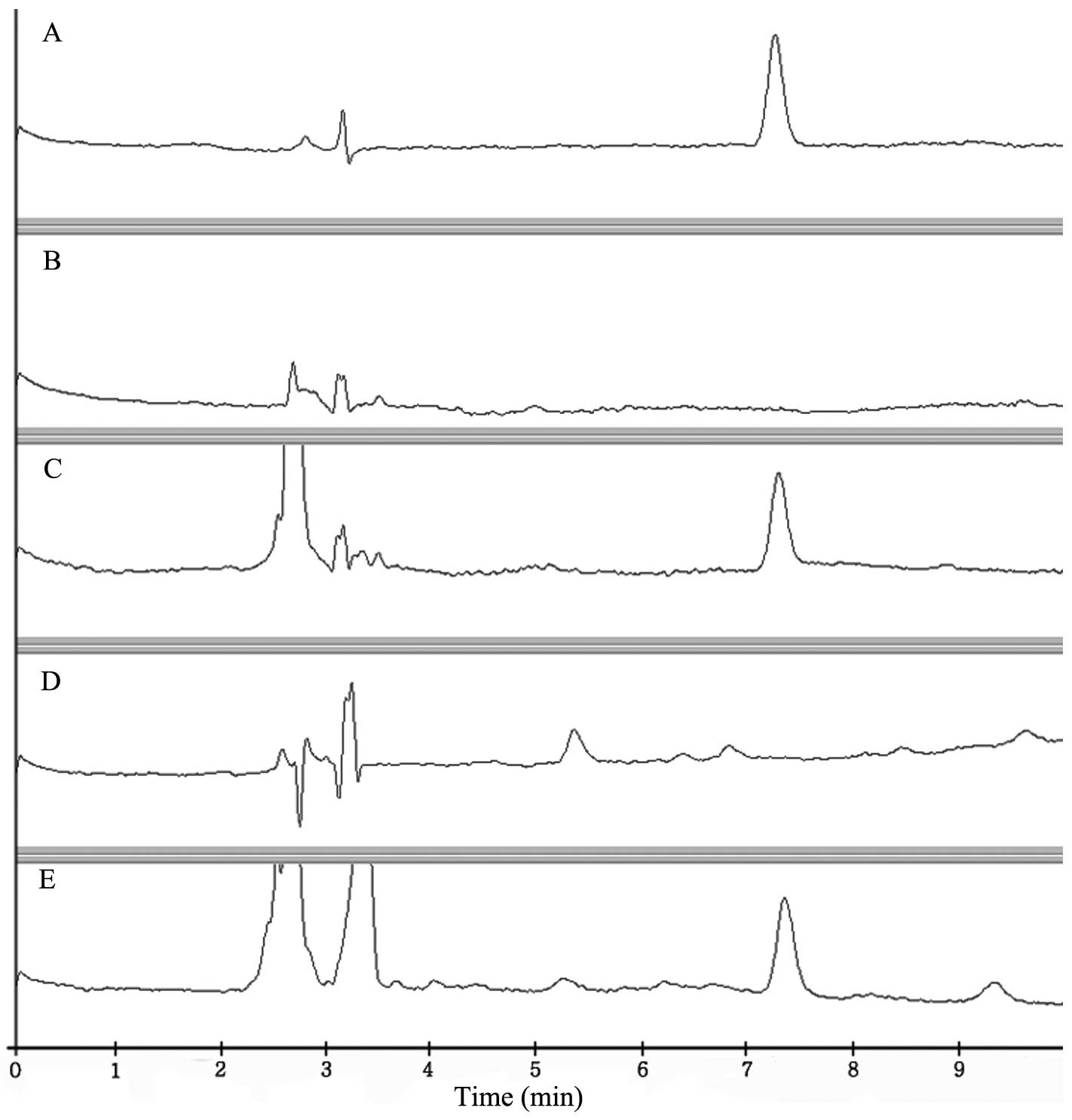
The specificity of the method for each sample was
evaluated by analyzing blank samples. None of these samples
exhibited interferences from beef and milk. As shown in Fig. 2, imidocarb was extracted
successfully from beef and milk.
The chromatographic method demonstrated linearity at
six concentration levels (0.5, 1, 2, 3, 4 and 5 times the permitted
MRL) (n=4, r≥0.99). The calibration data, as well as the LOD and
LOQ, are provided in Table II. The
LOQ were 0.15 mg/kg for beef and 0.025 mg/kg for commercial milk,
respectively. The LOQs in the matrices examined were lower than the
MRL imposed by the KFDA for these compounds (10).
 | Table IILinearity parameters of imidocarb in
spiked beef and milk. |
Table II
Linearity parameters of imidocarb in
spiked beef and milk.
| Sample | Range (mg/kg) | Slope | Intercept | R | LOD (mg/kg) | LOQ (mg/kg) |
|---|
| Beef | 0.15–1.5 | 40.814±0.773 | −0.180±0.353 | 0.998±0.002 | 0.05 | 0.15 |
| Milk | 0.025–0.25 | 235.668±16.651 | 0.178±0.996 | 0.998±0.002 | 0.008 | 0.025 |
The precision and accuracy (recovery) of the method
were determined using intra-day (n=3) and inter-day (n=5) methods
and three different concentrations. The results are shown in
Table III. Matrices were analyzed
at concentrations of 0.5, 1 and 2 times the limits permitted in
accordance with the CODEX guidelines. The RSD values (%) were
3.2–6.1 and 1.4–6.9 for the intra-day and inter-day precisions,
respectively. The accuracies of imidocarb in the spiked samples
were 80.4–82.2% in beef muscle and 80.1–89.5% in milk.
 | Table IIIPrecision and accuracy (recovery) of
imidocarb in spiked beef and milk. |
Table III
Precision and accuracy (recovery) of
imidocarb in spiked beef and milk.
| | Intra-day (n=3) | Inter-day (n=5) |
|---|
| |
|
|
|---|
| Sample | Concentration
(mg/kg) | Precision RSD
(%) | Accuracy recovery
(%) | Precision RSD
(%) | Accuracy recovery
(%) |
|---|
| Beef | 0.15 | 3.2 | 81.2 | 4.8 | 81.4 |
| 0.3 | 3.2 | 80.2 | 1.4 | 80.4 |
| 0.6 | 4.0 | 81.5 | 2.8 | 82.2 |
| Milk | 0.025 | 3.9 | 80.7 | 3.4 | 87.8 |
| 0.05 | 6.1 | 83.3 | 6.8 | 88.3 |
| 0.1 | 5.8 | 80.1 | 6.9 | 89.5 |
The accuracy and precision of the method in the
inter-laboratory context were expressed as a recovery value [mean
(%), RSD (%)]. Matrices were analyzed at concentrations of 1 time
the permitted MRLs. The mean recovery rates of imidocarb were
80.5–91.9% in the spiked beef samples and 86.9–94.3% in the spiked
milk samples (Table IV).
 | Table IVRecovery of imidocarb in beef and milk
(inter laboratories) at 1 mg/kg. |
Table IV
Recovery of imidocarb in beef and milk
(inter laboratories) at 1 mg/kg.
| | Recovery (n=5) |
|---|
| |
|
|---|
| Laboratory | Sample | RSD (%) | Mean (%) |
|---|
| Lab 1 | Beef | 4.0 | 84.2 |
| Milk | 4.1 | 90.5 |
| Lab 2 | Beef | 1.3 | 96.0 |
| Milk | 6.0 | 87.2 |
The proposed methods were applied to determine the
possible foundation of imidocarb in 10 different beef and milk
samples purchased from large markets in Seoul. Imidocarb was not
detected in any of the samples.
In conclusion, the HPLC analytical method described
in the present study has been applied successfully to separate and
detect imidocarb residues. The developed method has also
demonstrated acceptable precision and accuracy (recovery). The
procedure is simple and allows for high-sensitivity determination
of imidocarb residues in beef and milk.
Acknowledgements
This study was supported by KFDA (11162-002) in
2012.
References
|
1
|
Ayoob AL, Hackner SG and Prittie J:
Clinical management of canine babesiosis. J Vet Emerg Crit Care
(San Antonio). 20:77–89. 2010. View Article : Google Scholar
|
|
2
|
EMEA. The European Agency for the
Evaluation of Medicinal Products, Veterinary Medicines and
Information Technology. Imidocarb (Extension to sheep): Summary
report (3) - Committee for Veterinary Medicinal Products.
EMEA/MRL/881/03-FINAL https://www.ema.europa.eu.
|
|
3
|
Su D, Li XB, Wang ZJ, Wang L, Wu WX and Xu
JQ: Pharmacokinetics and bioavailability of imidocarb dipropionate
in swine. J Vet Pharmacol Ther. 30:366–370. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adaszek L, Winiarczyk S, Lukaszewska J and
Heile C: Feline babesiosis. Kleintierpraxis. 55:624–628. 2010.
|
|
5
|
Ishii R, Takahashi K and Matsumoto R:
Analysis of imidocarb in livestock and seafood products using
LC-MS/MS. Shokuhin Eiseigaku Zasshi. 52:34–39. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inoue K, Nunome M, Hino T and Oka H:
Determination of Imidocarb in bovine tissues and milk samples by
LC-MS/MS. J Liq Chromatogr Relat Technol. 34:2149–2156. 2011.
View Article : Google Scholar
|
|
7
|
Rashid A, Mubarak A and Hussain A:
Babesiosis in equines in Pakistan: a clinical report. Vet Ital.
45:391–395. 2009.PubMed/NCBI
|
|
8
|
Wang Z, Li X, Su D, Li Y, Wu L, Wang Y and
Wu W: Residue depletion of imidocarb in swine tissue. J Agric Food
Chem. 57:2324–2328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lewis KM, Cohn LA, Birkenheuer AJ and
Papich MG: Pharmacokinetics of diminazene diaceturate in healthy
cats. J Vet Pharmacol Ther. 35:608–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ministry of Food and Drug Safety. Republic
of Korea. Korean Food Standards Codex, appendix 7 MRLs for
veterinary drugs in foods: 2013–204. https://www.mfds.go.kr.
|
|
11
|
Tarbin JA and Shearer G: High-performance
liquid chromatographic determination of imidocarb in cattle kidney
with cation-exchange clean-up. J Chromatogr. 577:376–381. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Codex Alimentarius. Guidelines for the
design and implementation of national regulatory food safey
assurance programme associated with the use of veterinary drugs in
food producing animals: CAC/GL 71–209. http://www.codexalimentarius.org/standards/list-of-standards/enuri.
|
|
13
|
Christodoulou EA, Samanidou VF and
Papadoyannis IN: Validation of an HPLC-UV method according to the
European Union Decision 2002/657/EC for the simultaneous
determination of 10 quinolones in chicken muscle and egg yolk. J
Chromatogr B Analyt Technol Biomed Life Sci. 859:246–255. 2007.
View Article : Google Scholar : PubMed/NCBI
|