Introduction
Hepatocellular carcinoma (HCC) is the most common
primary malignant liver tumor and ranks the fifth most prevalent
malignant tumor worldwide. In addition, China has the highest
incidence of HCC, accounting for 55% of all new cases globally.
Although many risk factors, such as alcohol, aflatoxin, hepatitis,
genetic predisposition, obesity and diabetes have been identified,
the exact molecular mechanism has yet to be determined (1–3).
Oncoprotein 18 (op18), also known as op17, op19 and
stathmin, a ubiquitous 19-kDa cytosolic phosphoprotein, has been
reported to play a critical role in microtubulin destabilizing,
spindle assembly, chromosomal stability, cell shape, mitosis, and
other cell processes. It has been reported that op18 expression
correlates with tumorigenesis and tumor progression (4–7).
However, few studies have directly compared cell proliferation and
apoptosis in HCC cell lines transfected with op18. The mechanism of
op18 in HCC should be elucidated as well as the significance of the
upregulated expression of op18 in HCC cell lines. We therefore
analyzed cell proliferation, apoptosis and cell cycle in op18
overexpression SMMC7721 cells by stably transfecting
Flag-pcDNA3.1-op18 plasmid, compared with control SMMC7721 cells,
which were transfected with Flag-pcDNA3.1 vector. The aim of this
study was to examine the involvement of op18 in human
hepatocarcinogenesis and to evaluate its prognostic significance in
HCC.
Materials and methods
Cell lines and plasmids
Human HCC SMMC7721 cells were cultured in DMEM
supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA)
at 37°C in a humidified atmosphere of 5% CO2. The
Flag-pcDNA3.1-op18 plasmid was kindly provided by Dr Baldassarre
(Texas University, USA).
Stable transfection
The Flag-pcDNA3.1-op18 plasmid or Flag-pcDNA3.1
vector was transfected into SMMC7721 cells with Lipofectamine™ 2000
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s
instructions. Promycin (200 μg/ml) was used to select stably
transfected cell lines. The transfection efficiency was then
determined by western blot analysis.
Western blot analysis
Western blot analysis was performed as described
previously (8). Briefly, total cell
proteins were extracted from SMMC7721-op18 and control cells with
cell lysis buffer (Beyotime, Jiangsu, China), separated on 10%
SDS-PAGE gels and transferred onto PVDF membrane (Millipore,
Billerica, MA, USA). The primary antibody (1:10,000; Abcam,
Cambridge, MA, USA) was then added at 4°C overnight and bound with
HRP-conjugated secondary antibodies at room temperature for 1 h.
Chemiluminescent signaling was detected using an ECL kit
(Millipore) and autoradiography.
Cell proliferation assay
The cell counting kit-8 (CCK-8; Dojindo, Kumamoto,
Japan) colorimetric assay was used to measure cell proliferation
and viability with triplicate experiments for each set of
conditions. SMMC7721 control and SMMC7721-op18 cells were seeded in
96-well plates at a density of 5×103 cells per well. The
cells were cultured for 24 h, the supernatant was removed, and 100
μl of DMEM medium containing 10 μl of CCK8 was added to each well
for 1 h at 37°C. The absorbance at 450 nm was measured with a plate
reader (Multiskan GO Microplate Spectrophotometer; Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Cell cycle assay
Cells pellet were digested and collected by trypsin,
fixed in 70% ethanol on ice and then stained with 50 μg/ml
propidium iodide (PI) (Sigma, St. Louis, MO, USA) and 0.1 μg/ml
RNase A (Sigma). The cells were detected with flow cytometry using
FACStar Plus (FACSCalibur Flow cytometer; Becton-Dickinson,
Mountain View, CA, USA). Cell Quest software (BD CellQuest Pro
Software, BD Biosciences, San Diego, CA, USA) was used to analyze
the percentage of the cell population in each phase.
Cellular apoptosis assay
Apoptosis was assessed with the Annexin V-FITC kit
according to the manufacturer’s instructions. The cells were washed
twice with cold PBS, digested, collected, and resuspended to
binding buffer. Annexin V-FITC and PI were added (BioVision,
Milpitas, CA, USA), and the cells were incubated for 10 min at room
temperature in the dark. Then, 200 μl binding buffer was added, and
the cells were calculated with flow cytometry (FACScan, BD,
Germany). The percentage of apoptosis was analyzed using the
equation: 100 × [experimental apoptosis (%) - spontaneous apoptosis
(%)]/[100 - spontaneous apoptosis (%)].
Statistical analysis
Results are expressed as means ± SD of multiple
experiments. Statistical analysis was performed with the Student’s
t-test for comparison between two groups or an analysis of variance
(ANOVA) followed by Tukey’s t-test for comparison of multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
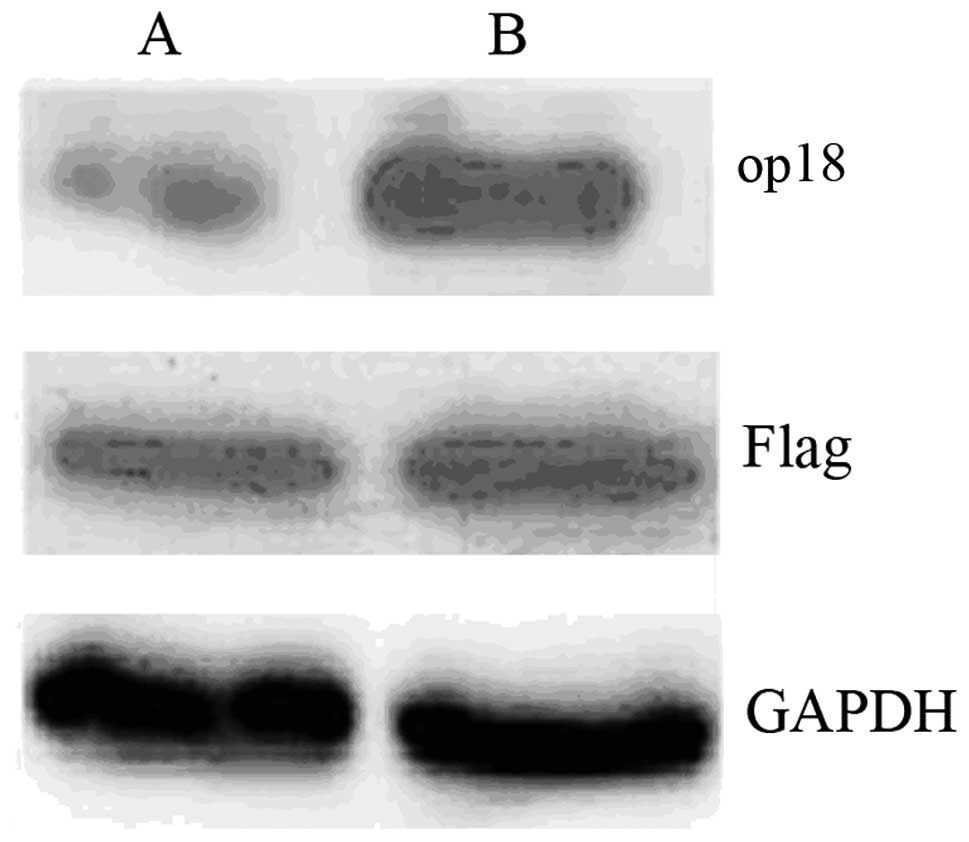
Establishment and identification of op18
overexpression SMMC7721 stable cell lines
Stable transfection method was used to establish
stably expressed Flag-pcDNA3.1 vector and Flag-pcDNA3.1-op18
plasmid human hepatocarcinoma SMMC7721 cell lines. Western blot
analysis results revealed that op18 expression was increased in
SMMC7721-op18 cells transfected with Flag-pcDNA3.1-op18 plasmid,
compared with control SMMC7721-transfected Flag-pcDNA3.1 plasmid
(Fig. 1).
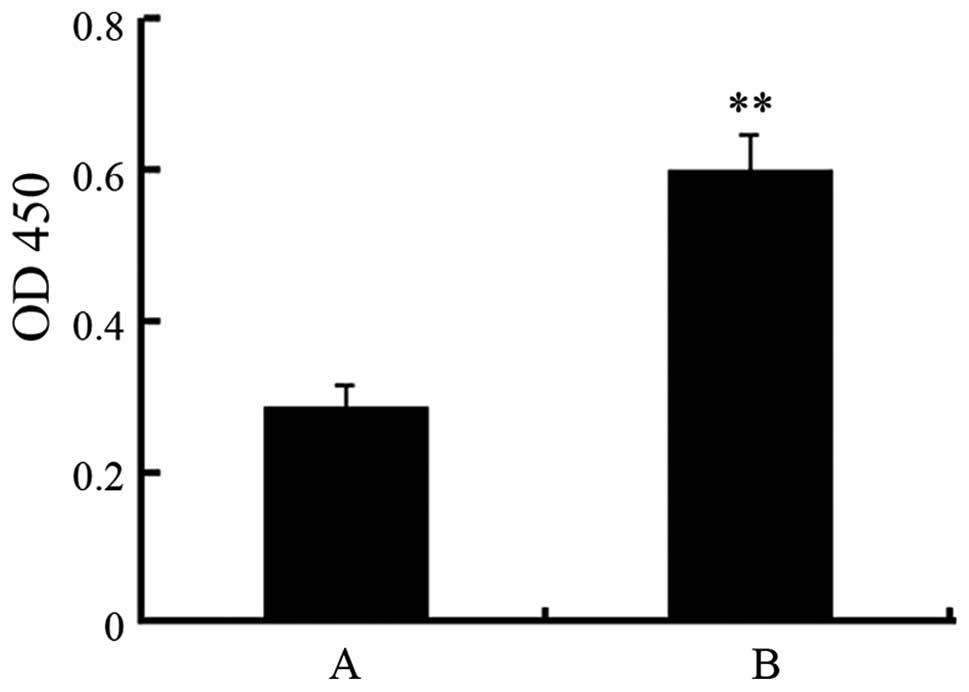
Increase of SMMC7721 cell proliferation
by op18 overexpression
To determine the effect of the upregulation of op18
expression on SMMC7721 cell proliferation, CCK8 assay was used to
analyze cell proliferation. The results showed that cell
proliferation was significantly increased in the op18
overexpression SMMC7721 cell group (0.60±0.05), compared with the
control group (0.29±0.03) at the absorbance of 450 nm (P<0.01,
Fig. 2).
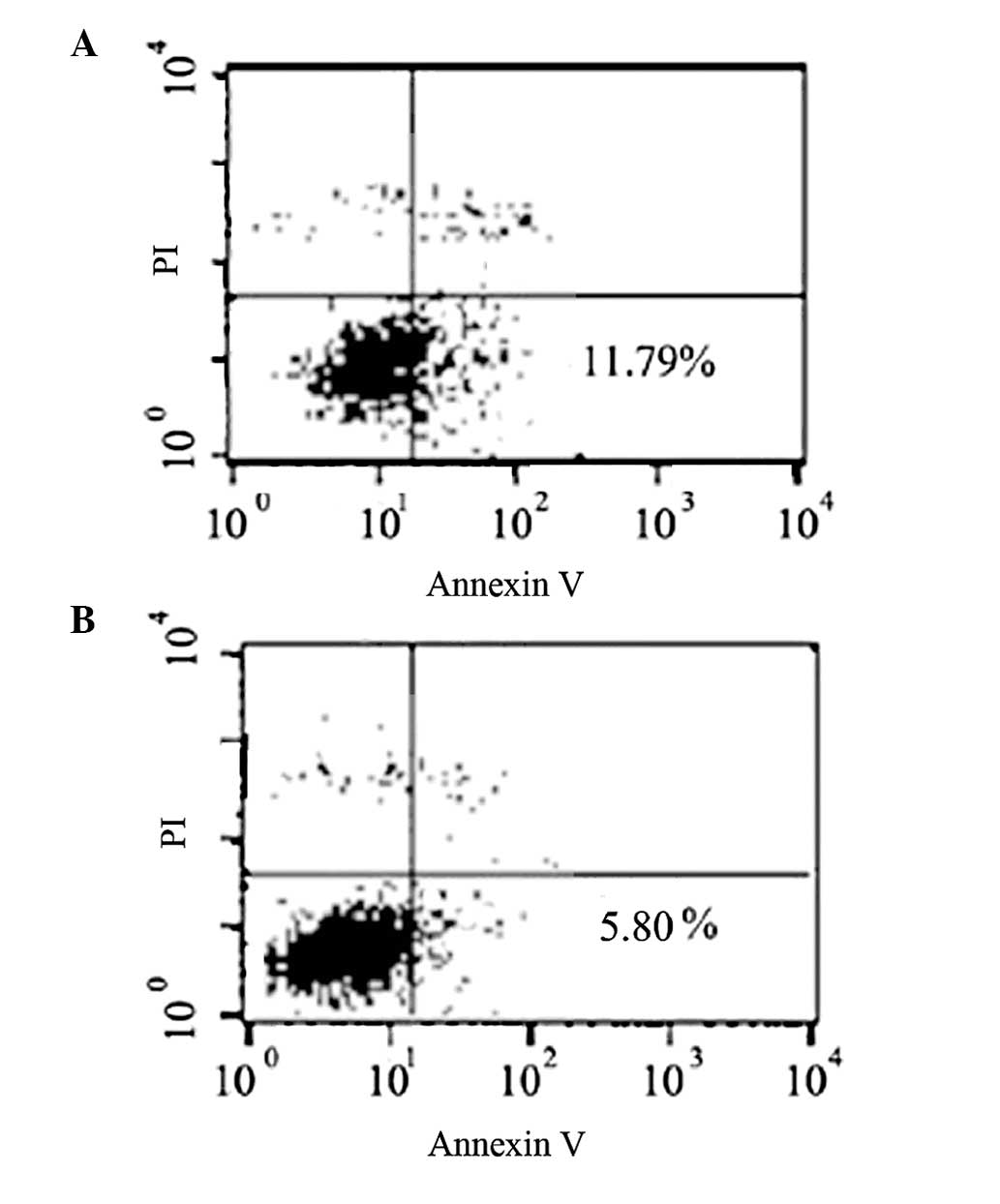
Inhibition of SMMC7721 apoptosis by
upregulating op18 expression
Flow cytometry was performed to test the effect of
op18 overexpression on SMMC7721 cell apoptosis through FITC-Annexin
V and PI labeling. The results revealed that the percentage of
apoptotic cells was inhibited to 5.80±0.33% in the op18
overexpression group, compared with 11.79±1.09% in the control
group (Fig. 3).
SMMC7721 cell cycle arrested at the phase
of G2/M by upregulation of op18 expression
To determine whether op18 plays a key role in the
progression of SMMC7721 cell cycle, we demonstrated the effect of
op18 overexpression in SMMC7721 cells on the cell cycle by single
cell analysis using FACS. The results showed that op18
overexpression induced cell cycle arrest by inhibiting progression
from G2 to M phase.
Discussion
op18 plays a crucial role in tumorigenesis and
metastasis. Numerous studies have demonstrated the upregulated
expression of op18 in several types of cancer, including breast,
prostate, lung, ovarian cancer and sarcoma (9–11),
which was associated with the malignant biological behavior of
cancer cells, as well as a potential predictor of worse prognosis
and poor treatment.
Recent studies have reported the association between
the expression of op18 and HCC (12–14).
Wang et al(12) found that
op18 distinctly expressed proteins identified in HCC cells treated
by gambogic acid (GA) by proteomic approach and western blotting.
Furthermore, it was reported that the overexpression of op18 in HCC
cells decreased their sensitivity, whereas small interfering RNAs
targeting op18 enhanced their sensitivity to GA, suggesting that
op18 is a potentially significant target for GA in combating HCC.
Results of a study by Chen et al(13) revealed that the upregulation of E2F1
and op18 proteins is associated with worse outcomes in patients
with HCC, and E2F1 significantly correlates with the op18 protein
level in HCC lesions and in vitro transactivation assays. It
was reported that op18 expression may be associated with HCC
metastasis, recurrence and prognosis, by detecting op18 mRNA
expression in normal liver, non-metastasis, metastasis and
recurrence in HCC tissues.
Our previous findings also suggested that the
consecutive upregulation of op18 expression was associated with
hepatocarcinogenesis by tissue microarray and IHC technology in
normal liver, hepatitis, hepatic cirrhosis and HCC tissue (8). To examine the role of op18 in
hepatocarcinogenesis in the present study, we established an op18
overexpression SMMC7721 cell model and detected the difference in
HCC cell proliferation, apoptosis and cell cycle in established
cell lines. The results demonstrated that the upregulation of op18
expression induced cell proliferation, inhibited cell apoptosis and
arrested the cell cycle from G2 to M phase in SMMC7721 cells,
showing that op18 overexpression was closely associated with HCC
tumorigenesis.
In an experimental model on HCC and lesions, Singer
et al(15) indicated that
the overexpression of op18 correlated with tumor progression,
proliferation and activation of a few pro-tumor factors, such as
p53. Results of that study demonstrated that op18 expression was
associated with HCC cell viability, migration and was mediated by
gain-of-function mutations in p53 (15). Accordingly, in an experimental model
of large-size HCC mice and in xenograft models of human hepatoma
tumors, Chen et al(16)
demonstrated an increase in op18-mediated tumor growth by
preventing its upregulation of EZH2 gene expression. However, any
underlying mechanistic association between op18 expression and
hepatocarcinogenesis is poorly understood, suggesting that
additional studies are required to investigate the mechanism by
which op18 is involved in HCC cell proliferation and apoptosis.
In conclusion, our findings suggest that op18
expression contributes to cell proliferation, represses cell
apoptosis and induces cell cycle arrest by inhibiting the
progression of G2 to M phase in human HCC SMMC7721-op18 cells.
Therefore, op18 is a potential predictor of prognosis in HCC.
Acknowledgements
This study was supported by the Project of the
Sichuan Provincial Department of Education (no. 11ZB125), the Youth
Foundation, and the National Natural Science Pre-Research
Foundation of Luzhou Medical College (no. 2012ZD-06, 202, 451).
References
|
1
|
Tsai CL, Koong AC, Hsu FM, Graber M, Chen
IS and Cheng JC: Biomarker studies on radiotherapy to
hepatocellular carcinoma. Oncology. 84(Suppl 1): 64–68. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Psyrri A, Arkadopoulos N, Vassilakopoulou
M, Smyrniotis V and Dimitriadis G: Pathways and targets in
hepatocellular carcinoma. Expert Rev Anticancer Ther. 12:1347–1357.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing, and treating hepatocellular carcinoma.
CA Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
D’Andrea S, Berton S, Segatto I, Fabris L,
Canzonieri V, Colombatti A, Vecchione A, Belletti B and Baldassarre
G: Stathmin is dispensable for tumor onset in mice. PLoS One.
7:e455612012.PubMed/NCBI
|
|
5
|
Chen J, Abi-Daoud M, Wang A, Yang X, Zhang
X, Feilotter HE and Tron VA: Stathmin 1 is a potential novel
oncogene in melanoma. Oncogene. 32:1330–1337. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tian X, Tian Y, Sarich N, Wu T and
Birukova AA: Novel role of stathmin in microtubule-dependent
control of endothelial permeability. FASEB J. 26:3862–3874. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nemunaitis J: Stathmin 1: a protein with
many tasks. New biomarker and potential target in cancer. Expert
Opin Ther Targets. 16:631–634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gan L, Guo K, Li Y, Kang X, Sun L, Shu H
and Liu Y: Up-regulated expression of stathmin may be associated
with hepatocarcinogenesis. Oncol Rep. 23:1037–1043. 2010.PubMed/NCBI
|
|
9
|
Belletti B and Baldassarre G: Stathmin: a
protein with many tasks. New biomarker and potential target in
cancer. Expert Opin Ther Targets. 15:1249–1266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Long M, Yin G, Liu L, Lin F, Wang X, Ren
J, Wei J, Dong K and Zhang H: Adenovirus-mediated Aurora A shRNA
driven by stathmin promoter suppressed tumor growth and enhanced
paclitaxel chemotherapy sensitivity in human breast carcinoma
cells. Cancer Gene Ther. 19:271–281. 2012. View Article : Google Scholar
|
|
11
|
Sabherwal Y, Mahajan N, Helseth DL,
Gassmann M, Shi H and Zhang M: PDEF downregulates stathmin
expression in prostate cancer. Int J Oncol. 40:1889–1899.
2012.PubMed/NCBI
|
|
12
|
Wang X, Chen Y, Han QB, Chan CY, Wang H,
Liu Z, Cheng CH, Yew DT, Lin MC, He ML, Xu HX, Sung JJ and Kung HF:
Proteomic identification of molecular targets of gambogic acid:
role of stathmin in hepatocellular carcinoma. Proteomics.
9:242–253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen YL, Uen YH, Li CF, Horng KC, Chen LR,
Wu WR, Tseng HY, Huang HY, Wu LC and Shiue YL: The E2F
transcription factor 1 transactives stathmin 1 in hepatocellular
carcinoma. Ann Surg Oncol. 20:4041–4054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsieh SY, Huang SF, Yu MC, et al:
Stathmin1 overexpression associated with polyploidy, tumor-cell
invasion, early recurrence, and poor prognosis in human hepatoma.
Mol Carcinog. 49:476–487. 2010.PubMed/NCBI
|
|
15
|
Singer S, Ehemann V, Brauckhoff A, Keith
M, Vreden S, Schirmacher P and Breuhahn K: Protumorigenic
overexpression of stathmin/Op18 by gain-of-function mutation in p53
in human hepatocarcinogenesis. Hepatology. 46:759–768. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Lin MC, Yao H, Wang H, Zhang AQ,
Yu J, Hui CK, Lau GK, He ML, Sung J and Kung HF:
Lentivirus-mediated RNA interference targeting enhancer of zeste
homolog 2 inhibits hepatocellular carcinoma growth through
down-regulation of stathmin. Hepatology. 46:200–208. 2007.
View Article : Google Scholar
|