Introduction
The introduction of Global Initiative for Asthma
(GINA) guidelines has enabled more patients with allergic asthma to
control their disease. However, a high percentage of asthmatic
patients are not efficiently controlled with the currently
available therapies and a high percentage of patients are not
compliant with inhaled treatments for asthma. Therefore, it is
crucial to improve our understanding of the pathophysiology of
asthma.
CD39 is a cell surface-located prototypic member of
the ectonucleoside triphosphate diphosphohydrolase (E-NTPDase)
family. This ectoenzymatic cascade in tandem with
ecto-5′-nucleotidase (CD73) generates adenosine and exerts
significant effects on adenosine 5′-triphosphate (ATP) and
adenosine receptor signalling (P2 and P1 purinergic receptors,
respectively). CD39 was first described as a B lymphocyte
activation marker (1). This
ectonucleotidase is also expressed on natural killer cells,
monocytes, dendritic cells (DCs) and subsets of activated T cells
(2). CD39 also catalyzes
extracellular ATP released by inflammatory, damaged and activated T
cells (3–7). However, extracellular ATP exerts
pro-inflammatory effects, such as promoting monocytes and
lymphocytes to secrete interleukin (IL)-1 and IL-8 (8–9),
promoting the differentiation and migration of DCs (10) and IL-17 expression and inducing the
apoptosis of regulatory T cells (11–12).
Therefore, CD39 may alleviate the inflammatory damage of tissue by
removing extracellular ATP.
However, the expression of CD39 in peripheral blood
mononuclear cells (PBMCs) in allergic asthma and its functions have
not been elucidated. Our study initially investigated the
expression of CD39 in the peripheral blood of patients with
allergic asthma and analyzed the correlations between CD39 mRNA and
serum IL-4, IL-17A, transforming growth factor β (TGF-β) and GATA3,
RAR-related orphan receptor γ (ROR-γt) and forkhead box P3 (FoxP3)
mRNA.
Materials and methods
Subjects and sample preparation
A total of 18 patients with chronic persistent
allergic asthma, as determined by the positive results of allergen
tests to house dust mites, were recruited in this study. Brief lung
function tests, including forced expiratory volume in the first
second (FEV1) (% predicted), were performed. The concentration of
DP.sIgE was measured. None of the patients had been treated with
systemic glucocorticoids within one month prior to the initiation
of the study and had never received other immunosuppressive agents
or desensitization therapy. A total of 19 age- and gender-matched
healthy donors, with normal pulmonary function and negative
allergen tests, were selected as normal controls. FEV1 (%
predicted) was markedly lower and DP.sIgE was markedly higher in
asthmatic patients compared to those in normal controls (Table I).
 | Table IGeneral characteristics of
subjects. |
Table I
General characteristics of
subjects.
| Index | Asthma | Control | P-value |
|---|
| No. | 18 | 19 | - |
| Age (years) | 37.06±11.67 | 38.11±11.37 | >0.05 |
| Gender (M/F) | 12/6 | 13/6 | >0.05 |
| FEV1 (%
predicted) | 80.05±16.72 | 92.27±6.54 | <0.01 |
| DP.sIgE (KU/ml) | 28.47±18.50 | 0.95±1.67 | <0.01 |
Written informed consent was obtained from all the
individuals and the study received ethical approval from the
Research Ethics Board of Ruijin Hospital, Shanghai Jiao Tong
University School of Medicine.
Main reagents and instruments
The human lymphocyte separation medium and the
SYBR-Green I quantitative polymerase chain reaction (qPCR) kit were
purchased from BioTNT, Shanghai, China; RPMI was provided by Gibco,
Carlsbad, CA, USA; the TRIzol mRNA extraction reagent and the ABI
ViiA 7 Real-Time PCR system were purchased from Life Technologies,
Carlsbad, CA, USA; the cDNA reverse transcription kit was obtained
from Promega Corporation, Madison, WI, USA; the human serum IL-4,
IL-17A and TGF-β enzyme-linked immunosorbent assay (ELISA) kits
were purchased from Anogen, Mississauga, Canada; the DP.sIgE ELISA
kit was provided by Dr. Fooke-Achterrath Laboratorien GmbH, Neuss,
Germany; and the Multiskan MS microplate reader was obtained from
Ani LabSystems, Ltd., Vantaa, Finland.
Methods
Heparinized peripheral venous blood (8 ml) was
collected from each participant. Plasma was isolated from
peripheral blood and stored at −80°C until used to measure the
concentrations of the cytokines. PBMCs were obtained from
peripheral blood by Ficoll-Hypaque density centrifugation (1,200 ×
g for 20 min at room temperature). The PBMCs were suspended at a
density of 2×106 cells/ml in 1 ml TRIzol reagent and
stored at −80°C.
The concentrations of DP.sIgE, IL-4, IL-17A and
TGF-β in the plasma were measured by ELISA, in accordance with the
manufacturer’s instructions. All the measurements were performed in
duplicate.
Relative qPCR
The total mRNA in the TRIzol reagent was
reverse-transcribed into cDNA. The primers were designed by Life
Technologies and synthesized by BioTNT, according to the
manufacturer’s instructions. For amplification, the SYBR-Green I
Real-Time PCR kit was used. Each reaction was run in triplicate on
the ABI ViiA 7 Real-Time PCR system and was normalized to
housekeeping gene β-actin transcripts. ΔCT values was recorded and
converted to 2−ΔΔCT, revealing a linear association
between the target concentrations and 2−ΔΔCT values (the
smaller the target concentrations, the smaller the
2−ΔΔCT values). The specific primers used are listed in
Table II.
 | Table IIPrimer sequences of human CD39, GATA3,
ROR-γt and Foxp3 gene. |
Table II
Primer sequences of human CD39, GATA3,
ROR-γt and Foxp3 gene.
| Gene | Sequences | Length (bp) |
|---|
| CD39 |
5′-CTGATTCCTGGGAGCACAT-3′
5′-GACATAGGTGGAGTGGGAGAG-3′ | 143 |
| GATA3 |
5′-GAGATGGCACGGGACACTAC-3′
5′-GTGGTTGTGGTGGTCTGACAGT-3′ | 151 |
| ROR-γt |
5′-GGCTCCCTGGATGAATAGAATG-3′
5′-AGGCAGAGGCAGAAAATGTAAAG-3′ | 190 |
| FoxP3 |
5′-ATGCGACCCCCTTTCACCTAC-3′
5′-TGGCGGATGGCGTTCTTC-3′ | 155 |
| β-actin |
5′-AAGGTGACAGCAGTCGGTT-3′
5′-TGTGTGGACTTGGGAGAGG-3′ | 195 |
Statistical analysis
GraphPad Prism 5 software was used for statistical
analysis. Normal distribution and homogeneity of variance in two
groups were first tested. If each group exhibited homogeneity,
statistical calculations were performed using the Student’s t-test
and the data are presented as means ± SD. When heteroscedasticity
was present in each group, the data were analyzed using the
Mann-Whitney U test and presented as medians (interquartile range).
Pearson’s correlation was used to analyze the relevance. P<0.05
was considered to indicate a statistically significant
difference.
Results
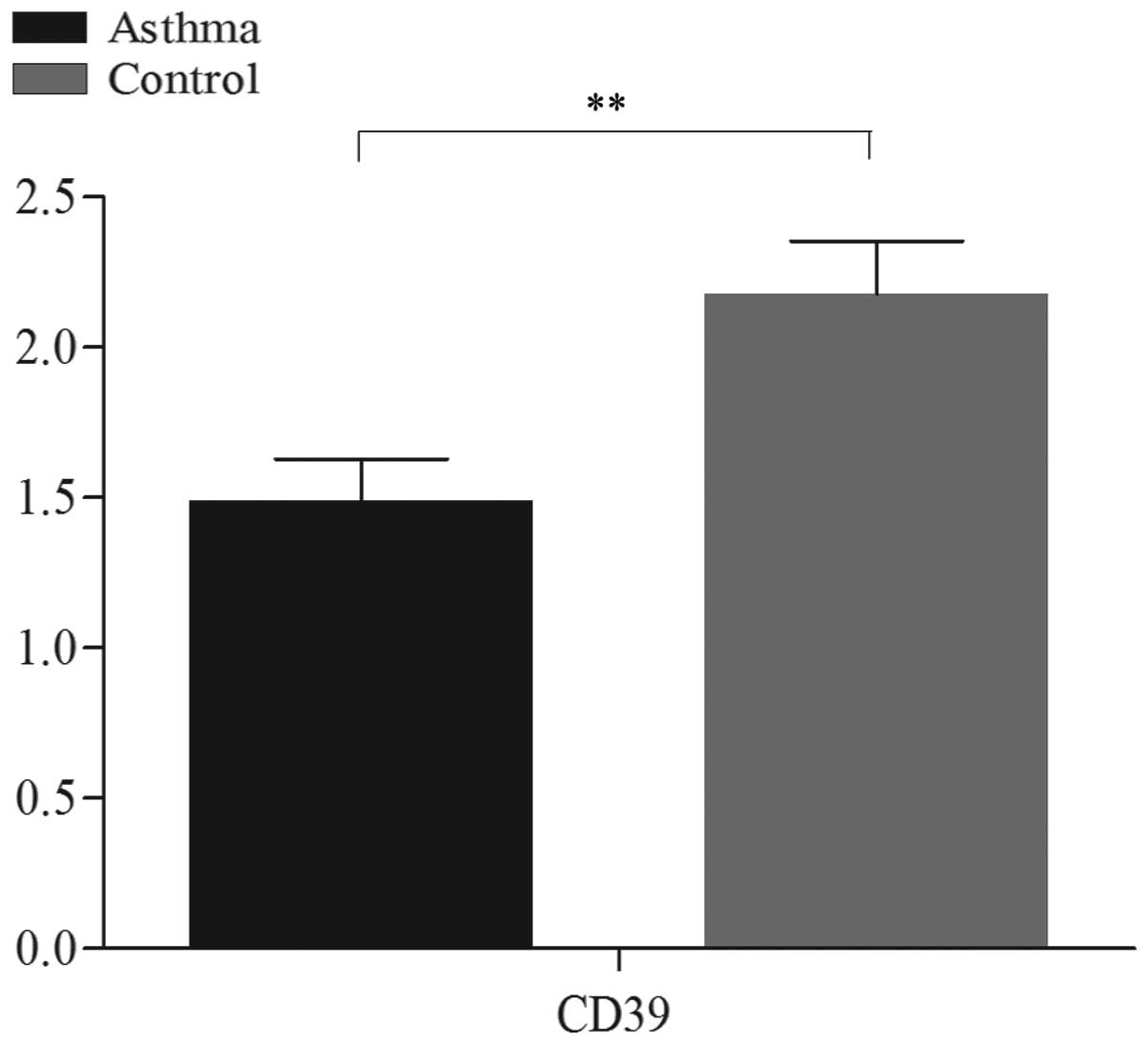
CD39 mRNA expression is decreased in the
peripheral blood of patients with allergic asthma
CD39 mRNA expression was markedly lower in patients
with allergic asthma compared to that in normal controls
[(1.49±0.59)×10−3 vs. (2.17±0.77)×10−3,
respectively; P<0.01] (Fig.
1).
Increased serum IL-4 and IL-17A levels
correlate with CD39 mRNA deficiency in PBMCs
The chronic inflammation of asthma is mediated by
several types of inflammatory cytokines, such as IL-4 and IL-17A
(13–14). In agreement with our previous
studies (15), the levels of IL-4
and IL-17A in the serum were markedly higher in patients with
allergic asthma compared to those in normal controls (Table III). We then analyzed the
correlation of CD39 mRNA with IL-4 and IL-17A and observed that
CD39 mRNA expression was negatively correlated with IL-4 and IL-17A
levels (r=−0.468, P<0.05; and r=−0.550, P<0.05,
respectively).
 | Table IIIComparison of serum IL-4, IL-17A and
TGF-β levels between patients with allergic asthma and normal
controls. |
Table III
Comparison of serum IL-4, IL-17A and
TGF-β levels between patients with allergic asthma and normal
controls.
| Cytokine | Asthma | Control | P-value |
|---|
| IL-4 | 137.11±187.42 | 3.03±3.01 | <0.01 |
| IL-17A | 54.42±47.15 | 15.90±23.31 | <0.01 |
| TGF-β | 66.15±33.30 | 164.44±42.61 | <0.01 |
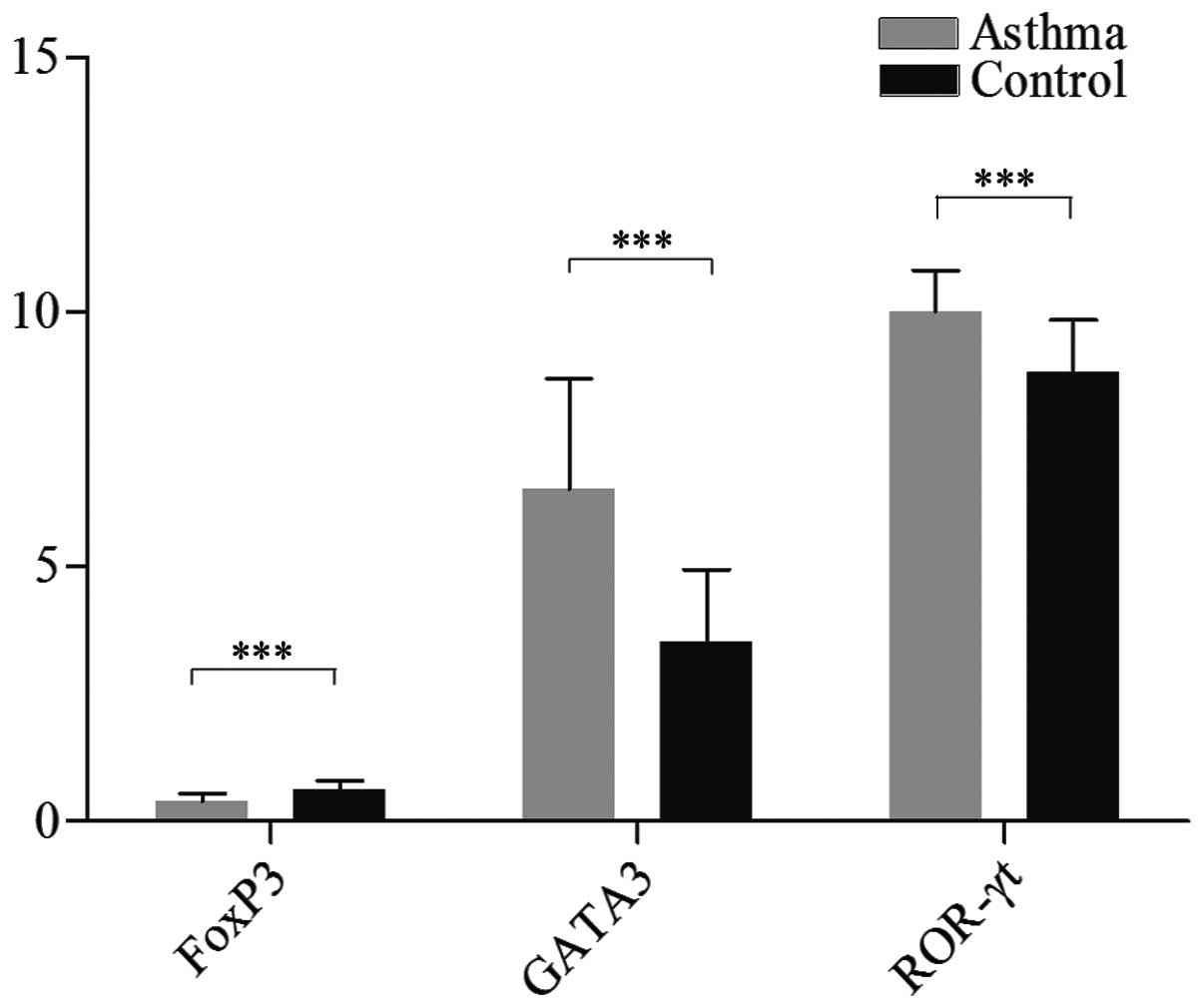
Correlation of CD39 mRNA with GATA3 and
ROR-γt mRNA
In our study, we further investigated GATA3 and
ROR-γt mRNA expression in PBMCs. We observed that the expression of
GATA3 and ROR-γt mRNA was markedly higher in patients with allergic
asthma compared to that in normal controls
[(6.53±2.17)×10−3 vs. (3.53±1.41)×10−3,
P<0.001; and (10.02±0.80)×10−3 vs.
(8.83±1.01)×10−3, P<0.001, respectively] (Fig. 2). These results were in agreement
with our previous study (16) and
confirmed the veracity of the selected asthmatic patients.
The correlation between GATA3 mRNA and IL-4, ROR-γt
mRNA and IL-17A was then analyzed. We observed that GATA3 mRNA
expression was positively correlated with serum IL-4 levels
(r=0.583, P<0.01), whereas there was no obvious correlation
between ROR-γt mRNA and serum IL-17A levels (r=0.197, P=0.406).
We also analyzed the correlation of CD39 mRNA with
GATA3 and ROR-γt mRNA expression and observed that CD39 mRNA
expression was negatively correlated with GATA3 mRNA (r=−0.424,
P<0.01) and exhibited no obvious correlation with ROR-γt mRNA
expression (r=−0.259, P=0.122).
Decreased FoxP3 and TGF-β levels are
associated with of CD39 mRNA deficiency
In our study, we also investigated the level of
serum TGF-β and FoxP3 mRNA expression in PBMCs and observed that
serum TGF-β and FoxP3 mRNA levels were markedly lower in patients
with allergic asthma compared to those in normal controls
[66.15±33.30 vs. 164.44±42.61, P<0.01; and
(0.40±0.15)×10−3 vs. (0.64±0.16)×10−3,
P<0.001, respectively] (Table
III and Fig. 2). Moreover, CD39
mRNA expression was positively correlated with serum TGF-β and
FoxP3 mRNA expression (r=0.425, P<0.05; r=0.373, P<0.05).
Discussion
In our study, we investigated the expression of CD39
mRNA in PBMCs. Our data indicated that CD39 mRNA expression was
downregulated in allergic asthma. We hypothesized that the
deficiency of CD39 mRNA in asthma, which leads to the absence of
the anti-inflammatory effects of CD39, may promote the occurrence
of asthma.
Imbalances in T helper (Th)1/Th2 and Th17/regulatory
T cells (Treg cells) were also detected in patients with allergic
asthma (15). Th2, Th17 and Treg
cells exhibit unique cytokine and specific transcription factor
profiles that instruct a specific differentiation program (13,14,17).
It is currently recognized that Th2-cell differentiation and IL-4
secretion is dependent on GATA3 (18). The differentiation and development
of Th17 cells, which mainly secrete IL-17A, require ROR-γt
(19–21), whereas Treg cells require FoxP3 and
mainly secrete TGF-β (22). It is
widely accepted that Th2 and Th17 cells are important contributors
to inflammatory responses and airway remodeling (18,23)
and Treg cells play a central role in regulating the self-tolerance
and homeostasis of the immune system (24). In our study, we observed that the
deficiency of CD39 mRNA in PBMCs was negatively correlated with
increased serum IL-4 and IL-17A levels and positively correlated
with decreased serum TGF-β levels. We hypothesized that the
decreased CD39 may result in inflammatory damages in asthma by
upregulating serum IL-4 and IL-17A, which mediate airway
inflammation in asthma, and downregulating serum TGF-β, which is a
soluble anti-inflammatory cytokine (21,24).
Previous studies demonstrated that increased GATA3 and ROR-γt and
decreased FoxP3 expression mediate the inflammatory responses in
asthma (20,25,26).
Moreover, CD73, another ectoenzyme, may act synergistically with
CD39 to catalyze the conversion of extracellular ATP into immune
suppressor adenosine. Subsequently, adenosine combines to the
adenosine receptor A2A, which is expressed on effector T (Teff)
cells and Treg cells, inhibiting the activation and functions of
Teff cells, such as inhibition of Th2 cell development and Th17
cell generation (27,28) and promoting the expression of FoxP3
by Treg cells (29). Accordingly,
we hypothesized that CD39 may regulate the levels of serum IL-4,
IL-17A and TGF-β by regulating GATA3, ROR-γt and FoxP3 expression.
However, we observed that CD39 was negatively correlated with GATA3
and positively correlated with FoxP3, whereas there was no obvious
correlation of CD39 with ROR-γt. The role of the signaling pathway
of CD39, CD73 and A2AR in asthma may differ from that in multiple
sclerosis and renal ischemia-reperfusion injury. Moreover, other
immune cells also express IL-17A in addition to the Th17 cells
(30). Thus we hypothesized that
CD39 may control IL-17A levels by regulating other IL-17A-producing
cells.
In conclusion, we demonstrated that CD39 mRNA
expression was downregulated in the peripheral blood of patients
with asthma, which was associated with serum IL-4 and IL-17A levels
and the expression of GATA3 and FoxP3 mRNA, whereas it was not
associated with ROR-γt mRNA expression. Therefore, the deficiency
of C0D39 mRNA may be involved in the occurrence and progression of
allergic asthma.
References
|
1
|
Maliszewski CR, Delespesse GJ, Schoenborn
MA, et al: The CD39 lymphoid cell activation antigen. Molecular
cloning and structural characterization. J Immunol. 153:3574–3583.
1994.PubMed/NCBI
|
|
2
|
Koziak E, Sevigny J, Robson SC, Siegel JB
and Kaczmarek K: Analysis of CD39/ATP diphosphohydrolase (ATPDase)
expression in endothelial cells, platelets and leukocytes. Thromb
Haemost. 82:1538–1544. 1999.PubMed/NCBI
|
|
3
|
Day YJ, Huang L, Ye H, Li L, Linden J and
Okusa MD: Renal ischemia-reperfusion injury and adenosine 2A
receptor-mediated tissue protection: the role of CD4+T
cells and IFN-gamma. J Immunol. 176:3108–3114. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohta A and Sitkovsky M: Role of
G-protein-coupled adenosine receptors in downregulation of
inflammation and protection from tissue damage. Nature.
414:916–920. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Erdmann AA, Gao ZG, Jung U, Foley J,
Borenstein T, Jacobson KA and Fowler DH: Activation of Th1 and Tc1
cell adenosine A2A receptors directly inhibits IL-2 secretion in
vitro and IL-2-driven expansion in vivo. Blood. 105:4707–4714.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sipka S, Kovacs I, Szanto S, Szegedi G,
Brugos L, Bruckner G and Jozsef Szentmiklosi A: Adenosine inhibits
the release of interleukin-1beta in activated human peripheral
mononuclear cells. Cytokine. 31:258–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lappas CM, Rieger JM and Linden J: A2A
adenosine receptor induction inhibits IFN-gamma production in
murine CD4+T cells. J Immunol. 174:1073–1080. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Imai M, Goepfert C, Kaczmarek E and Robson
SC: CD39 modulates IL-1 release from activated endothelial cells.
Biochem Biophys Res Commun. 270:272–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Warny M, Aboudola S, Robson SC, Sevigny J,
Communi D, Soltoff SP and Kelly CP: P2Y6nucleotide
receptor mediates monocyte interleukin-8 production in response to
UDP or lipopolysaccharide. J Biol Chem. 276:26051–26056. 2001.
|
|
10
|
la Sala A, Ferrari D, Corinti S, Cavani A,
Di Virgilio F and Girolomoni G: Extracellular ATP induces a
distorted maturation of dendritic cells and inhibits their capacity
to initiate Th1 responses. J Immunol. 166:1611–1617.
2001.PubMed/NCBI
|
|
11
|
Deaglio S, Dwyer KM, Gao W, et al:
Adenosine generation catalyzed by CD39 and CD73 expressed on
regulatory T cells mediates immune suppression. J Exp Med.
204:1257–1265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang S, Apasov S, Koshiba M and Sitkovsky
M: Role of A2a extracellular adenosine receptor-mediated signaling
in adenosine-mediated inhibition of T-cell activation and
expansion. Blood. 90:1600–1610. 1997.PubMed/NCBI
|
|
13
|
Zhou L, Chong MM and Littman DR:
Plasticity of CD4+T cell lineage differentiation.
Immunity. 30:646–655. 2009.
|
|
14
|
Dong C: TH17 cells in development: an
updated view of their molecular identity and genetic programming.
Nat Rev Immunol. 8:337–348. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi YH, Shi GC, Wan HY, et al: Coexistence
of Th1/Th2 and Th17/Treg imbalances in patients with allergic
asthma. Chin Med J (Engl). 124:1951–1956. 2011.PubMed/NCBI
|
|
16
|
Shi YH, Shi GC, Ma JY, AI XY, Zhu HX and
Wan HY: Expression of T-bet, GATA-3, RORγt and Foxp3 mRNA in the
peripheral blood of allergic asthma. Journal of Internal Medicine
Concepts and Practice. 6:113–116. 2011.(In Chinese).
|
|
17
|
Weaver CT, Harrington LE, Mangan PR,
Gavrieli M and Murphy KM: Th17: an effector CD4 T cell lineage with
regulatory T cell ties. Immunity. 24:677–688. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu J, Yamane H, Cote-Sierra J, Guo L and
Paul WE: GATA-3 promotes Th2 responses through three different
mechanisms: induction of Th2 cytokine production, selective growth
of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res.
16:3–10. 2006. View Article : Google Scholar
|
|
19
|
Chen Q, Yang W, Gupta S, et al:
IRF-4-binding protein inhibits interleukin-17 and interleukin-21
production by controlling the activity of IRF-4 transcription
factor. Immunity. 29:899–911. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ivanov II, McKenzie BS, Zhou L, et al: The
orphan nuclear receptor RORgammat directs the differentiation
program of proinflammatory IL-17+T helper cells. Cell.
126:1121–1133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tesmer LA, Lundy SK, Sarkar S and Fox DA:
Th17 cells in human disease. Immunol Rev. 223:87–113. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Josefowicz SZ and Rudensky A: Control of
regulatory T cell lineage commitment and maintenance. Immunity.
30:616–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao J, Lloyd CM and Noble A: Th17
responses in chronic allergic airway inflammation abrogate
regulatory T-cell-mediated tolerance and contribute to airway
remodeling. Mucosal Immunol. 6:335–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shevach EM:
CD4+CD25+suppressor T cells: more questions
than answers. Nat Rev Immunol. 2:389–400. 2002.
|
|
25
|
Erpenbeck VJ, Hagenberg A, Krentel H, et
al: Regulation of GATA3, c-maf and T-bet mRNA expression in
bronchoalveolar lavage cells and bronchial biopsies after segmental
allergen challenge. Int Arch Allergy Immunol. 139:306–316. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Curotto de Lafaille MA, Kutchukhidze N,
Shen S, et al: Adaptive Foxp3+regulatory T
cell-dependent and -independent control of allergic inflammation.
Immunity. 29:114–126. 2008.
|
|
27
|
Borsellino G, Kleinewietfeld M, Di Mitri
D, et al: Expression of ectonucleotidase CD39 by
Foxp3+Treg cells: hydrolysis of extracellular ATP and
immune suppression. Blood. 110:1225–1232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fletcher JM, Lonergan R, Costelloe L, et
al: CD39+Foxp3+regulatory T cells suppress
pathogenic Th17 cells and are impaired in multiple sclerosis. J
Immunol. 183:7602–7610. 2009.PubMed/NCBI
|
|
29
|
Ohta A, Kini R, Ohta A, Subramanian M,
Madasu M and Sitkovsky M: The development and immunosuppressive
functions of
CD4+CD25+FoxP3+regulatory T cells
are under influence of the adenosine-A2A adenosine receptor
pathway. Front Immunol. 3:1902012.
|
|
30
|
Michel ML, Pang DJ, Haque SF, Potocnik AJ,
Pennington DJ and Hayday AC: Interleukin 7 (IL-7) selectively
promotes mouse and human IL-17-producing γδ cells. Proc Natl Acad
Sci USA. 109:17549–17554. 2012.PubMed/NCBI
|